Phosphate theft: a path to fungal pathogenic success
Julianne T Djordjevic A B and Sophie Lev AA Centre for Infectious Diseases and Microbiology, Westmead Millennium Institute, 176 Hawkesbury Road, Westmead, NSW 2145, Australia
B Corresponding author. Tel: + 61 2 8627 3420, Email: julianne.djordjevic@sydney.edu.au
Microbiology Australia 36(2) 49-52 https://doi.org/10.1071/MA15018
Published: 19 March 2015
Inorganic phosphate/PO43–/Pi is an essential and major constituent of numerous cellular components in all eukaryotes, including fungi. These components include nucleic acids, phospholipids and ATP. Despite its abundance in organic compounds, Pi is relatively scarce in its free form. To become successful pathogens, fungi must therefore acquire free Pi from the host environment via enzyme-mediated hydrolysis of Pi-containing molecules and/or via more efficient use of their own Pi. Fungal adaptation to a Pi-limited environment is governed by the phosphate (PHO) system, a cellular pathway consisting of Pi transporters, Pi mobilising enzymes and regulatory elements, such as kinases and transcription factors that respond to Pi levels. This system is well studied in the model non-pathogenic yeast, Saccharomyces cerevisiae, but not in fungal pathogens. In this review we present what is known about the PHO system in the model fungal pathogen, Cryptococcus neoformans, including our identification and characterisation of a secreted acid phosphatase, Aph1, which serves as a valuable reporter for identifying the less well-conserved PHO elements, including transcription factors.
Cryptococcus neoformans has restricted access to phosphate (Pi) during host infection
Cryptococcus neoformans is a deadly fungal pathogen with a high rate of morbidity and mortality worldwide1. Its pathogenicity is attributable to the production of a multitude of virulence factors, including a polysaccharide capsule, melanin and the enzymes phospholipase B/C and acid phosphatase (Aph1), which collectively promote host invasion, protection against host-derived stress, evasion of the host immune system and nutrient acquisition2,3 (for review see Coelho et al.4). C. neoformans also provides a suitable model for understanding the PHO system in fungal pathogens. It has a fully sequenced genome that is highly amenable to manipulation and is currently being used by many laboratories around the world, including our own, to understand mechanisms of fungal virulence. C. neoformans, which infects the lungs and disseminates to the central nervous system via the blood stream and lymphatics, must obtain Pi from the host if it is to survive and become a successful pathogen, since mutant strains with reduced ability to either hydrolyse extracellular complexes containing Pi (acid phosphatase-deficient)5 or transport free Pi across the plasma membrane6 are hypovirulent in mice. Identifying the full repertoire of PHO responsive genes involved in the mobilisation of Pi from host and fungal sources, and their mode of regulation, is therefore of paramount importance. Knowledge of the PHO system in S. cerevisiae has led to the identification of some components of the PHO system in C. neoformans, including a secreted acid phosphatase Aph1, which is a useful reporter for identification of the less conserved elements, including transcription factors.
The PHO system in S. cerevisiae: a guide to understanding Pi homeostasis in fungal pathogens
The PHO system was extensively studied in S. cerevisiae and includes the high affinity membrane transporters (Pho84 and Pho89) system, polyphosphate (polyP) metabolising enzymes predominantly located in vacuoles, elements of the PHO regulation machinery and a number of PHO responsive genes involved in acquisition and storage of Pi. PHO-responsive genes include the high affinity Pi transporters and the secreted acid phosphatases, Pho5, Pho11 and Pho12, with Pho5 being the major secreted acid phosphatase. Excess cellular Pi is stored in vacuoles in chains of tens to hundreds of inorganic Pi molecules (polyPs), and is the first Pi storage source to be accessed during Pi deprivation. PolyP stores are believed to be exhausted prior to activation of the PHO system.
The S. cerevisiae PHO regulation machinery consists of the cyclin/cyclin-dependent kinase (CDK) complex Pho80/Pho85, the CDK inhibitor Pho81 and the transcription factor Pho4. During Pi deprivation, Pho81 represses the Pho80-Pho85 complex, preventing phosphorylation of Pho4. Hypophosphorylated Pho4 activates the expression of PHO responsive gene. Lee et al demonstrated that activation of the PHO system during Pi limitation paradoxically requires highly phosphorylated inositol species known as inositol pyrophosphates (PP-IPs)7,8. PP-IPs are comprised of inositol covalently linked to mono- and di-phosphate groups, and are derived from the mono-phosphorylated (IP) species. Exactly how PP-IPs are involved in PHO system regulation remains controversial. Lee et al established that the concentration of intracellular PP-IP5 (IP7) increased under Pi-deficient conditions and that IP7 allosterically modulates the CDK inhibitor Pho81 leading to a conformational change in the Pho85-Pho80-Pho81 complex and reduced phosphorylation of Pho47,8. However, Lonetti et al. demonstrated the opposite, with intracellular PP-IP levels decreasing by 80% during Pi deprivation9. Despite extensive investigation of the PHO pathway in S. cerevisiae, the mechanism for sensing intracellular Pi concentrations and the identity of the Pi sensor remain to be elucidated.
The PHO system in C. neoformans and its role in virulence
Only a few components of the PHO system have been identified in C. neoformans on the basis of their homology to PHO components from S. cerevisiae and responsiveness to the intracellular Pi status. These include the high-affinity Pi transporters (Pho840, Pho84 and Pho89)6, a family of secreted and intracellular acid phosphatases (Aph1, Aph3 and Aph4) (Lev et al.5 and our unpublished observation) that hydrolyse complex organic Pi sources, and the polyP polymerase Vtc4, which synthesises vacuolar polyPs6.
Pi transporters: A cryptococcal mutant defective in Pi uptake, Δpho840Δpho84Δpho89, is significantly attenuated for virulence in a mouse model of cryptococcosis, confirming that C. neoformans must acquire Pi from the host environment to reach its full virulence potential6.
Extracellular acid phosphatase Aph1: Extracellular Pi is often complexed to organic molecules and must be released by secreted acid phosphatases. The liberated Pi is then taken up by the Pi transporters. Acid phosphatase activity has been detected in the secretions of a large majority of C. neoformans strains isolated from patients with AIDS including the clinical type strain H9910. In a proteomic analysis of the H99 secretome, we identified the classically secreted acid phosphatase, Aph1, and deleted its encoding gene, creating Δaph15. Using a chromogenic enzyme assay which measures hydrolysis of the synthetic substrate, para-nitrophenol phosphate (pNPP), we found that the Δaph1 mutant was deficient in secreted acid phosphatase activity during Pi deprivation, confirming that Aph1 is the major secreted acid phosphatase in C. neoformans5. qPCR revealed that, similar to Pho5 from S. cerevisiae, Aph1 production during Pi deprivation is regulated at the transcriptional level. We also found that the concentration of Pi in a standard cell culture medium is sufficiently low to induce APH1 expression providing further evidence that C. neoformans encounters a low Pi environment during host infection. Δaph1 was less virulent in Galleria mellonella and mice, consistent with Pi mobilisation from complex sources being essential for virulence5. However, Aph1 deficiency had less of an impact on virulence than loss of the high affinity Pi transport system, suggesting that Aph1 hydrolyses only a proportion of the extracellular Pi sources available within the host. Other enzymes that potentially work in conjunction with Aph1 to achieve comprehensive mobilisation of Pi from complex sources include alkaline phosphatases, phosphodiesterases and serine/threonine/tryrosine phosphatases.
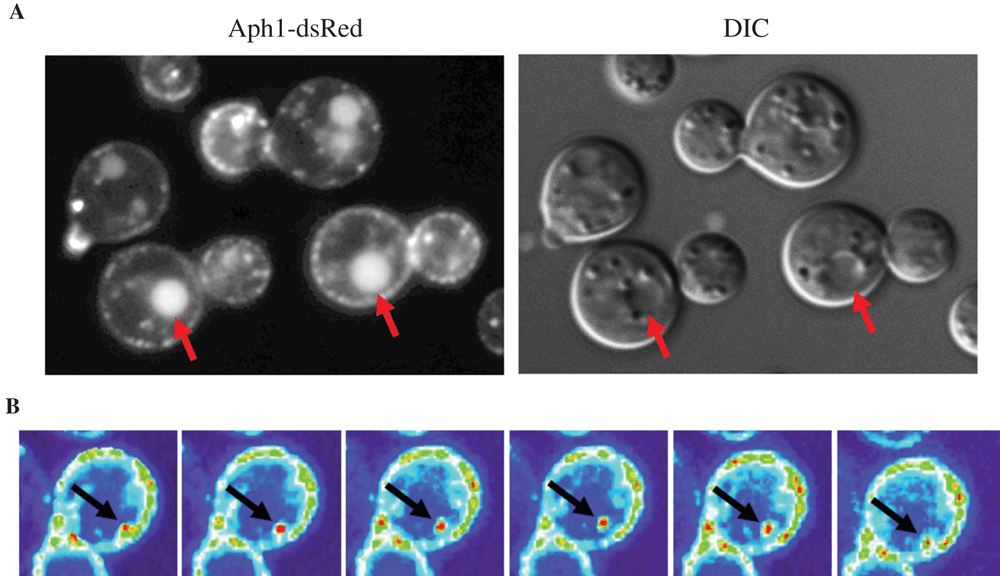
A role of intracellular Aph1 in Pi mobilisation: We found that Aph1 hydrolyses a broad range of substrates, including glucose-1-phosphate, β-glycerol phosphate, adenosine monophosphate (AMP) and mannose-6-phosphate and prefers the aromatic amino acid phosphotyrosine to phosphoserine/phosphothreonine5. By tagging Aph1 with the red fluorescent protein DsRed we observed its transport to vacuoles and the cell periphery via endosome-like structures (Figure 1A)5. Aph1-containing endosomes were highly mobile and were often observed transiently contacting the plasma membrane and vacuoles, reminiscent of the kiss-and-run mechanism observed for synaptic vesicle release11 (Figure 1B). Acid phosphatases are often found inside acidic vacuoles, an environment conducive to the working pH of Aph1 (pH 5). The dual targeting of Aph1 to vacuoles and the extracellular environment, and the broad substrate specificity of Aph1 is consistent with a role for Aph1 in releasing Pi from a wide range of both extra- and intracellular sources.
Vacuolar PolyP polymerases: Interestingly, the recruitment of Pi from polyPs is dispensable for fungal virulence since a VTC4 deletion mutant was as virulent as WT in a mouse infection model6.
Intracellular acid phosphatases: C. neoformans also produces three intracellular acid phosphatases (Aph2, Aph3 and Aph4). APH3 and APH4, but not APH2, are induced by low Pi (our unpublished observation). The decoupling of Aph2 from Pi regulation was unexpected given that Aph2 is most similar to Aph1. To test for potential up-regulation of other members of the APH family as a compensation for the loss of APH1, we measured APH 2, 3 and 4 mRNA in Δaph1, but found similar levels to WT (our unpublished observation), consistent with a lack of compensation. The contribution of each intracellular APH to cryptococcal virulence will be investigated by constructing single and combination deletion mutants.
Pho transcription regulatory machinery: Probable PHO regulatory components with similarity to the cyclin-dependent kinase Pho85, the cyclin Pho80 and the cyclin-dependent kinase inhibitor Pho81 have been identified in C. neoformans: (CNAG_07871), (CNAG_01922) and (CNAG_02541) respectively6. However their role in regulation of the PHO system remains to be determined. Interestingly, no Pho4 transcription factor homologue has been identified in C. neoformans. The Bahn laboratory (Yonsei University, Korea) has created a cryptococcal transcription factor knockout library. Use of Aph1 as a reporter for screening this library should determine the identity of transcription factor(s) regulating PHO gene expression in C. neoformans.
PP-IPs: Cryptococcal mutants deficient in IP7 production also fail to induce APH1 expression and secrete Aph1 during Pi deprivation (our unpublished observation). Via gene deletion analysis, we recently characterised the entire inositol polyphosphate biosynthesis pathway in C. neoformans, including the kinase directly responsible for IP7 synthesis (Kcs1). Using the full set of kinase deletion mutants we also observed that IP7 is essential for fungal virulence and dissemination to the brain in a mouse model (Lev et al.12 and our unpublished observation).
In summary, little is known about how Pi homeostasis is regulated in pathogenic fungi, as compared with S. cerevisiae. We have identified and extensively characterised the major secreted acid phosphatase in C. neoformans, Aph1, which will provide a valuable reporter for identifying PHO system regulators in this important fungal pathogen. As individual components of the PHO system have been demonstrated to play a role in virulence, the investigation of Pi homeostasis in C. neoformans may also provide unique opportunities for antifungal drug development.
References
[1] Park, B.J. et al. (2009) Estimation of the current global burden of cryptococcal meningitis among persons living with HIV/AIDS. AIDS 23, 525–530.| Estimation of the current global burden of cryptococcal meningitis among persons living with HIV/AIDS.Crossref | GoogleScholarGoogle Scholar | 19182676PubMed |
[2] Chayakulkeeree, M. et al. (2008) Role and mechanism of phosphatidylinositol-specific phospholipase C in survival and virulence of Cryptococcus neoformans. Mol. Microbiol. 69, 809–826.
| 1:CAS:528:DC%2BD1cXhtVehurzK&md5=4a429df9187cda2fdffe206f55212129CAS | 18532984PubMed |
[3] Cox, G.M. et al. (2001) Extracellular phospholipase activity is a virulence factor for Cryptococcus neoformans. Mol. Microbiol. 39, 166–175.
| Extracellular phospholipase activity is a virulence factor for Cryptococcus neoformans.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXlvVSmtA%3D%3D&md5=14c405524287f1b8f9156fae9c0c9b4bCAS | 11123698PubMed |
[4] Coelho, C. et al. (2014) The tools for virulence of Cryptococcus neoformans. Adv. Appl. Microbiol. 87, 1–41.
| The tools for virulence of Cryptococcus neoformans.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXhtVWktLvL&md5=fffd2ff198a2486cb375b9b865e2b703CAS | 24581388PubMed |
[5] Lev, S. et al. (2014) Identification of Aph1, a phosphate-regulated, secreted, and vacuolar acid phosphatase in Cryptococcus neoformans. mBio 5, e01649-14.
| Identification of Aph1, a phosphate-regulated, secreted, and vacuolar acid phosphatase in Cryptococcus neoformans.Crossref | GoogleScholarGoogle Scholar | 25227465PubMed |
[6] Kretschmer, M. et al. (2014) Defects in phosphate acquisition and storage influence virulence of Cryptococcus neoformans. Infect. Immun. 82, 2697–2712.
| Defects in phosphate acquisition and storage influence virulence of Cryptococcus neoformans.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXhsVWmu7jP&md5=d2ce949e3a1baaf113fdd6475e9b3ac1CAS | 24711572PubMed |
[7] Lee, Y.S. et al. (2008) Molecular basis of cyclin-CDK-CKI regulation by reversible binding of an inositol pyrophosphate. Nat. Chem. Biol. 4, 25–32.
| Molecular basis of cyclin-CDK-CKI regulation by reversible binding of an inositol pyrophosphate.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXhsVegtbbN&md5=bd35518502957e26cb7fe9f95e9fd102CAS | 18059263PubMed |
[8] Lee, Y.S. et al. (2007) Regulation of a cyclin-CDK-CDK inhibitor complex by inositol pyrophosphates. Science 316, 109–112.
| Regulation of a cyclin-CDK-CDK inhibitor complex by inositol pyrophosphates.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXjvVarsLg%3D&md5=3bce7e92384082b44991c767c6ed0910CAS | 17412959PubMed |
[9] Lonetti, A. et al. (2011) Identification of an evolutionarily conserved family of inorganic polyphosphate endopolyphosphatases. J. Biol. Chem. 286, 31 966–31 974.
| Identification of an evolutionarily conserved family of inorganic polyphosphate endopolyphosphatases.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhtFCju7%2FI&md5=3d87e1cc8655358b5db7397167138a2fCAS |
[10] Vidotto, V. et al. (2006) Extracellular enzymatic activities in Cryptococcus neoformans strains isolated from AIDS patients in different countries. Rev. Iberoam. Micol. 23, 216–220.
| Extracellular enzymatic activities in Cryptococcus neoformans strains isolated from AIDS patients in different countries.Crossref | GoogleScholarGoogle Scholar | 17388645PubMed |
[11] Wightman, R.M. and Haynes, C.L. (2004) Synaptic vesicles really do kiss and run. Nat. Neurosci. 7, 321–322.
| Synaptic vesicles really do kiss and run.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXisFCisrw%3D&md5=9cd3b2d827fdf95d058bddd345ea8e48CAS | 15048116PubMed |
[12] Lev, S. et al. (2013) Phospholipase C of Cryptococcus neoformans regulates homeostasis and virulence by providing inositol trisphosphate as a substrate for Arg1 kinase. Infect. Immun. 81, 1245–1255.
| Phospholipase C of Cryptococcus neoformans regulates homeostasis and virulence by providing inositol trisphosphate as a substrate for Arg1 kinase.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXlt1SltLs%3D&md5=a5704a13e6afdec773696baba5a02c9eCAS | 23381992PubMed |
Biographies
Dr Julie Djordjevic heads the Fungal Pathogenesis Group within the Centre for Infectious Diseases and Microbiology at the Westmead Millennium Institute, a research centre affiliated with the University of Sydney and Westmead Hospital. Using Cryptococcus neoformans as a model her research focuses on elucidating mechanisms used by fungi to cause systemic disease: specifically, understanding how fungal virulence factors get secreted and investigating the role of a series of newly described inositol polyphosphate kinases in a cellular pathway critical for production of virulence factors and phosphate homeostasis.
Dr Sophie Lev studied for her PhD degree in the Technion – Israel Institute of Technology, researching signal transduction in a fungal pathogen of corn. After completion of her PhD in 2003, she proceeded with post-doctoral training in the same University, and then in the University of California, Berkeley. She joined the Centre for Infectious Diseases and Microbiology in 2010, to work with Dr Julie Djordjevic and Professor Tania Sorrell to study signal transduction and virulence mechanisms in the medically important fungal pathogen, Cryptococcus neoformans.