Metabolic screening in primary care for patients with schizophrenia or schizoaffective disorder and taking antipsychotic medication
Rawiri Keenan 1 2 3 , Lynne Chepulis 1 , Joanna Ly 2 , Sally Carter 2 , Chunhuan Lao 1 , Muhammad Asim 2 , Abhijit Bhat 2 , Shivam Deo 2 , Kee Ping Lim 2 , Ruzaimah Mohammed 2 , Sophie Scarlet 2 , Ross Lawrenson 11 Medical Research Centre, University of Waikato, Hamilton, Waikato, New Zealand
2 Royal New Zealand College of General Practitioners, Wellington, New Zealand
3 Corresponding author. Email: rkeenan@waikato.ac.nz
Journal of Primary Health Care 12(1) 29-34 https://doi.org/10.1071/HC19023
Published: 24 February 2020
Journal Compilation © Royal New Zealand College of General Practitioners 2020 This is an open access article licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License
Abstract
INTRODUCTION: Life expectancy in patients with schizophrenia is 15–20 years less than the general population. A dominant cause of morbidity and mortality in these patients is cardiovascular disease. Adverse consequences of modifiable cardiovascular risk factors can be reduced by regular monitoring of metabolic outcomes and intervention if required.
AIM: To evaluate the metabolic screening in primary care for patients with schizoaffective disorders managed in primary care. To show the usefulness of combining simple practice audits in evaluating such areas of clinical practice.
METHODS: An audit was undertaken in eight general practices in the Waikato and Bay of Plenty regions of New Zealand. Specifically, the monitoring of patients with schizophrenia or schizoaffective disorder whose antipsychotic medication was prescribed by primary care doctors was audited. Patient monitoring was compared to the guideline recommendation of the Royal Australian and New Zealand College of Psychiatrists (RANZCP) and the Best Practice Advisory Centre (BPAC).
RESULTS: In total, 117 patients were included in the audit and none were fully monitored, as recommended by the RANZCP guidelines. Although two-thirds of patients had been evaluated for glycosylated haemoglobin (HbA1c), lipids, blood pressure, complete blood count and weight, <10% of patients had had prolactin, waist circumference or electrocardiogram measurements recorded. The proportion of patients having a HbA1c measured was also significantly higher in younger patients and patients who were non-Māori or enrolled with an urban practice (all P < 0.05). When using the simplified BPAC guidelines, half of all patients were correctly monitored.
DISCUSSION: These findings show there is room for improvement in the monitoring of patients receiving antipsychotic medication in primary care. This may indicate the need for clear guidance and general practitioner education around the monitoring requirements of these patients. Alternatively, a more simplified monitoring protocol may need to be developed. This audit has also shown that there is value in several practices completing the same audit and providing a larger cohort of patients for pooled data analysis.
| WHAT GAP THIS FILLS |
| What is already known: The gap in health care for people with long-term mental health conditions is significant and needs addressing. |
| What this study adds: This is an up-to-date New Zealand study to evaluate how well patients with psychotic disorders are being monitored against national recommendations. This audit suggests that there are significant inadequacies in monitoring of patients receiving antipsychotic medications, with inequity observed between Māori and non-Māori patients. Patients outside the usual cardiovascular risk assessment age groups are missing out on early identification of cardiovascular risks. With support, routine general practice audits can provide useful primary care research data. |
Introduction
Life expectancy in patients with schizophrenia is 15–20 years less than the general population.1–9 A dominant cause of morbidity and mortality in these patients is cardiovascular disease. Some studies involving patients with schizophrenia show that over two-thirds die of coronary heart disease, compared with approximately one half in the general population.2 The pro-arrhythmic and metabolic side-effects of antipsychotics are also well known and contribute to the risk of cardiovascular disease through weight gain as well as lipid and glucose abnormalities.2,10–13
The adverse consequences of these modifiable risk factors may be reduced by regular monitoring of metabolic measures and intervention as required. There are several relevant guidance documents for monitoring metabolic effects of antipsychotic medication. As these are potentially modifiable risk factors, recommendations by the Royal Australian and New Zealand College of Psychiatrists (RANZCP) provide guidance for the baseline and ongoing monitoring of people on these medications.14 The consensus statement of the American Psychiatric Association (APA) and American Diabetes Association (ADA) on the same subject gives fewer recommendations in regards to the extent of monitoring.15 Specifically, they suggest monitoring personal and family history, weight (body mass index; BMI), waist circumference (WC), blood pressure (BP), fasting plasma glucose (HBA1c: glycosylated haemoglobin) and fasting lipid profile. RANZCP recommends the same, but also includes monitoring of prolactin (PRL), complete blood count (CBC), electrocardiogram (ECG) and an ophthalmological review. For primary care in New Zealand, the Best Practice Advisory Centre (BPAC) produced guidelines in 2007 that recommend BP, weight, lipids and glucose monitoring only.16 Although BPAC includes fewer monitoring standards overall, they recommend that weight should be recorded 3-monthly. Similarly, the National Institute for Health and Care Excellence (NICE) have less explicit guidance around monitoring of these patients, but offer recommendations for general lifestyle support of people taking antipsychotics.17
Where patient care is shared, there is often confusion over whose role it is to provide ongoing patient monitoring.18 This is particularly relevant in an environment where increasing amounts of what has traditionally been ‘secondary care’ is being shifted into primary care. For example, there has been an increased focus and acceptance of mental health issues in the wider community in recent times, in turn increasing the number of people seeking support with mild-moderate mental health conditions in primary care.
Therefore, the primary aim of this study was to investigate how well the recommended guidelines were being adhered to for the monitoring of patients with schizophrenia or schizoaffective disorder who are prescribed antipsychotics in primary care. The audit was completed as a collective group of clinical audits of practice, as part of ongoing Royal New Zealand College of General Practitioners (RNZCGP) training requirements.
Methods
As the RANZCP guidelines incorporate the recommendations of the APA and ADA, and were the most recent (2016), these were initially used as the monitoring reference standard against which general practice monitoring performance was assessed.
A standardised template for data collection was created, based on RANZCP practice guidelines for managing schizophrenia and related disorders. Specifically, our template included BMI, WC, BP, HBA1c, lipid profile, PRL, CBC and ECG. Ophthalmologic review was not included because this is generally not routine, even in secondary care. Additional information collected included patient gender, ethnicity and age, and information describing the practice (urban or rural location and whether the practice attracts the Very Low-Cost Access (VLCA) funding).
Data for this study were collected by eight different investigators, including two experienced general practitioner (GP) fellows and six year one GP registrars at eight different general practices (seven in the Waikato Region and one in the Bay of Plenty). At each clinic, the same ‘Medtech 32©’ (patient management software; Medtech Global, Auckland, New Zealand) query was used to identify registered patients who have the Read Code of schizophrenia or schizoaffective disorder. Investigators then determined by brief review of the clinical notes (primarily the medications tab within Medtech 32©) which of these patients were prescribed any antipsychotics by the practice in the previous 12 months. Patients were included in the dataset for analysis if they were prescribed antipsychotic medication by their practice between 1 April 2016 and 31 March 2017. Patients whose most recent prescriptions were given by secondary care mental health team(s) were excluded. All data were de-identified before analysis.
The proportion of patients who had received appropriate monitoring according to the RANZCP guidelines was determined by subgroups of age, gender, ethnicity, rural or urban location and practice funding, with differences examined with a chi-squared test. Logistic regression was used to estimate the odds ratios of monitoring of Māori patients compared to non-Māori patients after adjustment for age, gender, rural or urban location and practice funding. A modified secondary analysis was then completed to determine how many patients were being monitored correctly according to the BPAC guidelines (including HBA1c, lipids, BP and weight (or WC) only). All data analyses were performed in IBM SPSS statistics 25 (New York, United States). This study was completed as an audit of clinical activity and was deemed out of scope for a formal ethics review.
Results
There were 120 patients with a diagnosis of schizophrenia or schizoaffective disorder identified across the eight general practices. Three patients were excluded as no medication name was recorded in their primary care records. The analysis therefore included 117 patients: 67 (57.3%) male and 50 (42.7%) female patients, with a mean patient age of 48 years (range 22–82 years). The ethnicity composition was 53 Māori (45.3%) and 64 non-Māori (54.7%). The non-Māori group included 55 New Zealand European (NZE) and nine patients of another ethnicity. Twenty-five patients (21%) were from rural practices and the remaining 92 (78.6%) were from urban-based practices. Four practices were VLCA funded and the remaining four had standard funding support.
Monitoring against the full RANZCGP guidelines in the 12 months of the audit is shown in Table 1. Overall, no patients were fully monitored according to RANZCP guidelines. Although at least two-thirds of patients had been evaluated for HbA1c, lipids, BP, CBC and weight, <10% of patients had had PRL, WC or ECG measurements recorded.
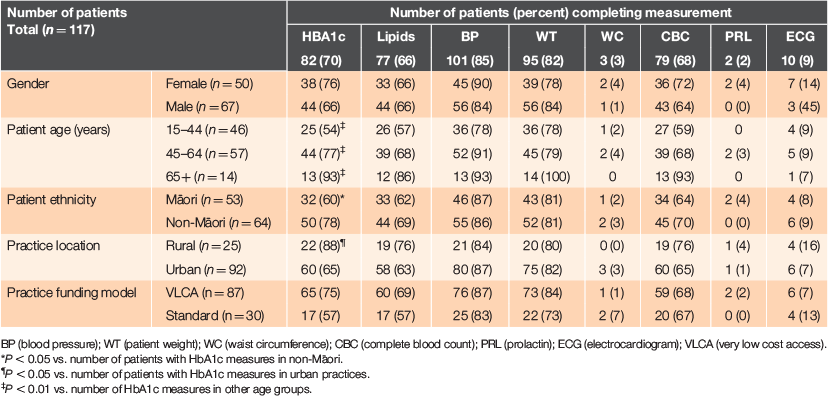
|
The proportion of patients who had their HbA1c levels measured did not differ by gender or funding model, but did differ by age, ethnicity and practice location. Patients were more likely to have an HbA1c measurement if they were non-Māori (78% vs. 60% of Māori; P < 0.05) or enrolled in a rural practice (88% vs. 65% of patients in urban practices; P < 0.05). The proportion of patients receiving HbA1c testing was lowest in patients aged 15–44 years (54%), but increased to 77% in patients aged 45–64 years and was highest (93%) in patients aged >65 years. No significant differences between subgroups were observed for any of the other metabolic measures.
When using the BPAC monitoring guidelines as a reference, more patients were fully monitored than implied by the previous analysis using RANZCP guidelines, although the proportion of complete monitoring remained low. Approximately half of all men (51%) and women (52%) were screened appropriately, although this differed by age. Patients aged >65 years were most likely to be fully monitored (79%) compared to patients aged 15–44 years (45%) and 45–64 years (60%; P = 0006). However, there was no difference in the proportion of Māori and non-Māori patients being fully monitored (45% vs. 56%; P = 0.237) or by rurality (P = 0.595) or practice funding model (P = 0.152).
With logistic regression, there was no difference for the proportion of Māori and non-Māori patients being monitored for the different metabolic endpoints for either the RANZCP or BPAC guidelines.
Discussion
These findings suggest that there is scope to improve the monitoring of patients receiving antipsychotic medication in primary care. Neither the RANZCP nor BPAC guidance were followed to any great level. This is a similar to a finding in 2011 when a meta-analysis found similar numbers and no significant difference between database audits like ours and complete case studies.19 Even if we had used the ADA–APA statement, only one patient would have received full monitoring.
Monitoring in the general practices appeared to be more likely to be undertaken in line with the older 2007 guidance from BPAC. This is likely to be due to the fact that these recommendations are targeted specifically at primary care. Regardless, the completeness was ~50%, but clearly less in the younger age group.
Overall, it appears the concerns around metabolic monitoring are generally known, given the highnumber who had diabetes and cholesterol monitoring. Although age appeared to be the main factor in increased likelihood of HBA1c tests, it is possible that the high number of these tests was in fact due to awareness in cardio-vascular risk assessment (CVRA).
One of the main measures missing was weight (or WC). Improvement in recording this measure would take us to near complete monitoring within this patient group. It is possible that weight is recorded, just not as regularly as the guidance suggests. We suspect that if we included weight within 5 years, monitoring rates would improve, although this timeframe would allow for significant changes for the patient. Five years is the usual time frame for a CVRA, and these checks are part of practice and regional performance programmes. A high number of patients receiving HBA1c and lipid checks were in the eligible group for this (≥35 years, depending on certain criteria).20
The other investigations (CBC, PRL and ECG) are possibly conducted on initiation of antipsychotic medicines and, overall, it seems some refreshed guidance for primary care is required. It would also be worth seeking clarification from psychiatrists and the literature regarding the ongoing frequency of tests such as CBC and PRL. Equally, if GPs’ behaviour is to change, clearer reasons are required as to why WC is needed in addition to weight alone. This would also help in explaining the need for these assessments to patients.
It is possible a more pragmatic approach to the guidance could be sought and implemented given the high rates of general monitoring in primary care for patients prescribed antipsychotics. The use of ECG on initiation of these medications would be an interesting though more challenging audit to complete. The recording of clinical procedures such as an ECG is less systematic (blood tests are in a defined area of the electronic record but ECGs are not), but also the cost of an ECG is often prohibitive to patients and they are not easily obtained in the community.
Importantly, this study highlights the fact that there is a need for either a more simplified monitoring policy or for improved GP education. As with many areas of medicine now, there is an increasing expectation for primary care to manage health conditions that in the past have been the domain of the secondary care services. Mild-to-moderate depression has always been part of primary care, but initiation and ongoing titration of antipsychotic medication once was in the realm of secondary care providers. In the current study, we excluded patients whose medication had been prescribed in secondary care, as full medication information was not always available in the primary care records. This is a study limitation; we recognise that GPs have a role in the ongoing monitoring of cardiometabolic issues in these patients. However, the aim of this paper was to evaluate the monitoring of patients with schizophrenia or schizoaffective disorder whose antipsychotic medication is prescribed in primary care. Patients under the care of a psychiatrist still require monitoring, but in our region at least this is often undertaken by the community mental health team.
In addition to the devolution of services, we also acknowledge that there are now wider uses for some of these medications. In particular, medications such as quetiapine, which has fewer extrapyramidal side-effects, are being increasingly used in lower doses for moderate and treatment-resistant depression.11,21,22 Although we did not include patients in our study who were taking antipsychotic medications for other reasons, this does ultimately mean that GPs and primary care teams need to become increasingly aware of ongoing monitoring requirements for a variety of different patients. An opportunity to review this more widely, investigating the monitoring in all patients taking this group of medications, would be useful.
GPs in New Zealand are required to complete audits for their training and ongoing competence and continual professional standards.23 Such audits usually focus on an individual doctor’s or practice’s care. Results are rarely pooled to give larger numbers, so provide limited ability to understand any trends. For this study, a local group of trainees agreed to undertake the same audit in their own practices and these data were then combined for analysis. This allowed us to better understand how well patients with schizophrenia or schizoaffective disorder are being metabolically monitored, rather than collecting data from only one GP or practice, which may produce a biased dataset. Importantly, however, this clinical audit was only completed once, and there is no way to assess whether these results (individual GP or pooled data) have led to a change in GP practice or patient care. Ideally, there should be a second audit cycle to assess for improvement, and this should be followed up in further studies.
Competing interests
The authors declare no competing interests.
Funding
This research did not receive any specific funding.
References
[1] Dembling BP, Chen DT, Vachon L. Life expectancy and causes of death in a population treated for serious mental illness. Psychiatr Serv. 1999; 50 1036–42.| Life expectancy and causes of death in a population treated for serious mental illness.Crossref | GoogleScholarGoogle Scholar | 10445651PubMed |
[2] Newcomer JW. Metabolic considerations in the use of antipsychotic medications: a review of recent evidence. J Clin Psychiatry. 2007; 68 20–7.
| 17286524PubMed |
[3] Chang CK, Hayes RD, Perera G, et al. Life expectancy at birth for people with serious mental illness and other major disorders from a secondary mental health care case register in London. PLoS One. 2011; 6 e19590
| Life expectancy at birth for people with serious mental illness and other major disorders from a secondary mental health care case register in London.Crossref | GoogleScholarGoogle Scholar | 21747961PubMed |
[4] Laursen TM, Wahlbeck K, Hallgren J, et al. Life expectancy and death by diseases of the circulatory system in patients with bipolar disorder or schizophrenia in the Nordic countries. PLoS One 2013; 8 e67133
| Life expectancy and death by diseases of the circulatory system in patients with bipolar disorder or schizophrenia in the Nordic countries.Crossref | GoogleScholarGoogle Scholar | 24223877PubMed |
[5] Khasawneh FT, Shankar GS. Minimizing cardiovascular adverse effects of atypical antipsychotic drugs in patients with schizophrenia. Cardiol Res Pract. 2014; 2014 273060
| Minimizing cardiovascular adverse effects of atypical antipsychotic drugs in patients with schizophrenia.Crossref | GoogleScholarGoogle Scholar | 24649390PubMed |
[6] Ringen PA, Engh JA, Birkenaes AB, et al. Increased mortality in schizophrenia due to cardiovascular disease - a non-systematic review of epidemiology, possible causes, and interventions. Front Psychiatry. 2014; 5 137
| Increased mortality in schizophrenia due to cardiovascular disease - a non-systematic review of epidemiology, possible causes, and interventions.Crossref | GoogleScholarGoogle Scholar | 25309466PubMed |
[7] Lêng CH, Chou MH, Lin SH, et al. Estimation of life expectancy, loss-of-life expectancy, and lifetime healthcare expenditures for schizophrenia in Taiwan. Schizophr Res. 2016; 171 97–102.
| Estimation of life expectancy, loss-of-life expectancy, and lifetime healthcare expenditures for schizophrenia in Taiwan.Crossref | GoogleScholarGoogle Scholar | 26811230PubMed |
[8] Ilyas A, Chesney E, Patel R. Improving life expectancy in people with serious mental illness: should we place more emphasis on primary prevention? Br J Psychiatry. 2017; 211 194–7.
| Improving life expectancy in people with serious mental illness: should we place more emphasis on primary prevention?Crossref | GoogleScholarGoogle Scholar | 28882826PubMed |
[9] Te Pou o te Whakaaro Nui. The physical health of people with mental health conditions and/or addiction: Summary evidence update - December 2017. Auckland, NZ: Te Pou o te Whakaaro Nui; 2017.
[10] Casey DE. Dyslipidemia and atypical antipsychotic drugs. J Clin Psychiatry. 2004; 65 27–35.
| 15600382PubMed |
[11] Van Gaal LF. Long-term health considerations in schizophrenia: metabolic effects and the role of abdominal adiposity. Eur Neuropsychopharmacol. 2006; 16 S142–8.
| Long-term health considerations in schizophrenia: metabolic effects and the role of abdominal adiposity.Crossref | GoogleScholarGoogle Scholar | 16863690PubMed |
[12] Bell SG, Newcomer SF, Bachrach C, et al. Challenges in replicating interventions. J Adolesc Health. 2007; 40 514–20.
| Challenges in replicating interventions.Crossref | GoogleScholarGoogle Scholar | 17531757PubMed |
[13] Mangurian C, Chaudhry S, Capitelli L, et al. Implementation of a weight loss program for Latino outpatients with severe mental illness. Community Ment Health J. 2013; 49 150–6.
| Implementation of a weight loss program for Latino outpatients with severe mental illness.Crossref | GoogleScholarGoogle Scholar | 22447345PubMed |
[14] Royal Australian and New Zealand College of Psychiatrists Royal Australian and New Zealand College of Psychiatrists clinical practice guidelines for the management of schizophrenia and related disorders. Aust N Z J Psychiatry. 2016; 50 1–117.
[15] American Diabetes Association, American Psychiatric Association Consensus development conference on antipsychotic drugs and obesity and diabetes. Diabetes Care. 2004; 27 596–601.
| Consensus development conference on antipsychotic drugs and obesity and diabetes.Crossref | GoogleScholarGoogle Scholar | 14747245PubMed |
[16] Best Practice Advisory Centre. Monitoring for metabolic disorders in patients taking antipsychotic drugs. Dunedin, NZ: Best Practice Advisory Centre; 2007. [cited 2018 December]. Available from: https://bpac.org.nz/magazine/2007/february/antipsychotics.asp
[17] National Institute for Health and Care Excellence. Psychosis and schizophrenia in adults: prevention and management. London, UK: NIHCE; 2014; [cited 2018 December]. Available from: https://www.nice.org.uk/guidance/cg178/chapter/1-Recommendations#care-across-all-phases
[18] Mangurian C, Giwa F, Shumway M, et al. Primary care providers’ views on metabolic monitoring of outpatients taking antipsychotic medication. Psychiatr Serv. 2013; 64 597–9.
| Primary care providers’ views on metabolic monitoring of outpatients taking antipsychotic medication.Crossref | GoogleScholarGoogle Scholar | 23728604PubMed |
[19] Mitchell AJ, Delaffon V, Vancampfort D, et al. Guideline concordant monitoring of metabolic risk in people treated with antipsychotic medication: systematic review and meta-analysis of screening practices. Psychol Med. 2012; 42 125–47.
| Guideline concordant monitoring of metabolic risk in people treated with antipsychotic medication: systematic review and meta-analysis of screening practices.Crossref | GoogleScholarGoogle Scholar | 21846426PubMed |
[20] Ministry of Health. Cardiovascular Disease Risk and Assessment Management for Primary Care. Wellington, NZ: Ministry of Health; 2018.
[21] Han C, Wang SM, Kato M, et al. Second-generation antipsychotics in the treatment of major depressive disorder: current evidence. Expert Rev Neurother. 2013; 13 851–70.
| Second-generation antipsychotics in the treatment of major depressive disorder: current evidence.Crossref | GoogleScholarGoogle Scholar | 23898855PubMed |
[22] Wang SM, Han C, Lee SJ, et al. Second generation antipsychotics in the treatment of major depressive disorder: an update. Chonnam Med J. 2016; 52 159–72.
| Second generation antipsychotics in the treatment of major depressive disorder: an update.Crossref | GoogleScholarGoogle Scholar | 27689026PubMed |
[23] Royal New Zealand College of General Practitioners. Continuing Professional Development Programme 2014–2017. Wellington, NZ: RNZCGP; 2013.


