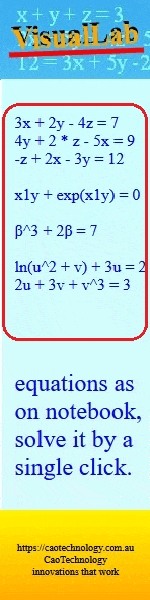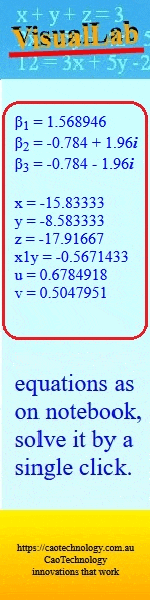
Australian Journal of Chemistry
Volume 65 Number 8 2012
RESEARCH FRONT: 33rd Australasian Polymer Symposium

This introduction sets the background to this special issue, which arose from the 33rd Australasian Polymer Symposium (33APS) held in Hobart, Tasmania, from 12 to 15 February 2012. The APS is the flagship meeting for polymer science in Australia and, over the years, the symposium has developed a strong reputation for bringing together the latest polymer research from top international and Australian polymer scientists. This special issue includes some of the best contributions to 33APS.
CH12194On the Origins of Nitroxide Mediated Polymerization (NMP) and Reversible Addition–Fragmentation Chain Transfer (RAFT)

Nitroxide mediated polymerization (NMP) and reversible addition–fragmentation chain transfer (RAFT) polymerization put Australian polymer science at the forefront of research into free radical polymerization. This and related work was largely responsible for the transformation of free radical polymerization from a mature science in the 1960–1970s to the great activity we see today.
CH12194 Abstract | CH12194 Full Text | CH12194PDF (3.6 MB) Open Access Article
CH12192Introducing the Azlactone Functionality into Polymers through Controlled Radical Polymerization: Strategies and Recent Developments

The strategies and recent developments used to prepare reactive azlactone-containing polymers using controlled radical polymerization (ATRP and RAFT) and thiol-Michael addition ‘click’ reaction are summarized. The ability of these well defined (co)polymers to react with amines under mild conditions, without the formation of by-product, makes them promising candidates for bioconjugation methodologies.
CH12251Peptide-Based Star Polymers: The Rising Star in Functional Polymers

Peptide-based star polymers show great potential as the next-generation of functional polymers due to their structure-related properties. The peptide component augments the polymer’s properties by introducing biocompatible and biodegradable segments, and enhancing their functionalities and structural ordering, which make peptide-based star polymers an attractive candidate in the field of nanomedicine. This article provides a brief summary of the recent developments of peptide-based star polymers.
CH12295Living Radical Polymerization by the RAFT Process – A Third Update

This paper provides a third update to the review of reversible deactivation polymerization radical polymerization (RDRP) achieved with thiocarbonylthio compounds (ZC(=S)SR) by a mechanism of reversible addition–fragmentation chain transfer (RAFT). This period has witnessed further significant developments, particularly in the areas of novel RAFT agents, techniques for end-group transformation, the production of micro/nanoparticles and modified surfaces, and biopolymer conjugates both for therapeutic and diagnostic applications.
CH12295 Abstract | CH12295 Full Text | CH12295PDF (24.4 MB) Open Access Article
CH12152Probing the RAFT Process Using a Model Reaction between Alkoxyamine and Dithioester

A small-molecular model reaction between alkoxyamine and dithioester was designed and performed to probe the reversible addition–fragmentation chain transfer (RAFT) process through Monte Carlo simulation fitting to the measured reaction kinetics. Possible reactions of the intermediates are discussed.
CH12164Heck Reactions in Aqueous Miniemulsions

Organic reaction in water-based nanoreactors eliminates the need for organic solvents. In this work, we demonstrate the successful miniemulsion reaction using the well known Heck cross-coupling reaction, resulting in high conversions with high trans stereoisomers.
CH12158One-Pot Endgroup-Modification of Hydrophobic RAFT Polymers with Cyclodextrin by Thiol-ene Chemistry and the Subsequent Formation of Dynamic Core–Shell Nanoparticles Using Supramolecular Host–Guest Chemistry

A thermo-responsive core–shell nanoparticle system has been created by supramolecular chemistry. The water-soluble polymer hairs are anchored to the surface of a solid PMMA core by a cyclodextrin–adamantane host–guest system. At high temperatures the particle loses its hydrophilic shell and starts precipitating, a process that can be reversed at low temperature. The PMMA nanospheres with cyclodextrin were created by a one-pot reaction involving a RAFT made polymer, heating, and a subsequent thiol-ene reaction.
CH12177Voltammetric Detection of Hg2+ Using Peptide-Functionalized Polymer Brushes

Peptide-functionalized polymer brushes are explored as a sensory coating for the voltammetric detection of Hg2+. The polymer brush based coatings allow a selective detection of Hg2+ in the subnanomolar range.
CH12182Enhanced Spin-capturing Polymerization and Radical Coupling Mediated by Cyclic Nitrones

A series of cyclic nitrones have been tested for their spin-trapping activity in enhanced spin-capturing polymerization (ESCP) of styrene and in nitrone-mediated radical coupling (NMRC) reactions. rac-2-Isopropyl-2,3-dimethyl-1-oxy-2,3-dihydro-imidazol-4-one was found to be the most efficient nitrone, allowing efficient control of polymerizations and almost ideal coupling of macroradicals.
CH12176Star Polymers of Sodium Styrenesulfonate Prepared by One-Pot TEMPO-Controlled SFRP

Using a single-pot procedure, low-dispersity star-like polymers of sodium styrenesulfonate possessing controlled, monodisperse arm lengths can be prepared. The methodology can be applied to monomers that are amenable to SFRP.
CH12185Synthesis, Characterization, and Self-Assembly of Poly(N-vinylpyrrolidone)-block-poly(vinyl acetate)

Poly(N-vinylpyrrolidone)-block-poly(vinyl acetate) (PVP-b-PVAc) block copolymers are amphiphilic materials. The amphiphilic character induces self-assembly, where the hydrophilic PVP forms the biocompatible corona. The polymers possess properties that make them potentially interesting for drug delivery applications.
CH12247Investigations on the Combination of Cationic Ring Opening Polymerization and Single Electron Transfer Living Radical Polymerization to Synthesize 2-Ethyl-2-Oxazoline Block Copolymers

Poly(2-alkyl-2-oxazoline)s are an important class of polymers and combination of these polymers with radically polymerizing vinyl monomers have been a challenge. In this report, we combine cationic ring opening polymerization and single electron transfer living radical polymerization techniques using heterofunctional initiators to prepare various block copolymers.
CH12163Cold Plasma Metallization of Supported Metal Salt Layers

Non-isothermal (cold) H2 plasma reduction of supported silver nitrate or palladium acetate leads to the formation of metallic films.
CH12232Synthesis of Poly(2-methyl-2-oxazoline) Star Polymers with a β-Cyclodextrin Core

The synthesis of star poly(2-methyl-2-oxazoline)s with a β-CD core was investigated through the arm-first and core-first strategies in order to obtain controlled structures. The behaviour of the polymers in solution was then investigated by measuring the diffusion coefficients of the polymers by DOSY NMR and by performing viscosity experiments.
CH12211Characterization of Gellan Gum by Capillary Electrophoresis

Gellan gum is a food thickener and emulsifier, and it has potential in tissue engineering. Free-solution capillary electrophoresis reveals the presence of oligomers and also separates the gellan gum according to its composition and conformation, even in the presence of aggregates. It provides a tool to assess natural variability, dissolution, ageing, and sonication.
CH12278Synthesis of Symmetrical, Substituted (alkane-α,ω-diyl)(bis[3,3′-allyl dithioethers]) Monomers for Photoplastic Polymer Networks

Symmetrical (alkane-α,ω-diyl)(bis[3,3′-allyl dithioethers]) monomer compounds, designed for enhanced photoplastic polymer networks when cured, have been synthesised from (alkane-α,ω-diyl)bis([2-{chloromethyl}allyl]sulfane) precursors. One oxygen atom in the (alkane-α,ω-diyl)-moiety is essential for high purity of precursors and final products.
CH12291RAFT Copolymerization of Styrene/Divinylbenzene in Supercritical Carbon Dioxide

The effect on polymer network formation of adding a RAFT controller in the copolymerization of styrene/divinylbenzene carried out in supercritical carbon dioxide is studied. The polymerization rate and the onset of the gelation point are both delayed, and the degree of swelling is increased as the concentration of controller is increased. Looser polymer networks are produced in scCO2, compared with bulk copolymerization. Apparently less heterogeneous polymer networks are possible.
CH12252Honeycomb Films from Perfluoropolyether-Based Star and Micelle Architectures

A perfluoropolyether-b-poly(tert-butyl acrylate) (PFPE-b-PtBA) block copolymer was used to prepare both star polymers and micelles. The star polymers were synthesised via atom transfer radical polymerisation (ATRP) and the arm-first approach, and possessed a PFPE outer shell and PtBA inner shell. In comparison, the micelles have a reversed structure with PtBA shell and PFPE core. Both micelle and CCS polymer can be fabricated into non-cracking honeycomb (HC) patterned films on both planar and non-planar surfaces.