Twenty-five practical recommendations in primary care dermoscopy
Antonio Chuh 1 2 3 8 , Vijay Zawar 3 4 , Regina Fölster-Holst 3 5 , Gabriel Sciallis 3 6 , Thomas Rosemann 3 71 Department of Family Medicine and Primary Care, The University of Hong Kong and Queen Mary Hospital, Pokfulam, Hong Kong.
2 JC School of Public Health and Primary Care, The Chinese University of Hong Kong and Prince of Wales Hospital, Shatin, Hong Kong.
3 The Hong Kong Society of Primary Care Dermoscopy, Hong Kong.
4 Department of Dermatology, Godavari Foundation Medical College and Research Center, Dr Vasantrao Pawar Medical College, Nashik, India.
5 Universitätsklinikum Schleswig-Holstein, Campus Kiel, Dermatologie, Venerologie und Allergologie, Germany.
6 Emeritus, Department of Dermatology, Mayo Medical School, Minnesota, USA.
7 Institute of Primary Care, University of Zürich, Zurich, Switzerland.
8 Corresponding author. Email: antonio.chuh@yahoo.com.hk
Journal of Primary Health Care 12(1) 10-20 https://doi.org/10.1071/HC19057
Published: 15 January 2020
Journal Compilation © Royal New Zealand College of General Practitioners 2020 This is an open access article licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License
Abstract
Dermoscopy in primary care enhances clinical diagnoses and allows for risk stratifications. We have compiled 25 recommendations from our experience of dermoscopy in a wide range of clinical settings. The aim of this study is to enhance the application of dermoscopy by primary care clinicians. For primary care physicians commencing dermoscopy, we recommend understanding the aims of dermoscopy, having adequate training, purchasing dermoscopes with polarised and unpolarised views, performing regular maintenance on the equipment, seeking consent, applying contact and close non-contact dermoscopy, maintaining sterility, knowing one algorithm well and learning the rules for special regions such as the face, acral regions and nails. For clinicians already applying dermoscopy, we recommend establishing a platform for storing and retrieving clinical and dermoscopic images; shooting as uncompressed files; applying high magnifications and in-camera improvisations; explaining dermoscopic images to patients and their families; applying toggling; applying scopes with small probes for obscured lesions and lesions in body creases; applying far, non-contact dermoscopy; performing skin manipulations before and during dermoscopy; practising selective dermoscopy if experienced enough; and being aware of compound lesions. For clinicians in academic practice for whom dermatology and dermoscopy are special interests, we recommend acquiring the best hardware available with separate setups for clinical photography and dermoscopy; obtaining oral or written consent from patients for taking and publishing recognisable images; applying extremely high magnifications in search of novel dermoscopic features that are clinically important; applying dermoscopy immediately after local anaesthesia; and further augmenting images to incorporate messages beyond words to readers.
KEYwords: Basal cell carcinoma; epiluminescence; melanoma; skin cancer; skin microscopy; squamous cell carcinoma
| WHAT GAP THIS FILLS |
| What is already known: Many primary care practitioners would like to apply dermoscopy for their patients, but there are not yet any systematic recommendations for these health-care practitioners specific for their stages of applying dermoscopy. |
| What this study adds: We compiled 25 practical recommendations for primary care practitioners; for clinicians contemplating using dermoscopy, for those already applying dermoscopy, and for clinicians with an academic interest in primary care dermoscopy. Most of our recommendations are original and, where possible, we substantiate our recommendations by demonstrating dermoscopic images. |
Introduction
Dermoscopy is efficient in the early diagnosis of melanoma1–3 and possibly efficient in diagnosing keratinocyte carcinomas.4 We have previously reported novel applications of dermoscopy applied in primary care.5–7 In line with investigators in secondary care,8,9 we reported that dermoscopy enhances the diagnosis of vascular skin lesions,10 infections11 and infestations in primary care.12
We have pioneered dermoscope-guided surgical procedures performed in primary care.13–15 We further reported a procedure-control study, providing evidence that dermoscope guidance lowers the risk of incomplete excision and obvious scars 6 months after the surgical procedures.16
The use of dermoscopy in primary care varies widely in different parts of the world.17,18 We formulate here 25 recommendations from our experiences in various clinical settings. We aim to arouse the interests of primary care physicians in dermoscopy as an aid in making diagnoses and formulating plans of management.
Recommendations for primary care physicians commencing dermoscopy
1. Understand the aims of dermoscopy
Although melanoma makes up only 4% of all skin cancers, 80% of deaths from skin cancers are due to melanoma.19 Predisposing factors are: genetics, family history, sun exposure and Familial Atypical Multiple-Mole Melanoma Syndrome.20,21 Melanomas rarely arise from melanocytic naevi, but early stages of melanoma can resemble dysplastic naevi.21 The principal aim of dermoscopy is therefore to detect and diagnose melanoma. It is not to monitor naevi to see whether they might become melanomas.18
Other aims of dermoscopy include: detecting keratinocyte carcinomas,4 diagnosing other skin diseases,5–12 facilitating biopsies13–15 and complementing dermoscope-guided surgical procedures.16
2. Get yourself prepared first
Dermoscopy in the hands of inadequately trained clinicians can be dangerous. False-positive interpretations may lead to further unnecessary and invasive procedures. False-negative interpretations might delay definitive treatment.18
Participate in courses and join online societies in dermoscopy. Most courses have a clinical component in the examination. If you pass the examination, buy a scope and start using it.
3. Purchase a scope showing both unpolarised and polarised views
Dermoscopes with cross-polarisation have two polarising filters installed at 90° to each other. They obscure light reflected from skin surfaces, thus allowing for subsurface evaluations of lesions down to the papillary dermis. However, unpolarised dermoscopy is useful to evaluate skin surfaces for diseases such as scabies or seborrhoeic keratosis. The two views complement each other.
The left-hand dermoscope in Figure 1 delivers polarised views only. The other two scopes deliver both polarised and unpolarised modes. All of these models deliver clear images. The wireless dermoscope with a tablet in Figure 2 gives a live dermoscopic view of the lesion with image quality that is very near to the best in the present state of technology.

|

|
4. Maintain your equipment regularly and meticulously
Keep your hardware clean and fresh. Weak batteries, blurry lenses, opaque tissues, tissues with debris, blood and pus can obscure a good evaluation.
5. Seek consent
For medical procedures such as venepuncture, implicit consent is probably adequate as patients know what the procedure involves. However, many patients are unaware of the indications and limitations of dermoscopy. Informed oral consent usually suffices. For children and adolescents, Gillick competence18,22 should be adequate.
6. Use contact and close non-contact dermoscopy
We pragmatically define close non-contact dermoscopy as dermoscopy, with the distance between the probe and the lesion being ≤3 cm. This requires stability of the lesion and your scope, but usually yields clear views. The dermoscope in the left of Figure 1a is designed for non-contact dermoscopy. All the scopes in Figures 1 and 2 allow for both.
7. Maintain a sterile field
For contact dermoscopy, we need protection not only from bacteria and fungi, but also from viruses such as extragenital variants of human papillomaviruses.23 Simple sanitation measures such as applying alcohol swabs are therefore inadequate. We recommend placing a disposable glass slide between the probe and the skin.24 Take extra care to prevent the sides of the slide from injuring the skin. Otherwise the vasculature will be attenuated.
8. Know one algorithm, and know it well
Multiple algorithms have been established and validated for the dermoscopic evaluation of skin cancers. Become familiar with them, but choose one that works for you. We advocate the Chaos and Clues Algorithm established and validated by Clues Algorithm25–27 because this approach is simple, easy to learn and evidence-based. It is applicable to all pigmented skin lesions. The results will indicate whether a biopsy is required.
However, this algorithm does not provide an exact diagnosis. If you can ascertain if the lesion is a seborrhoeic keratosis, you can override the algorithm.25–27 For adults, if a lesion is changing, nodular or with grey circles seen on the head and neck, we would excise and not apply the algorithm. If the pigmentation is patterned as parallel lines on the ridges of acral regions (palms and soles), we also recommend excision.
Your clinical experience and common sense are invaluable. If your clinical diagnosis is incompatible with the outcome suggested by the algorithm, apply other algorithms. For indeterminate lesions, it is best to biopsy.
9. Understand the rules for special regions
Apart from the trunk and limbs, there are special rules for interpreting dermoscopic images from the scalp, face, acral regions, nails, genitalia and mucosal surfaces. Understand these rules. You may later find that interpreting dermoscopic images from these special regions is actually easier than interpreting trunk and limb lesions.
Recommendations for primary care physicians already applying dermoscopy
10. Establish a platform for storing and retrieving images
Backup your clinical and dermoscopic images regularly, if not concomitantly. Your computer should allow you to retrieve images according to the dates and times the images were taken. You can then locate the clinical photos by the dates and times from your clinical records; the additional image files are your dermoscopic images for the same patient.
11. Shoot as raw files and keep these files
Always retain the original uncompressed images. This is important clinically and for medical–legal reasons. This is analogous to ‘strikethrough’, striking through wrongly written words in the clinical records, with the original words still being readable.
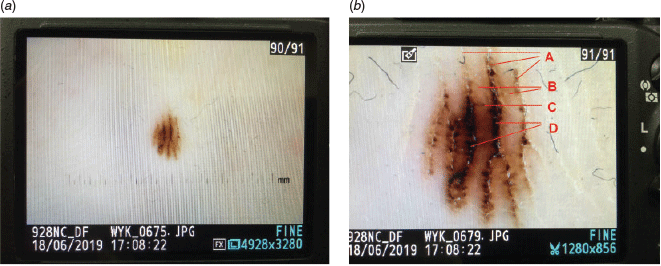
12. Apply high magnifications and other in-camera augmentations
Apply the highest magnification possible. Figure 3a is a camera shooting the back of another camera. A polarised dermoscopic image capturing a melanocytic lesion on the sole is seen. Figure 3b shows this image being magnified. The image is clearer, and interpretation is easier.
Apply other in-camera improvisations such as adjustments of contrast, hue and colour saturations. In experienced hands, augmented images can be much more easily interpreted.
13. Show augmented images to patients and their families
Whenever feasible, show augmented dermoscopic images to patients and their families. This will help them develop deeper understanding regarding the diseases and the treatment options.18
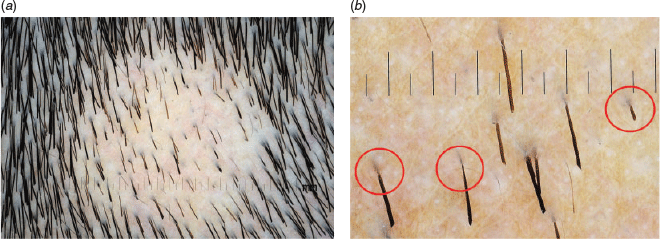
One example was a girl with a clinical diagnosis of trichotillomania. Despite detailed discussions, her mother found it difficult to accept the diagnosis. We captured polarised dermoscopic images, such as Figure 4a, where the features of trichotillomania are not obvious. When shown augmented images such as Figure 4b, with broken and kinking hairs at variable stages of hair growth, and an inflammatory background, but with no exclamation mark, the mother accepted our diagnosis. We then referred the girl for psychiatric assessments and kept providing primary care longitudinally for the family.
14. Practise toggling
Toggling means viewing dermoscopic images taken in the same position and magnification with and without cross-polarisation alternatively and repeatedly at one or two frames per second (Figure 5a, b).18 Some clinicians find that this manoeuvre enhances their conceptualisation of the three-dimensional architecture of lesions, thus facilitating diagnosis and plans for surgical procedures.
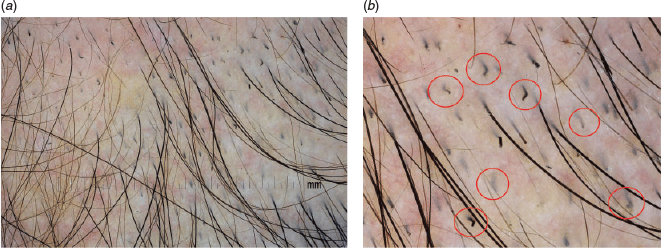
15. Use dermoscopes with small probes for obscured lesions and creases
Skin lesions in hair-bearing and concave areas such as interdigital sites and skin creases may not allow for proper focusing. In such sites, apply dermoscopes with small probes, such as the one in Figure 2. Figures 6a and 6b demonstrate the results.
16. Use far, non-contact dermoscopy
For lesions in skin creases, far (>3 cm), non-contact dermoscopy is another option, although minute movements of the patient or dermoscope will jeopardise the focus. Patients might therefore need to be lying down on an examination couch. The dermoscope would be secured by stands and clamps vertically and directly above the lesion, with the probe heading down. Far, non-contact dermoscopy is mandatory for performing dermoscope-guided surgical procedures.13–16
17. Manipulate the skin before and during dermoscopy
Stretching and contracting the skin may offer improved images for cysts, mucin-containing basal cell carcinomas, and vascular lesions. Compression of vascular lesions can help to distinguish vasculitis from simple telangiectasias. Better margin identification can also be discerned.
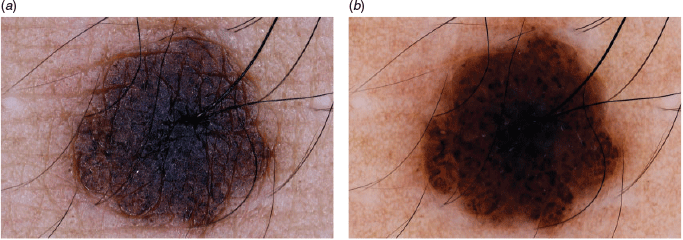
18. Practise selective dermoscopy appropriately
Acquired melanocytic naevi on an adult usually look alike. We can call them signature naevi (Figures 7a and 7b). For experienced clinicians, selective dermoscopy prevents unnecessary biopsies, particularly for patients with multiple atypical naevi.28 This assists in identifying a worrisome lesion – the ugly duckling (such as Figure 7c).

|
19. Be aware of compound lesions
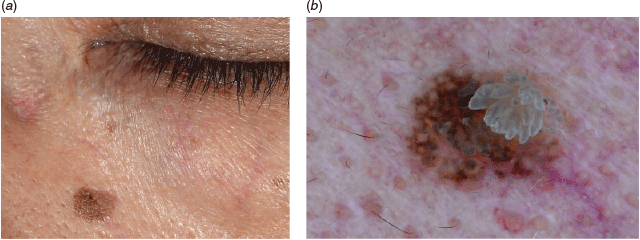
Figures 8a and 8b depict a melanocytic naevus on the face. The pattern is a pseudo-network, meaning that the pigmentation spares holes for small and dense hair follicles on the face. A viral wart, as multiple chambers with blood vessels seen inside, is extending from the right upper part to the centre of the naevus. This is therefore a compound lesion with two skin diseases bearing different aetiologies, presentations and management options. Look out for these multiple lesions in numerous combinations.
|
Recommendations for primary care physicians in academic practice with dermatology and dermoscopy as special interests
20. Use the best dermoscope models available
For taking publishable dermoscopic images, we suggest camera-lens-like dermoscopes (left, Figure 9).

|
21. Assemble separate sets of hardware for clinical photography and dermoscopy
For clinicians performing dermoscopy frequently, we recommend having two camera bodies – one for clinical photography (right, Figure 9) and the other for dermoscopy (left, Figure 9). This saves precious time otherwise spent on switching lenses, mounting adapters and other equipment, and modifying settings in the camera bodies. The risk of dust and fungal collection inside the cameras is also minimised.
22. Obtain written consent from patients for potentially publishable images
For all clinical and dermoscopic images with a possibility of future publication, especially images on the face or external genitalia, informed consent – preferably written – is necessary. For images of children and adolescents, we strongly advocate seeking informed written consent from the parents or legal guardians.
23. Apply magnification to the extremes
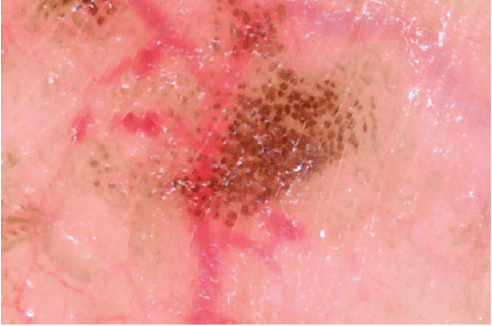
Dermatopathologists talk about ‘nests’ of melanocytes but what is a nest? Figure 10 demonstrates a polarised dermoscopic image for a lentigine on the scrotal wall. Each pigmented spot denotes one nest of melanocytes. Dermoscopic features of skin diseases seen in this order of magnification – if clinically significant – provide ample opportunities for clinical research.
|
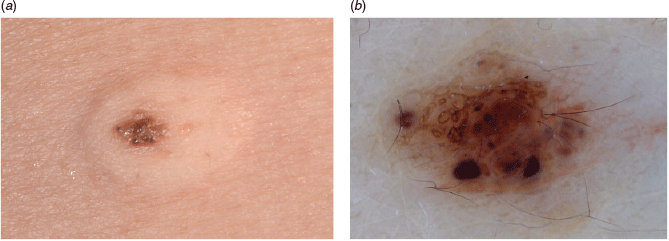
24. Apply dermoscopy after local anaesthesia
Take dermoscopic images immediately after the application of intralesional or perilesional anaesthesia. This can offer diagnostic clues of depth and vascular patterns.
Owing to the asymmetry of patterns and colours,25–27 we decided to biopsy a melanocytic naevus. We applied perilesional anaesthesia, and the lesion ‘floated’ above the deeper tissues but was still attached (Figure 11a). The dermoscopic image (Figure 11b) is crisp and sharp, and of publishable quality.
Obviously, this could only be done after the decision to biopsy has been made. The value of this manoeuvre is that the architecture and parameters of lesions are more clearly perceived by clinicians before the surgery.
25. Augment, annotate and send a message
Figure 12a is a polarised dermoscopic image from a lesion of a patient with alopecia areata. Nothing is missing but a message. We magnified, cropped, augmented and annotated, resulting in Figure 12b. The message of exclamation marks is thus lucid beyond words.
Discussion
Skin cancer is globally on the rise. Early detection is a challenge, even in countries that have health-care systems with adequate economic resources and medical staff.29 Campaigns to raise awareness and foster prevention have been ongoing for more than a decade.30,31 Due to its superficial growth and natural course in preclinical stages, skin cancer is highly amenable to screening interventions with potential economical and health benefits.32,33
In many health-care systems, primary care physicians play an important role as the first contact person and a gatekeeper. They are usually aware of skin cancer risk factors such as family history and sun exposure, so they play a key role in the early detection of skin cancer.
In a survey of 500 primary care clinicians,17 44.4% felt unsure in detecting potentially malignant lesions, and slightly more declared that they would refer all patients with suspicious skin lesions to dermatologists. In some health-care systems, the time-lags in referral actions are long. These delays can adversely affect prognoses.18
In contrast, as many as 79% of primary care practitioners expressed the wish for training in the early detection of skin cancer.17 We thus advocate for more resources to train these clinicians in applying dermoscopy.
The 25 recommendations above are the results of our decades of experience performing dermoscopy in a wide range of clinical settings and in multiple parts of the world. Each one may not be applicable to all clinicians. An example is toggling – we witnessed many clinicians performing such a manoeuvre frequently, while other clinicians found it to be of no impact at all both during the formations of clinical diagnoses and before biopsy. Yet, we would like to share our experience so that more investigators might report their experiences.
To the best of our knowledge, each of these 25 pieces of advice is our original idea or exemplification out of common sense, except for toggling, skin manipulation, selective dermoscopy, signature naevi and ugly ducklings. The toggling and skin manipulation are well-accepted practices. Unfortunately, despite an exhaustive search, we have not been able to find the original publications concerned; however, selective dermoscopy, signature naevi and ugly ducklings have been reported.3,28
Clinical medicine embraces the virtues of science, technology, art and craft. We believe that craftsmanship plays a predominate part in dermoscopy. Where possible, we substantiate some of the recommendations by dermoscopic images. As to the usefulness of some of our advice, only time can tell.
We thus conclude that our recommendations may enhance the diagnostic accuracy made by primary care physicians when confronted by skin cancer and other skin diseases. Taking into account the prominent role of primary care in most health-care systems, we believe that these recommendations might facilitate the early diagnosis of skin cancer and other skin diseases in many parts of the world.
Competing interests
Apart from such sponsorship, we received no other benefit – financial or otherwise – from these two manufacturers (Dermlite, 3Gen Inc. San Juan Capistrano, CA, USA; and Firefly Global, Belmont, MA, USA). We strongly recommend readers to consider obtaining dermoscopes from all manufacturers, not only from these two brands.
Acknowledgements
We thank DermLite and Firefly Global for sponsoring some of the dermoscopes discussed and demonstrated in this article.
References
[1] Adler NR, Kelly JW, Guitera P, et al. Methods of melanoma detection and of skin monitoring for individuals at high risk of melanoma: new Australian clinical practice. Med J Aust. 2019; 210 41–7.| Methods of melanoma detection and of skin monitoring for individuals at high risk of melanoma: new Australian clinical practice.Crossref | GoogleScholarGoogle Scholar | 30636296PubMed |
[2] Hartman RI, Lin JY. Cutaneous melanoma – a review in detection, staging, and management. Hematol Oncol Clin North Am. 2019; 33 25–38.
| Cutaneous melanoma – a review in detection, staging, and management.Crossref | GoogleScholarGoogle Scholar | 30497675PubMed |
[3] Dinnes J, Deeks JJ, Chuchu N, et al. Dermoscopy, with and without visual inspection, for diagnosing melanoma in adults. Cochrane Database Syst Rev. 2018; 12 CD011902
| Dermoscopy, with and without visual inspection, for diagnosing melanoma in adults.Crossref | GoogleScholarGoogle Scholar | 30521691PubMed |
[4] Dinnes J, Deeks JJ, Chuchu N,, et al. Visual inspection and dermoscopy, alone or in combination, for diagnosing keratinocyte skin cancers in adults. Cochrane Database Syst Rev. 2018; 12 CD011901
| Visual inspection and dermoscopy, alone or in combination, for diagnosing keratinocyte skin cancers in adults.Crossref | GoogleScholarGoogle Scholar | 30521691PubMed |
[5] Chuh AAT, Zawar V. Demonstration of residual perifollicular pigmentation in localized vitiligo – a reverse and novel application of digital epiluminescence dermoscopy. Comput Med Imaging Graph. 2004; 28 213–7.
| Demonstration of residual perifollicular pigmentation in localized vitiligo – a reverse and novel application of digital epiluminescence dermoscopy.Crossref | GoogleScholarGoogle Scholar |
[6] Chuh A, Zawar V. Pseudofolliculitis barbae – epiluminescence dermatoscopy enhanced patient compliance and achieved treatment success. Australas J Dermatol. 2006; 47 60–2.
| Pseudofolliculitis barbae – epiluminescence dermatoscopy enhanced patient compliance and achieved treatment success.Crossref | GoogleScholarGoogle Scholar | 16405487PubMed |
[7] Chuh A, Lee A, Wong W, et al. Diagnosis of pediculosis pubis – a novel application of digital epiluminescence dermatoscopy. J Eur Acad Dermatol Venereol. 2007; 21 837–8.
| Diagnosis of pediculosis pubis – a novel application of digital epiluminescence dermatoscopy.Crossref | GoogleScholarGoogle Scholar | 17567326PubMed |
[8] Jaworek-Korjakowska J. A deep learning approach to vascular structure segmentation in dermoscopy colour images. BioMed Res Int. 2018; 2018 5049390
| A deep learning approach to vascular structure segmentation in dermoscopy colour images.Crossref | GoogleScholarGoogle Scholar | 30515404PubMed |
[9] Verzì AE, Lacarrubba F, Dinotta F, Micali G. Dermatoscopy of parasitic and infectious disorders. Dermatol Clin. 2018; 36 349–58.
| Dermatoscopy of parasitic and infectious disorders.Crossref | GoogleScholarGoogle Scholar | 30201144PubMed |
[10] Chuh A, Zawar V, Sciallis G. Does dermoscopy facilitate the detection and diagnosis of vascular skin lesions? – a case-control study. J R Coll Physicians Edinb. 2018; 48 210–6.
| Does dermoscopy facilitate the detection and diagnosis of vascular skin lesions? – a case-control study.Crossref | GoogleScholarGoogle Scholar | 30191908PubMed |
[11] Chuh A, Zawar V, Ooi C, Lee A. A case-control study on the roles of dermoscopy in infectious diseases affecting the skin Part I – Viral and bacterial infections. Skinmed. 2018; 16 247–54.
| 30207527PubMed |
[12] Chuh A, Zawar V, Ooi C, Lee A. A case-control study on the roles of dermoscopy in infectious diseases affecting the skin Part II – Mycologic infections and ectoparasitic infestations. Skinmed. 2018; 16 315–9.
| 30413225PubMed |
[13] Chuh A, Fölster-Holst R, Zawar V. Dermoscope-guided lesional biopsy to diagnose EMA+ CK7+ CK20+ extramammary Paget’s disease with an extensive lesion. J Eur Acad Dermatol Venereol. 2018; 32 e92–e94.
| 28846155PubMed |
[14] Chuh A, Klapper W, Zawar V, Fölster-Holst R. Dermoscope-guided excisional biopsy in a child with CD68+ and S100– juvenile xanthogranuloma. Eur J Pediatr Dermatol. 2017; 27 134–7.
[15] Chuh A. Dermoscope-guided suturing for an open wound adjacent to the lacrimal sac and the nasolacrimal duct. Australas J Dermatol. 2018; 59 153–4.
| Dermoscope-guided suturing for an open wound adjacent to the lacrimal sac and the nasolacrimal duct.Crossref | GoogleScholarGoogle Scholar | 28891080PubMed |
[16] Chuh A, Zawar V, Sciallis G, Fölster-Holst R. Outcomes of dermoscope-guided surgical procedures in primary care. J Prim Health Care. 2019; 11 54–63.
| Outcomes of dermoscope-guided surgical procedures in primary care.Crossref | GoogleScholarGoogle Scholar | 31039990PubMed |
[17] Badertscher N, Senn O, Rossi PO, et al. Skin cancer in primary care: frequency, need to further education and subjective diagnostic certainty. A cross sectional survey among general practitioners in Canton of Zurich. Praxis. 2013; 102 1353–9.
| Skin cancer in primary care: frequency, need to further education and subjective diagnostic certainty. A cross sectional survey among general practitioners in Canton of Zurich.Crossref | GoogleScholarGoogle Scholar | 24169480PubMed |
[18] Chuh A. Roles of epiluminescence dermoscopy beyond the diagnoses of cutaneous malignancies and other skin diseases. Int J Trop Dis Health. 2017; 24 1–10.
| Roles of epiluminescence dermoscopy beyond the diagnoses of cutaneous malignancies and other skin diseases.Crossref | GoogleScholarGoogle Scholar |
[19] Miller AJ, Mihm MC. Melanoma. N Engl J Med. 2006; 355 51–65.
| Melanoma.Crossref | GoogleScholarGoogle Scholar | 16822996PubMed |
[20] Rastrelli M, Tropea S, Rossi CR, Alaibac M. Melanoma: epidemiology, risk factors, pathogenesis, diagnosis and classification. In Vivo. 2014; 28 1005–11.
| 25398793PubMed |
[21] Burns D, George J, Aucoin D, et al. The pathogenesis and clinical management of cutaneous melanoma: an evidence-based review. J Med Imaging Radiat Sci. 2019; 50 460–69.
| The pathogenesis and clinical management of cutaneous melanoma: an evidence-based review.Crossref | GoogleScholarGoogle Scholar | 31204313PubMed |
[22] Austin G. Righting a child’s right to refuse medical treatment: Section 11 of the New Zealand Bill of Rights Act and the Gillick competent child. Otago Law Rev. 1992; 7 578–96.
| 11659776PubMed |
[23] Penso-Assathiany D, Gheit T, Prétet JL, et al. Presence and persistence of human papillomavirus types 1, 2, 3, 4, 27, and 57 on dermoscope before and after examination of plantar warts and after cleaning. J Am Acad Dermatol. 2013; 68 185–6.
| Presence and persistence of human papillomavirus types 1, 2, 3, 4, 27, and 57 on dermoscope before and after examination of plantar warts and after cleaning.Crossref | GoogleScholarGoogle Scholar | 23244381PubMed |
[24] Mun JH, Park SM, Ko HC, et al. Prevention of possible cross-infection among patients by dermoscopy: a brief review of the literature and our suggestion. Dermatol Pract Concept. 2013; 3 33–4.
| Prevention of possible cross-infection among patients by dermoscopy: a brief review of the literature and our suggestion.Crossref | GoogleScholarGoogle Scholar | 24282661PubMed |
[25] Rosendahl C, Tschandl P, Cameron A, Kittler H. Diagnostic accuracy of dermatoscopy for melanocytic and nonmelanocytic pigmented lesions. J Am Acad Dermatol. 2011; 64 1068–73.
| Diagnostic accuracy of dermatoscopy for melanocytic and nonmelanocytic pigmented lesions.Crossref | GoogleScholarGoogle Scholar | 21440329PubMed |
[26] Rosendahl C, Cameron A, McColl I, Wilkinson D. Dermatoscopy in routine practice – “chaos and clues”. Aust Fam Physician. 2012; 41 482–7.
| 22762066PubMed |
[27] Weber P, Tschandl P, Sinz C, Kittler H. Dermatoscopy of neoplastic skin lesions: recent advances, updates, and revisions. Curr Treat Options Oncol. 2018; 19 56
| Dermatoscopy of neoplastic skin lesions: recent advances, updates, and revisions.Crossref | GoogleScholarGoogle Scholar | 30238167PubMed |
[28] Tromme I, Devleesschauwer B, Beutels P, et al. Selective use of sequential digital dermoscopy imaging allows a cost reduction in the melanoma detection process: a Belgian study of patients with a single or a small number of atypical nevi. PLoS One. 2014; 9 e109339
| Selective use of sequential digital dermoscopy imaging allows a cost reduction in the melanoma detection process: a Belgian study of patients with a single or a small number of atypical nevi.Crossref | GoogleScholarGoogle Scholar | 25313898PubMed |
[29] Lutz JM, Francisci S, Mugno E, et al. Cancer prevalence in Central Europe: the EUROPREVAL Study. Ann Oncol. 2003; 14 313–22.
| Cancer prevalence in Central Europe: the EUROPREVAL Study.Crossref | GoogleScholarGoogle Scholar | 12562661PubMed |
[30] Heinzerling LM, Dummer R, Panizzon RG, et al. Prevention campaign against skin cancer. Dermatology. 2002; 205 229–33.
| Prevention campaign against skin cancer.Crossref | GoogleScholarGoogle Scholar | 12399668PubMed |
[31] van der Leest RJ, de Vries E, Bulliard JL, et al. The Euromelanoma skin cancer prevention campaign in Europe: characteristics and results of 2009 and 2010. J Eur Acad Dermatol Venereol. 2011; 25 1455–65.
| The Euromelanoma skin cancer prevention campaign in Europe: characteristics and results of 2009 and 2010.Crossref | GoogleScholarGoogle Scholar | 21951235PubMed |
[32] Choudhury K, Volkmer B, Greinert R, et al. Effectiveness of skin cancer screening programmes. Br J Dermatol. 2012; 167 94–8.
| Effectiveness of skin cancer screening programmes.Crossref | GoogleScholarGoogle Scholar | 22881593PubMed |
[33] Gordon LG, Rowell D. Health system costs of skin cancer and cost-effectiveness of skin cancer prevention and screening: a systematic review. Eur J Cancer Prev. 2015; 24 141–9.
| Health system costs of skin cancer and cost-effectiveness of skin cancer prevention and screening: a systematic review.Crossref | GoogleScholarGoogle Scholar | 25089375PubMed |