Candida and macrophages: a deadly affair
Timothy Tucey A , Thomas Naderer A and Ana Traven A BA Department of Biochemistry and Molecular Biology, Building 76, 23 Innovation Walk, Monash University, Clayton, Vic. 3800, Australia
B Corresponding author. Email: ana.traven@monash.edu
Microbiology Australia 36(2) 53-56 https://doi.org/10.1071/MA15019
Published: 17 March 2015
The human fungal pathogen Candida albicans is a significant cause of invasive disease in hospital patients. Treatments are inadequate resulting in high financial costs and mortality rates that approach 50%1–5. Over the past decades, extensive use of immunosuppressive therapies and invasive medical procedures has exacerbated the problem6. Recent advances have shed light on the intimate relationship between Candida and innate immune cells, which triggers rapid fatal infections7–10. In this review we focus on the dynamic interaction between C. albicans and macrophages, which act as front line defense against invading pathogens, and discuss a newly discovered deadly affair.
Developmental transitions allow C. albicans to adapt and survive in host niches
The capacity to undergo a reversible switch between a yeast and hyphal mode of growth is linked to the virulence of C. albicans11,12. Although other yeast species, such as the model yeast Saccharomyces cerevisiae, are capable of transitioning between different cell types, unlike S. cerevisiae, C. albicans not only grows as yeast and pseudohyphae, but it also makes true hyphae – highly elongated tubular cells with no constrictions between mother and daughter cells and a primary septum that is not degraded during cell division13. Hyphae enable C. albicans to conquer new environments, and mediate pathogenesis-related functions such as invasion of epithelial tissue during colonisation of mucosal surfaces and the movement of C. albicans from the gastrointestinal tract to the bloodstream7,13.
Regulated gene expression is at the core of cellular pathways enabling the yeast-to-hyphae morphogenetic switch, and it is thought that the interchange between these cell types is critical for pathogenesis13–16. Although yeast cells are considered better suited for transport via the blood in disseminated disease, whereas hyphal cells have invasive capacity, there are still a lot of questions about the specific functions of these developmental states and how they are triggered. Hyphae can switch back to yeast which is seen as yeast cells budding from the hyphal filaments17. Genetic mutants that cannot revert to growth as budding yeast from the hyphal forms are attenuated in virulence17, suggesting that the reverse transition is also important for pathogenicity.
In the human body, Candida must adapt readily to new environments as it transitions between host niches. It does so by orchestrating gene expression programs that control stress resistance, metabolic adaptation and morphogenesis18,19. The innate immune response is the primary and immediate response against candidiasis, and one leukocyte in particular, the macrophage, plays an important role7,20. The ability of Candida to switch between morphogenetic types is crucial for evasion of innate immunity7,14,20. When yeast forms of C. albicans are engulfed by macrophages, they can evade this line of defense by switching to hyphal growth, which leads to the hyphal filament bursting out of the macrophage and killing the host cell in the process7,14,20. Although it is tempting (and dramatic) to speculate that these hyphae kill by exerting pressure on the macrophage membrane and physically breaking through, recent reports from our lab and others suggest that other mechanisms, related to regulation of the host–pathogen ‘synapse’, mediate host cell death and pathogen escape from macrophages9,10.
Live cell assay of Candida-induced death of macrophages
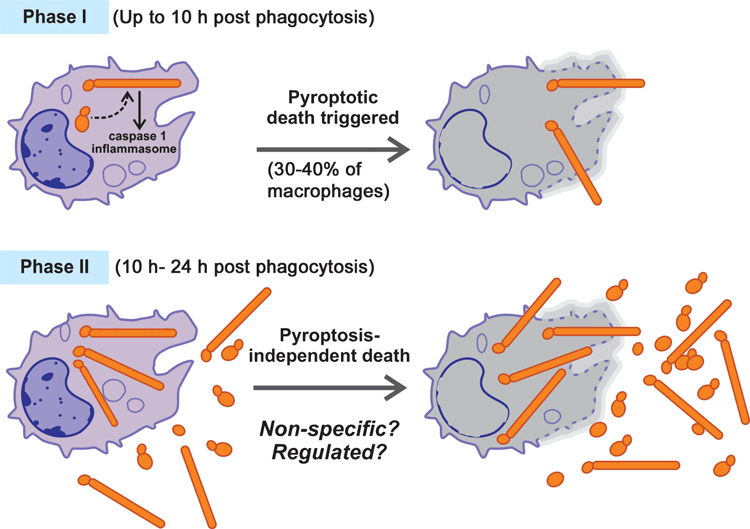
Our lab has recently established powerful live-cell imaging to monitor the interaction of C. albicans with host macrophages using primary bone-derived macrophages from mice9. Using this ex vivo assay we could observe functionally distinct events over time in the C. albicans-macrophage interaction. When yeast forms of C. albicans are added at the start of the assay, they are rapidly phagocytosed by macrophages. Shortly afterwards, a switch from yeast to hyphae inside macrophages triggers macrophage death and, concomitantly, fungal hyphae become extracellular. This initial phase of macrophage killing is followed by a second phase of host cell death (Figure 1). We and others have recently identified genetic mutations in the host and in the pathogen responsible for the first phase of macrophage killing, as outlined below. Our ultimate goal is to understand precisely which Candida and host molecules are important to induce macrophage death, when they act temporally as the infection progresses, and what their contribution is to pathology and disease outcomes.
Phase I: C. albicans manipulation of the host macrophage
The key result that showed that C. albicans does not simply break macrophages, but rather induces a more regulated mechanism of killing, came with the realisation that macrophages derived from mice deficient in a central host immune pathway – the caspase 1 inflammasome – were protected from killing by C. albicans in the first few hours post-phagocytosis9,10. This was despite normal formation of fungal hyphae, and therefore it argued that physical disruption of macrophages by fungal filaments is not the only mechanism of host cell death. Instead, it appears that the initial hyphal formation triggers the macrophage to commit suicide by a programmed cell death pathway called pyroptosis, which is enacted by caspase-1 and occurs before the filament has extended to the surface of the macrophage (Figure 1)9,10. In addition to caspase-1, the inflammasome subunits ASC and NLRP3 are also involved in Candida-induced pyroptosis10. Pyroptosis is lytic, leading to bursting of the macrophage cell and release of Candida. We note that the macrophage-like cell line RAW264.7, which is commonly used to study Candida-macrophage interactions, does not express ASC21, and therefore it is protected from pyroptosis following incubation with C. albicans9. While RAW264.7 cells, similar to caspase-1 deficient macrophages, are eventually killed by intracellular Candida9, it is clear that an important component of Candida-macrophage interaction is not recapitulated in this model macrophage system. Pyroptosis is triggered as part of the inflammatory response to intracellular pathogens, to deplete the host niche needed for their replication and cause further immune activation22,23. Pyroptosis per se does not appear to be involved in the antifungal mechanisms exerted by macrophages, as C. albicans can survive in macrophages to the same extent in the presence or absence of pyroptosis10. As we have proposed, it appears that some pathogens, like C. albicans, can take advantage of this host response to evade being destroyed by macrophages9,24. In light of our studies, we suggest that the role of pyroptosis in Candida infections may need to be revisited, as caspase-1 triggers potent inflammatory signals that activate anti-fungal immune responses, but the same events are also associated with the pathology in fungal infections.
By using live-cell imaging we have observed that Candida kills macrophages in two phases (I and II) that are mechanistically quite different (Figure 1)9. The duration of the caspase-1-dependent Phase I of macrophage death is dependent on the infectious load of C. albicans: the bigger the ratio of Candida to macrophages, the faster transition to Phase II occurs (Wellington et al.10 and our unpublished data). In our experiments, where six Candida cells per one macrophage were co-incubated, Phase I lasted for about 8–10 hours9. While this phase causes significant macrophage death, it is not complete. Approximately 30–40% of macrophages are killed, and these dead cells display subsequent nucleation of long hyphal projections outward as the imaging continues. Therefore, triggering of macrophage death is not uniform across all cells9. Inactivation of pyroptosis reduces macrophage death in Phase I, but does not fully protect9,10, showing that C. albicans uses multiple means to escape from macrophages in the first phase of the interaction.
Key questions remain about how exactly C. albicans filaments are recognised by the caspase-1 inflammasome to trigger pyroptosis. An intriguing C. albicans genetic mutation identified in our lab is in the Srb9 subunit of the Mediator complex (a central eukaryotic transcriptional regulator). While this mutant strain is able to transition to long hyphal filaments in the macrophage during Phase I, these filaments cannot fully exert their function in causing macrophage death9. We have shown that Mediator is required for proper structuring of the cell wall9,25. The hyphae made by the srb9 mutant displayed lower levels of exposed β-1,3 glucan on their cell surface, which is the main component of the fungal cell wall and is immunogenic7,26. The srb9 mutant hyphae also showed altered biophysical properties9, but how these cell surface changes are responsible for less macrophage cell death remains to be determined. Similarly, the transcriptional activator Upc2 is required for the ability of hyphal filaments to trigger pyroptosis10. Understanding the structural and physical properties of hyphae that are needed for signaling to the host macrophage to activate pyroptosis is the subject of current work. These factors may be reprogrammed by transcriptional regulators like the Mediator complex, as the developmental transition to hyphae begins inside the macrophage.
Phase II: macrophage necrosis or a regulated response?
Following Phase I, there is a second, quite rapid phase of macrophage killing that results in the death of the majority of remaining macrophages9. Very little is known about the mechanism of this second phase of killing. The second phase of killing can occur in the absence of caspase 1 or the alternative pyroptotic regulator, caspase 119,10. This excludes pyroptosis as a mechanism of macrophage death in Phase II. It has been proposed that this second phase occurs when C. albicans numbers increase, as a non-specific process10. It is also possible that, like Phase I, Phase II is a regulated mechanism executed by a programmed host cell death pathway9,24. On the pathogen’s side, it appears that hyphal filaments are functionally involved not only in the first phase, but also in the second phase of macrophage death. The Mediator mutant med31, which is delayed in making hyphae in macrophages, triggers significantly slower Phase II of macrophage death9. When assayed in caspase 1/caspase 11 mutant macrophages, the srb9 mutant of C. albicans also induced a slower rate of macrophage killing in Phase II, although much faster than the hyphae-defective med319. Studying genetic mutations that uncouple hyphal morphogenesis from the ability to kill macrophages is likely to lead to better understanding of how C. albicans induces Phase II of macrophage death.
Concluding remarks
Candida albicans is a commensal organism of the skin and intestinal mucosa in approximately 50% of individuals. Breakdown of the physical barrier, due to surgery, burns or long-term use of antibiotics that reduces the numbers of competing microorganisms can lead to invasive Candida infections, even in immuno-competent patients. There is increasing evidence that systemic Candida infections trigger immunopathological reactions that contribute to the high mortality rate despite the use of state of the art antifungal therapy. Dissecting the fungal factors that foster the transitions between yeast and hyphal forms, and host factors that recognise these forms, will undoubtedly reveal novel insights into the host–fungal pathogen synapse. By following these interactions on the molecular and cellular level, we will come to understand how our relationship with Candida can suddenly turn deadly.
Acknowledgements
The work on Candida-macrophage interactions in the Traven and Naderer labs is supported by a Project grant from the National Health and Medical Research Council (APP1081072).
References
[1] Perlroth, J. et al. (2007) Nosocomial fungal infections: epidemiology, diagnosis, and treatment. Med. Mycol. 45, 321–346.| Nosocomial fungal infections: epidemiology, diagnosis, and treatment.Crossref | GoogleScholarGoogle Scholar | 17510856PubMed |
[2] Delaloye, J. and Calandra, T. (2014) Invasive candidiasis as a cause of sepsis in the critically ill patient. Virulence 5, 161–169.
| Invasive candidiasis as a cause of sepsis in the critically ill patient.Crossref | GoogleScholarGoogle Scholar | 24157707PubMed |
[3] Zaoutis, T.E. et al. (2005) The epidemiology and attributable outcomes of candidemia in adults and children hospitalized in the United States: a propensity analysis. Clin. Infect. Dis. 41, 1232–1239.
| The epidemiology and attributable outcomes of candidemia in adults and children hospitalized in the United States: a propensity analysis.Crossref | GoogleScholarGoogle Scholar | 16206095PubMed |
[4] Spellberg, B. (2008) Novel insights into disseminated candidiasis: pathogenesis research and clinical experience converge. PLoS Pathog. 4, e38.
| Novel insights into disseminated candidiasis: pathogenesis research and clinical experience converge.Crossref | GoogleScholarGoogle Scholar | 18282100PubMed |
[5] Brown, G.D. et al. (2012) Hidden killers: human fungal infections. Sci. Transl. Med. 4, 165rv13.
| Hidden killers: human fungal infections.Crossref | GoogleScholarGoogle Scholar | 23253612PubMed |
[6] Samaranayake, L.P. et al. (2002) Fungal infections associated with HIV infection. Oral Dis. 8, 151–160.
| Fungal infections associated with HIV infection.Crossref | GoogleScholarGoogle Scholar | 12164650PubMed |
[7] Gow, N.A. et al. (2012) Candida albicans morphogenesis and host defence: discriminating invasion from colonization. Nat. Rev. Microbiol. 10, 112–122.
| 1:CAS:528:DC%2BC3MXhs1aitr%2FK&md5=588e7420bcd309172a4857162b3f2783CAS |
[8] Zwolanek, F. et al. (2014) The non-receptor tyrosine kinase Tec controls assembly and activity of the noncanonical caspase-8 inflammasome. PLoS Pathog. 10, e1004525.
| The non-receptor tyrosine kinase Tec controls assembly and activity of the noncanonical caspase-8 inflammasome.Crossref | GoogleScholarGoogle Scholar | 25474208PubMed |
[9] Uwamahoro, N. et al. (2014) The pathogen Candida albicans hijacks pyroptosis for escape from macrophages. MBio 5, e00003–e00014.
| The pathogen Candida albicans hijacks pyroptosis for escape from macrophages.Crossref | GoogleScholarGoogle Scholar | 24667705PubMed |
[10] Wellington, M. et al. (2014) Candida albicans triggers NLRP3-mediated pyroptosis in macrophages. Eukaryot. Cell 13, 329–340.
| Candida albicans triggers NLRP3-mediated pyroptosis in macrophages.Crossref | GoogleScholarGoogle Scholar | 24376002PubMed |
[11] Saville, S.P. et al. (2003) Engineered control of cell morphology in vivo reveals distinct roles for yeast and filamentous forms of Candida albicans during infection. Eukaryot. Cell 2, 1053–1060.
| Engineered control of cell morphology in vivo reveals distinct roles for yeast and filamentous forms of Candida albicans during infection.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXos1Gkur0%3D&md5=6fd0487168fe322a2aca059875bbf3e2CAS | 14555488PubMed |
[12] Kumamoto, C.A. and Vinces, M.D. (2005) Contributions of hyphae and hypha-co-regulated genes to Candida albicans virulence. Cell. Microbiol. 7, 1546–1554.
| Contributions of hyphae and hypha-co-regulated genes to Candida albicans virulence.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXhtFGkt7vP&md5=00051fa64083a7824802f7dde856b47bCAS | 16207242PubMed |
[13] Sudbery, P.E. (2011) Growth of Candida albicans hyphae. Nat. Rev. Microbiol. 9, 737–748.
| Growth of Candida albicans hyphae.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhtVWrtLzN&md5=c4d8119fdbad3458affe1977906f2506CAS | 21844880PubMed |
[14] Lo, H.J. et al. (1997) Nonfilamentous C. albicans mutants are avirulent. Cell 90, 939–949.
| Nonfilamentous C. albicans mutants are avirulent.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2sXmtV2htro%3D&md5=2e2917d560a376d3e0b96c0a8ff320d1CAS | 9298905PubMed |
[15] Braun, B.R. et al. (2000) Identification and characterization of TUP1-regulated genes in Candida albicans. Genetics 156, 31–44.
| 1:CAS:528:DC%2BD3cXntVylsb8%3D&md5=e119d7a4d7ab5088f444e36cf328cdb9CAS | 10978273PubMed |
[16] Braun, B.R. and Johnson, A.D. (1997) Control of filament formation in Candida albicans by the transcriptional repressor TUP1. Science 277, 105–109.
| Control of filament formation in Candida albicans by the transcriptional repressor TUP1.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2sXksVygsLo%3D&md5=3f804f77d7aeea5ee3a98d7532363579CAS | 9204892PubMed |
[17] Shen, J. et al. (2008) The Candida albicans pescadillo homolog is required for normal hypha-to-yeast morphogenesis and yeast proliferation. Proc. Natl. Acad. Sci. USA 105, 20918–20923.
| The Candida albicans pescadillo homolog is required for normal hypha-to-yeast morphogenesis and yeast proliferation.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXksFWisA%3D%3D&md5=ca590021125d7d7e3967d16deacf0fb1CAS | 19075239PubMed |
[18] Fradin, C. et al. (2003) Stage-specific gene expression of Candida albicans in human blood. Mol. Microbiol. 47, 1523–1543.
| Stage-specific gene expression of Candida albicans in human blood.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXitlOgsbw%3D&md5=99973eac6a0748d1a86469d7440b578dCAS | 12622810PubMed |
[19] Lorenz, M.C. and Fink, G.R. (2001) The glyoxylate cycle is required for fungal virulence. Nature 412, 83–86.
| The glyoxylate cycle is required for fungal virulence.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXlt1CrurY%3D&md5=e33c781d667729005f0f14df81123065CAS | 11452311PubMed |
[20] Miramón, P. et al. (2013) Thriving within the host: Candida spp. interactions with phagocytic cells. Med. Microbiol. Immunol. (Berl.) 202, 183–195.
| Thriving within the host: Candida spp. interactions with phagocytic cells.Crossref | GoogleScholarGoogle Scholar |
[21] Pelegrin, P. et al. (2008) P2X7 receptor differentially couples to distinct release pathways for IL-1beta in mouse macrophage. J. Immunol. 180, 7147–7157.
| P2X7 receptor differentially couples to distinct release pathways for IL-1beta in mouse macrophage.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXmtVantL0%3D&md5=f6c473c7723ee25893bec5e98f268be8CAS | 18490713PubMed |
[22] Lamkanfi, M. and Dixit, V.M. (2010) Manipulation of host cell death pathways during microbial infections. Cell Host Microbe 8, 44–54.
| Manipulation of host cell death pathways during microbial infections.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXptlylur0%3D&md5=e6744e900233d0e38818d413b9e8a5f8CAS | 20638641PubMed |
[23] Fink, S.L. and Cookson, B.T. (2005) Apoptosis, pyroptosis, and necrosis: mechanistic description of dead and dying eukaryotic cells. Infect. Immun. 73, 1907–1916.
| Apoptosis, pyroptosis, and necrosis: mechanistic description of dead and dying eukaryotic cells.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXjtFygt78%3D&md5=6c808ed8a77ce4bf30f3b39382333cb1CAS | 15784530PubMed |
[24] Traven, A. and Naderer, T. (2014) Microbial egress: a hitchhiker’s guide to freedom. PLoS Pathog. 10, e1004201.
| Microbial egress: a hitchhiker’s guide to freedom.Crossref | GoogleScholarGoogle Scholar | 25057992PubMed |
[25] Uwamahoro, N. et al. (2012) The functions of Mediator in Candida albicans support a role in shaping species-specific gene expression. PLoS Genet. 8, e1002613.
| The functions of Mediator in Candida albicans support a role in shaping species-specific gene expression.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XlslejtLw%3D&md5=212104b929c99d3fd6a815a52143a1cfCAS | 22496666PubMed |
[26] Sun, L. and Zhao, Y. (2007) The biological role of dectin-1 in immune response. Int. Rev. Immunol. 26, 349–364.
| The biological role of dectin-1 in immune response.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXhtlWrtL7L&md5=ac6e385b7f1d9b19c8732846df9643acCAS | 18027205PubMed |
Biographies
Tim Tucey is a Research Fellow at Monash University. He obtained his PhD from the University of California, San Diego, where he studied telomerase in the budding yeast Saccharomyces cerevisiae. He is now applying his molecular and cell biology background to understanding the live cell dynamics of the pathogenic yeast Candida albicans.
Thomas Naderer leads the Macrophage-Pathogen Interactions laboratory in the Department of Biochemistry and Molecular Biology at Monash University. The Naderer lab focuses on understanding how microbial pathogens modulate host responses to egress from host cells and their contribution to disease.
Ana Traven heads the Laboratory for fungal pathogenesis in the Department of Biochemistry and Molecular Biology at Monash University. The research programs in the lab aim to decipher how Candida albicans, a common human pathogen, adapts to its environment and resists antifungal and host-derived attacks through metabolic control and by remodeling gene regulatory networks.