Serial tests of T-cell function predict long-term survival in an elderly cohort from a Scottish general practice
J. C. Murdoch 1 3 , M. Elwood 2 , Phyu Sin Aye 21 Department of General Practice and Rural Medicine, Dunedin School of Medicine, University of Otago, New Zealand
2 University of Auckland, Auckland, New Zealand
3 Corresponding author. Email: camgp@xtra.co.nz
Journal of Primary Health Care 12(1) 21-28 https://doi.org/10.1071/HC19079
Published: 30 March 2020
Journal Compilation © Royal New Zealand College of General Practitioners 2020 This is an open access article licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License
Abstract
INTRODUCTION: The care of the elderly presents serious challenges to general practice. In 1979, the first author took over the care of a general practice in Scotland where 21% of registered patients were elderly. This resulted in a high workload and prompted research into how this might be mitigated.
AIM: To measure serial tests of T-cell function in these individuals in order to identify those whose immune response was impaired and assess the effect of this in a long term follow up.
METHODS: This research comprised two phases. In the assessment phase (1979–82), patients were invited to have a 3-monthly visit from a research nurse where clinical measurements were made and blood taken for immunological tests of lymphocyte proliferation after culture with phytohaemagglutinin (PHA). For each patient, all records were surveyed and problems identified. In the follow-up phase (post 1982), all deaths were assessed with complete life-long follow up.
RESULTS: Of 405 people originally invited to participate in this research, 314 (78%) agreed and 246 (153 female, 93 male) entered the follow-up phase and were followed for 36.5 years. Factors significantly associated with lower survival were age, male sex, diastolic blood pressure, current smoking and poor immune function, as demonstrated by the percentage of negative responses in at least six PHA tests. Considered in four groups by percentage of failing tests, the lowest group had a life span 4 years shorter than the highest (P < 0.01). The four groups did not differ significantly in general practitioner workload, diagnosed problems or causes of death.
DISCUSSION: Poor cellular immune function was associated with poor survival over lifetime follow up of >30 years. A sensitive, specific and longitudinally consistent measure of T-cell function is required to predict who may be at risk of poorer survival within our practices.
KEYwords: General practice; ageing; cohort study; immunology; primary health care; aged; longitudinal studies; death certificates; immune function phenomena.
Introduction
It has been claimed over the last 40 years that ‘declining function of the immune system, termed immunosenescence, leads to a higher incidence of infection, cancer and autoimmune disease related mortalities in the elderly populations’.1,2 However, this claim has never been supported by longitudinal studies in humans.
In 1977, the first author was appointed to an academic position with a view to developing a Medical School Teaching Practice. The vision behind this initiative was that academics would be able to frame research questions that were relevant to a contemporary general practice in which the proportion of patients aged ≥65 years in the practice was 21%, well above the average at the time. The research question raised was whether biomedical factors observed in this elderly population could identify people at risk of poor survival. This led to the planning of a study comparing the in vitro behaviour of lymphocytes of people who consented to have blood samples taken, with their in vivo morbidity and mortality.
The outcomes of the planned study’s 3-year assessment period assessed were acute hospital admission in the years 1979–82 and mortality from 1979–83.3 The results showed that there was no association between abnormal response to phytohaemagglutinin (PHA) and mortality, nor was there any link between these findings and the prevalence of serious infections such as acute bronchitis, pneumonia or urinary tract infection. It was therefore decided to continue the research by extending the link with mortality to the date of death for each patient. We report this extended study here.
Methods
All patients born before 1 January 1915 who were living at home and registered with the practice on 1 January 1979 were invited to participate in the initial study. They were offered a home visit every 3 months, when questionnaires would be completed, measurements made and blood taken. It was made clear that participation was entirely voluntary and that care by the practice would continue as usual, regardless of their decision regarding involvement in this research.
All general practice records of the patients agreeing to participate were analysed and serious problems were recorded. In the assessment period from 1 July 1979 to 30 June 1982, all contacts with the practice’s general practitioners were also recorded. New cardiovascular and cancer problems were noted and particular search was made for the diagnoses of acute bronchitis, pneumonia, herpes zoster and urinary tract infection, in view of the hypothesis that patients with immunological defects could be predisposed to infectious disease. Acute referrals to hospital were also recorded.
An initial assessment was made by the research nurse asking about smoking habits, mobility and dependency on others. At this examination, the nurse also measured height and weight to enable body mass index (BMI) calculation, and systolic and diastolic blood pressure using a standard sphygmomanometer. Venepuncture was performed and blood samples taken for laboratory testing of autoantibody screen and immune function.
After preparation in the laboratory, activation of lymphocytes was measured by volume spectroscopy and replicative growth by liquid scintillation according to the methods described by Gibbs et al.4 and Brown et al.5 Lymphocyte activation was regarded as abnormal when the percentage of cells responding to phytohaemagglutinin was <55%. Autoantibody tests were for antinuclear antibody, thyroid autoantibody, rheumatoid factor and antigastric antibody. We counted tests as positive if any autoantibody was present in a dilution of 1:32 or more.
At 30 June 1982, participating patients who had less than six venepunctures, had moved from the practice and who had died were excluded and the research outcome for this follow-up study changed to death certification from that date. For each death, we recorded the death date and location (home or community, rest home, acute hospital bed, long stay bed) and whether the death had happened in Dundee, Tayside, Scotland or elsewhere in the United Kingdom. The first cause of death on the certificate was recorded and whether a post mortem examination had been held. The causes were further classified into four groups: respiratory, cardiovascular, cancer and others. From 1979 to 1992, these records were made by the practice reception staff and from 1992 onwards, search was made for death certificates of survivors through the General Register Office for Scotland.
Data analysis
Data analyses were performed using Stata 13 (StataCorp, College Station, TX, USA). Descriptive analyses, including survival analysis, summarises the data. As all study participants were followed to death, there were no censored observations. Univariable linear regression analyses were performed to investigate the effect of each variable of interest on the survival time outcome (in years). The β coefficients and their 95% confidence intervals were calculated. The β coefficient is interpreted as the change in the dependent variable (survival in years) for a one unit change in the independent variable (the predictor). A two-sided P-value of <0.05 was considered significant.
To show the independent effects of each factor, multiple regression analyses were performed by using backward stepwise selection of the variables. The variable with the highest P-value was removed from the model at each step, leaving the final model with statistically (and clinically) significant variables. To guard against non-normality of the survival time, analyses using log and square root transformations, and a Cox regression on survival, were also conducted. The results presented here give a direct measure of the change in survival time associated with each factor.
Ethics
The project was funded by the Biomedical Research Committee of the Chief Scientist, Scottish Home and Health Department on 5 March 1979. At that time, there was no well-developed system of research ethics review and none took place for this study and in the institution (University of Dundee). However, the first author (J. C. Murdoch) was required to ensure that any personal data collected during the project was always securely held and handled and that the anonymity of people to whom the data refer would be preserved in any report or publication. J. C. Murdoch is required to sign and submit an annual declaration, the last of which was signed in December 2018.
Results
Of the 405 patients (258 female, 147 male) invited to participate in the initial study in 1979, 314 patients (190 female, 124 male) agreed to take part (response rate female 74%, male 84%). By the end of the assessment period at 30 June 1982, 246 patients (153 female, 93 male) fulfilled the inclusion criteria described above, with 46 having died, 12 defaulted, seven entered geriatric care and three having moved away. The mean age at 1 July 1982 of the males was 75 years (range 67.8 – 88.1 years) and the females’ mean age was 77 years (range 67.8 – 94.3 years). All were living in the community and had been under the care of the general practice team for the previous 3-year period. They had a mean of 11.2 examinations and blood tests performed during the initial study period.
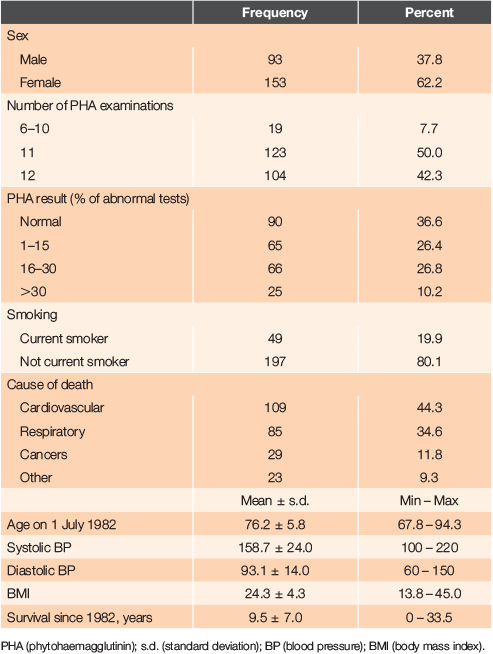
The last patient died in 2016 aged 103.3 years, having survived 33.5 years since the start of the initial study. The mean age at death of the 153 females was 87.1 years (range 71.9 – 102.3 years) and of the 93 males, the mean age was 83.5 years (range 68.0 – 103.3 years). Thirty-six percent died in the community (16% in rest homes, 8% in bed in their own home and 12% suddenly in the community). Forty-three percent died after admission to an acute hospital bed and 21% died in geriatric care. The characteristics of patients in the study are shown in Table 1.
|
In the assessment period, it was confirmed that lymphocyte response to PHA was inconsistent in individual patients, as had been previously reported.6 The range of abnormal responses, defined as <55% varied from 18.0% (9th quarter) to 6.6% (10th quarter). It was therefore decided to present the results as a percentage of abnormal tests per number of 3-monthly tests conducted (PHA percent), classified further into groups with no abnormal tests, 1–15%, 16–30% and >30% abnormal tests (Table 1).
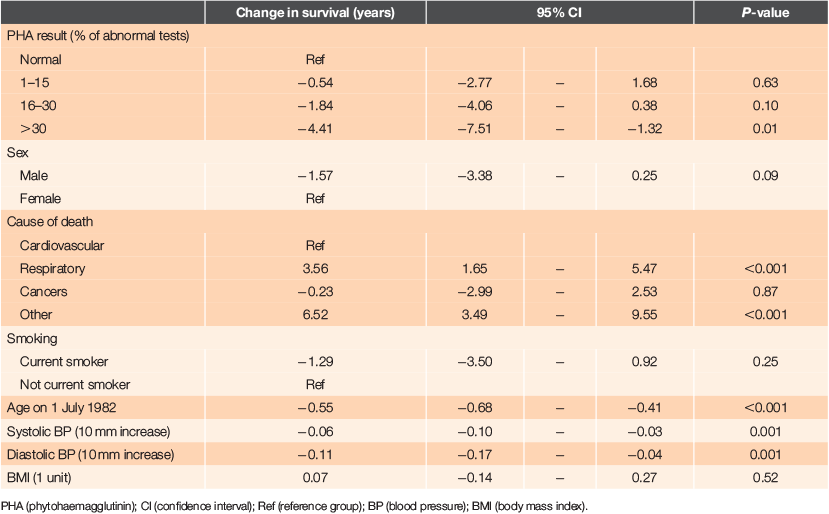
The primary analysis was of factors associated with length of survival (Table 2). There were statistically significant associations with survival in single factor analysis with PHA percent, and a dose–response effect seen with greater reductions in survival with increasing proportions of abnormal PHA tests. Significant reductions in survival were also seen with increases in age, and with both systolic and diastolic blood pressure. While reductions in survival were associated with male sex, current smoking and decreased BMI, these were not statistically significant. There were also significant associations with several other factors: dependency, mobility, the number of problems recorded initially and during the 3-year period, electrocardiogram (ECG) abnormalities, numbers of home visits and total consultations. However, none of these variables were associated with PHA percent, so they did not affect the association between survival and PHA percent.
|
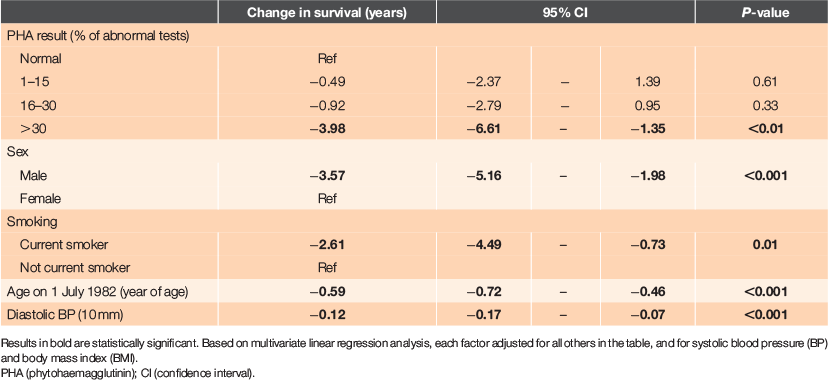
The effects of factors independently, adjusted for all other factors, are shown in Table 3, derived from multiple linear regression analysis. The reductions in survival with increasing PHA percent being abnormal and the dose–response seen, persisted after control of other factors. Having >30% abnormal tests was associated with 4.0 years’ shorter survival from study entry to death (P < 0.01). Having 16–30% and 1–15% of tests abnormal showed ~1 year and a half year shorter survival, respectively, but these effects were not statistically significant.
|
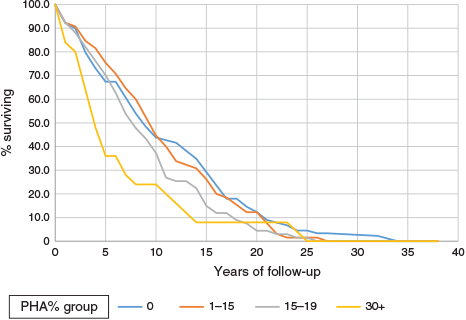
The regular trend of decreasing survival with increasing proportions of negative tests is also shown in the survival curves of Figure 1, which shows significant differences (log rank = 8.1, P < 0.005) in survival for the four groups by PHA percent. The lower two groups by PHA percent had lower survival. Survival differences are clear up to 15 years. The differences are not just in the early years. Ignoring differences before 5 years’ survival, the analysis still showed significant differences in longer-term survival: 165 patients, log rank 4.5, P = 0.03. Analysis of later periods is limited as the numbers under follow up became quite low.
|
Other independent significant effects strongly associated with shorter survival were age at study entry (an older age conferring shorter survival), male sex, increased diastolic blood pressure and current smoking. Systolic blood pressure and BMI had no significant effect on survival.
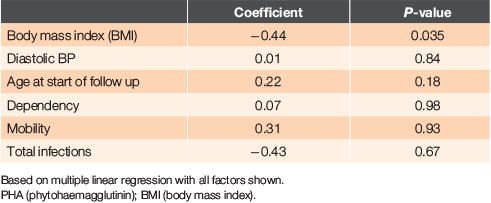
We also assessed factors associated with PHA using a multiple linear regression with PHA percent as the dependent variable (Table 4). PHA percent decreased by 0.44 with one unit increase in BMI, after controlling for other factors (P = 0.04). Thus, patients with a high BMI tended to have a lower frequency of abnormal immune function tests. However, BMI itself was not associated with survival. The association between PHA percent and survival persisted even if these other variables were controlled. This analysis also showed that PHA percent did not vary significantly with age.
|
Discussion
This study of 246 patients aged 68–94 years showed a significant association between lowered immune response measured in the assessment phase using T-cell proliferative response to PHA and reduced overall survival until the death of each individual (P < 0.01 in the most abnormal group). The analysis in Table 3 used four arbitrary groups of increasing percent of abnormal tests. Older age at study entry, male sex, smoking and higher diastolic blood pressure were most associated with shorter survival. Systolic blood pressure and BMI had no significant effect.
The association between ageing, immune response and mortality was first suggested by Roberts-Thomson et al. in 1974.7 However, the link demonstrated in their study was between negative delayed-type hypersensitivity reactions and mortality measured over a 2-year period in 52 people aged >80 years. Proliferative response to PHA was measured once in 20 people aged >60 years and 20 people aged <25 years and no link with poor survival was either looked for or established.
A further study evaluated mitogen-induced lymphocyte proliferation one to six times in 403 elderly individuals aged 70–106 years over a 25-month period.8 The evaluation revealed that 18% had a negative response to PHA, Con A and pokeweed mitogen, defined as a mean liquid scintillation count that was <10% of the mean response of the 20- to 39-year-old controls who were assayed concurrently with the elderly subjects. The mortality of the whole cohort over a 25-month period was 15% and the negative responders demonstrated twice the mortality of the positive responders. The authors suggested that the inability of lymphocytes to proliferate in response to mitogens was a predictor of death not only from malignancy or immunosuppressive therapy, but also in terms of all-cause mortality. The short follow up is a major limitation to generalising from this study.
Another study related initial immunologic function to all-cause mortality, cancer mortality, cancer incidence and pneumonia incidence over a 9-year period in 273 healthy elderly individuals.9 In this research, response to PHA was again found to be either low or normal when comparing results from the elderly to healthy young controls. People with PHA levels less than two standard deviations below the mean level of the controls were considered to have low PHA levels and all others were considered to have normal PHA levels. In a 10-year follow up, the comparable percentages for all-cause mortality for low and normal PHA were 22% and 15% respectively; a difference consistent with our findings but not statistically significant.
Further studies have been done in Sweden as part of an ongoing longitudinal investigation examining the relationship between survival and immune function.10–12 T-cell responses to activation by the mitogen, Concanavalin A, were used when 102 very old individuals (86–92 years) were studied. This revealed a subgroup associated with non-survival, whose characteristics included poor T-cell proliferative response and high CD8, low CD4 and CD19 percentages. In subsequent studies, poor proliferative response to mitogen stimulation has ceased to be measured because it was believed that changes in CD4 and CD8 subpopulations of lymphocytes alone were able to identify individuals at risk. However, a recent review found that there is still confusion, with the description of marked variations in the factors associated with mortality in elderly populations, even within Northern Europe.13
Studies of ageing in younger people using potential biomarkers such as leucocyte telomere length,14,15 C-reactive protein and many other measures16 suggest that the pace of ageing differs in young adult cohorts at an age when they are still young enough to implement potential anti-ageing therapies. The results of our study lend support to this notion because, even in this elderly cohort, ageing was not uniform. There was no significant difference in the mean values of PHA percent between the 26 individuals who were aged >85 years and the 37 who were aged <70 years at 30 June 1982. This would seem to support the findings from the Dunedin study showing that the individuals in a cohort of 38-year-olds were ageing at different rates.17
We found that the only variable associated with PHA percent in a regression analysis was BMI – with PHA percent decreasing by 0.53 with one unit increase in BMI. In other words, individuals with high BMI are likely to have lower frequency of abnormal immune function tests, but BMI itself is not associated with survival in this study. This association has not previously been reported; indeed, the opposite has been claimed.18
This was not primarily an immunological study but a general practice study. We were looking for a way we could improve the latter part of the life of patients born before 1914, in the practice we had inherited. The most important aim stated in the research application for the study in 1978 was ‘Can the results of immunological tests be used to identify patients at particular risk?’. We did identify a group of 25 patients whose in vitro T-cell behaviour predicted a significant shortening of their lifespan and a statistical difference in the whole group, but paradoxically, death was required to clarify the issue.
So how could we have gone about identifying this vulnerable group earlier? The most immunodeficient group (3) was not older but was mainly female. Other factors were compared. While no other differences were statistically significant, group 3 had a higher rate of autoimmunity and of acute admission to hospital, and had higher rates of cardiovascular causes and cancer on their death certificates. They consulted their general practitioner less and had fewer new health problems and acute infections such as pneumonia, herpes zoster and urinary tract infection than other groups. So, the ‘immune-deficient’ group was invisible in the practice and it would have required some more sophisticated screening test to identify them. Obviously, what was needed was a sensitive, specific and longitudinally consistent measure of T-cell function, as even using the methods devised 40 years ago, it is apparent that these individuals’ lymphocytes held the key to the length of their survival.
While discussion of ways that immune factors could be modified is beyond the scope of this paper, it would be very important to pay attention to identifying issues such as diastolic blood pressure and smoking prevention in patients found to have immune deficiency, as would be the provision of continuity of care by doctors. A 2018 systematic review has demonstrated that continuity of care is also associated with improved survival.19
At the heart of the mission of general practice is the efficient surveillance of a patient population. The conclusions of this study became visible only after 40 years of observations, using the only tests available 40 years ago, which required patients to be bled on multiple occasions. Muller and Pawelec20 asked the question ‘Does slippage of quality control in the immune system lead to collateral damage?’. Most of the diseases causing significant morbidity and mortality at the end of life, such as arterial disease, cancer and infections are thought to have an immunological basis. This study has shown evidence of such slippage in a long-term follow up.
Sir James Mackenzie 100 years ago described four stages of disease: the first, the predisposing stage, was when individuals were free from disease but liable to be ‘attacked’ either from some inherent weakness or from an outside source.21 We propose that one inherent weakness is immune function, and that there is a need not only to develop and evaluate sensitive, specific and longitudinally stable methods of measuring immune deficiency in primary care, but also to respect the lesson expressed by Pawelec13 and by primary care practitioners through the ages that ‘even seemingly very similar populations are actually quite different.’
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgements
The study was funded by the Chief Scientist Office of the Scottish Home and Health Department. The late Professor John Swanson Beck (1928–2007) was the supervisor of this project and the late Professor Jimmy Knox (1927–2010) believed that care of real individuals was the proper setting for academic general practice. This publication is dedicated to them and the memory of their wonderful support to a (then) young colleague.
References
[1] Stahl EC, Brown BN. Cell therapy strategies to combat immunosenescence. Organogenesis. 2015; 11 159–72.| Cell therapy strategies to combat immunosenescence.Crossref | GoogleScholarGoogle Scholar | 26588595PubMed |
[2] Pawelec G. Does the human immune system ever really become “senescent?” F1000Res. 2017; 6 1323
| Does the human immune system ever really become “senescent?”Crossref | GoogleScholarGoogle Scholar |
[3] Murdoch JC. Characteristics of the elderly in an urban general practice. PhD Thesis. University of Dundee; 1983.
[4] Gibbs JH, Brown RA, Robertson AJ, et al. A new method of testing for mitogen-induced lymphocyte stimulation: measurement of the percentage of growing cells and of some aspects of their cell kinetics with an electronic particle counter. J Immunol Methods. 1979; 25 147–58.
| A new method of testing for mitogen-induced lymphocyte stimulation: measurement of the percentage of growing cells and of some aspects of their cell kinetics with an electronic particle counter.Crossref | GoogleScholarGoogle Scholar | 422855PubMed |
[5] Brown RA, McWalter R, Slidders W, Gibbs J. Beck Measurement by Quantimet 720 of the proportion of actively growing cells in tissue cultures of human lymphocytes. J Microsc. 1979; 115 51–63.
| Beck Measurement by Quantimet 720 of the proportion of actively growing cells in tissue cultures of human lymphocytes.Crossref | GoogleScholarGoogle Scholar | 370399PubMed |
[6] Dionigi R, Zonta A, Albertario F, et al. Cyclic variation in the response of lymphocytes to phytohemagglutinin in healthy individuals. Transplantation. 1973; 16 550–7.
| Cyclic variation in the response of lymphocytes to phytohemagglutinin in healthy individuals.Crossref | GoogleScholarGoogle Scholar | 4755096PubMed |
[7] Roberts-Thomson IC, Whittingham S, Youngchaiyud U, Mackay IR. Ageing, immune response and mortality. Lancet. 1974; 304 368–70.
| Ageing, immune response and mortality.Crossref | GoogleScholarGoogle Scholar |
[8] Murasko DM, Weiner P, Kaye D. Association of lack of mitogen-induced lymphocyte proliferation with increased mortality in the elderly. Aging: Immunol Infect Dis. 1988; 1 1–6.
[9] Wayne SJ, Rhyne RL, Garry PJ, Goodwin JS. Cell-mediated immunity as a predictor of morbidity and mortality in subjects over 60. J Gerontol. 1990; 45 M45–8.
| Cell-mediated immunity as a predictor of morbidity and mortality in subjects over 60.Crossref | GoogleScholarGoogle Scholar | 2313042PubMed |
[10] Ferguson FG, Wikby A, Maxson P, et al. Immune parameters in a longitudinal study of a very old population of Swedish people: a comparison between survivors and nonsurvivors. J Gerontol A Biol Sci Med Sci. 1995; 50A B378–82.
| Immune parameters in a longitudinal study of a very old population of Swedish people: a comparison between survivors and nonsurvivors.Crossref | GoogleScholarGoogle Scholar |
[11] Wikby A, Maxson P, Olsson J, et al. Changes in CD8 and CD4 lymphocyte subsets, T cell proliferation responses and non-survival in the very old: the Swedish longitudinal OCTO-immune study. Mech Ageing Dev. 1998; 102 187–98.
| Changes in CD8 and CD4 lymphocyte subsets, T cell proliferation responses and non-survival in the very old: the Swedish longitudinal OCTO-immune study.Crossref | GoogleScholarGoogle Scholar | 9720651PubMed |
[12] Olsson J, Wikby A, Johansson B, et al. Age-related change in peripheral blood T-lymphocyte subpopulations and cytomegalovirus infection in the very old: the Swedish longitudinal OCTO immune study. Mech Ageing Dev. 2000; 121 187–201.
| 11164473PubMed |
[13] Pawelec G. Immune signatures associated with mortality differ in elderly populations from different birth cohorts and countries even within northern Europe. Mech Ageing Dev. 2019; 177 182–5.
| Immune signatures associated with mortality differ in elderly populations from different birth cohorts and countries even within northern Europe.Crossref | GoogleScholarGoogle Scholar | 29654793PubMed |
[14] Cawthon RM, Smith KR, O’Brien E, et al. Association between telomere length in blood and mortality in people aged 60 years or greater. Lancet. 2003; 361 393–95.
| Association between telomere length in blood and mortality in people aged 60 years or greater.Crossref | GoogleScholarGoogle Scholar | 12573379PubMed |
[15] Mather KA, Jorm AF, Parslow RA, Christensen H. Is telomere length a biomarker of aging? A review. J Gerontol A Biol Sci Med Sci. 2011; 66A 202–13.
| Is telomere length a biomarker of aging? A review.Crossref | GoogleScholarGoogle Scholar |
[16] Barron E, Lara J, White M, Mathers JC. Blood borne biomarkers of mortality risk: systematic review of cohort studies. PLOS ONE. 2015; 10 e0127550
| Blood borne biomarkers of mortality risk: systematic review of cohort studies.Crossref | GoogleScholarGoogle Scholar | 26372210PubMed |
[17] Belsky DW, Caspi A, Houts R, et al. Quantification of biological aging in young adults. Proc Natl Acad Sci USA. 2015; 112 E4104–10.
| Quantification of biological aging in young adults.Crossref | GoogleScholarGoogle Scholar | 26150497PubMed |
[18] Frasca D, Diaz A, Romero M, Blomberg BB. Ageing and obesity similarly impair antibody responses. Clin Exp Immunol. 187 64–70.
| Ageing and obesity similarly impair antibody responses.Crossref | GoogleScholarGoogle Scholar |
[19] Pereira Gray DJ, Holdaway-Lee K, White E, et al. Continuity of care with doctors-a matter of life and death? A systematic review of continuity of care and mortality. BMJ Open. 2018; 8 e021161
| Continuity of care with doctors-a matter of life and death? A systematic review of continuity of care and mortality.Crossref | GoogleScholarGoogle Scholar | 29959146PubMed |
[20] Müller L, Pawelec G. As we age: does slippage of quality control in the immune system lead to collateral damage? Ageing Res Rev. 2015; 23 116–23.
| As we age: does slippage of quality control in the immune system lead to collateral damage?Crossref | GoogleScholarGoogle Scholar | 25676139PubMed |
[21] Mackenzie J. in The Future of Medicine. London: Oxford Medical Publications; 1919.


