Tackling superbugs in their slime castles: innovative approaches against antimicrobial-resistant biofilm infections
Katharina RichterRichter Lab
Department of Surgery
Basil Hetzel Institute for Translational Health Research
The Queen Elizabeth Hospital and
University of Adelaide
37a Woodville Road
Woodville South, SA 5011, Australia
Tel: +61 8 8222 7541
Email: Katharina.Richter@adelaide.edu.au
Microbiology Australia 40(4) 165-168 https://doi.org/10.1071/MA19049
Published: 11 November 2019
The rise of ‘superbugs’ like methicillin-resistant Staphylococcus aureus threatens human health on a global scale. Bacteria have established many ways to withstand antimicrobial treatments, evade the immune system and protect them from external stressors. Current medical care frequently fails to eradicate multi-drug resistant bacteria, microbial biofilms and small colony variants that can hide from antibiotic treatments inside human cells, therefore, drug discovery and drug development to improve healthcare is pivotal. In this article, novel antimicrobial strategies with extensive activity against multi-drug resistant staphylococci are described, including:
-
Trojan Horse approaches
-
multi-pronged strategies
-
metals
-
repurposing of drugs
-
phage therapy.
Preclinical validation confirmed safety and efficacy against biofilms and small colony variants in vitro and in vivo. This was the foundation for the translation of two strategies into phase I clinical trials using: (1) deferiprone and gallium-protoporphyrin to disrupt bacterial iron metabolism; and (2) colloidal silver nanoparticles as topical treatments for staphylococcal biofilm-related infections.
Bugs and drugs
The rise of multidrug-resistant bacteria has large implications for society, the economy and the environment on a global scale1. While antibiotic-resistance is on the rise, the drug discovery and drug development pipeline has declined over decades. Complicating the situation is the fact that bacteria form biofilms, which protect bacteria making them less susceptible to antibiotic treatments and attacks from the immune system2. Moreover, the overuse and misuse of antibiotics is progressively rendering antibiotic therapy ineffective, therefore, new antibacterial strategies against resistant bacteria and biofilms will be instrumental in improving patient outcomes.
New antimicrobial approaches include (but are not limited to):
-
Trojan Horse approaches
-
multi-pronged strategies
-
metals
-
repurposing of drugs
-
phage therapy.
Trojan Horse approaches
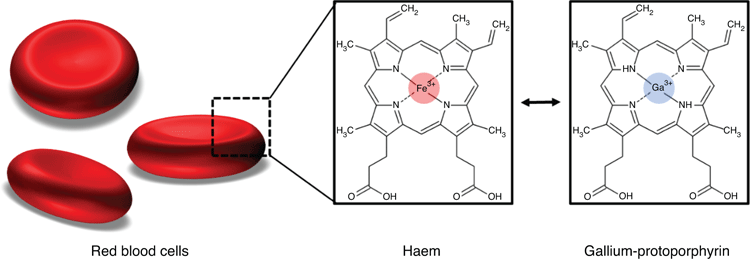
Trojan Horse approaches are treatments that mimic a specific compound bacteria favour to take up. An example is the patented combination of deferiprone (Def) and gallium-protoporphyrin (GaPP). While Def is an iron chelator approved for the treatment of thalassemia major, GaPP shows chemical similarity to haem (Figure 1), thereby mimicking the preferred iron source of many bacteria, including staphylococci3–5.
The underlying mechanism of this Trojan Horse approach lies in disrupting bacterial iron metabolism, which is typically unaffected by antibiotics. Iron is vital for cellular processes, such as respiration, DNA synthesis, energy generation, biofilm formation and for the protection against toxic radicals, for which higher iron levels are needed than for vegetative growth6.
While bacteria can establish resistance to antibiotics, they still require iron for growth, survival and virulence6. Therefore, bacteria possess multiple iron acquisition systems, including the iron-regulated surface determinant system, to obtain iron or haem (i.e. iron protoporphyrin IX, the most abundant iron source in the human body) from the host5. Interfering with bacterial iron metabolism has the potential to improve the efficacy of antimicrobial therapy, even when bacteria established antibiotic resistance.
In vitro studies with multidrug-resistant Staphylococcus aureus confirmed the efficacy of the Def-GaPP treatment7–9. While monotherapy with either Def or GaPP showed limited antibiofilm activity, a consecutive treatment of both compounds exhibited synergistic effects7. Def induces iron starvation in bacteria causing upregulation of iron transporter proteins that are utilised by GaPP. The haem analogue GaPP is actively taken up into starved bacterial cells or penetrates through the bacterial cell wall6,10. GaPP occupies intracellular iron/haem binding sites, but in contrast to haem, GaPP cannot transfer electrons and fails to induce redox reactions, thereby inhibiting respiration, ATP production and other essential cellular pathways. This ultimately increases the production of toxic radicals in bacteria which is lethal10,11.
Pre-clinical studies confirmed these effects in particular against staphylococcal biofilms, including highly resistant S. aureus small colony variants (SCVs) and clinical isolates of methicillin-resistant S. aureus7–9 (Figure 2). Interestingly, the combination of Def-GaPP also showed in vitro activity against Pseudomonas aeruginosa strains8. Moreover, in a biofilm wound model that utilised an artificial dermis made of collagen and hyaluronic acid, Def-GaPP exhibited antibacterial effects even in the presence of human blood and plasma8,9. When Def12 or Def-GaPP8,9 was combined with antibiotics, the activity of ciprofloxacin, gentamicin, clindamycin and vancomycin was potentiated against staphylococci, indicating the potential to improve standard antibiotic therapy.
|
Furthermore, the Def-GaPP treatment showed activity against intracellular S. aureus SCVs in an infected cell culture model. Considering that most antibiotics fail to effectively eradicate intracellular SCVs, this is of significant interest for invasive, antibiotic-refractory infections, such as chronic implant infections and chronic wounds (manuscript in preparation).
In addition, animal studies in nematodes (Caenorhabditis elegans) and sheep using Def-GaPP in a surgical wound healing gel confirmed in vivo safety and efficacy9,13. The Def-GaPP gel progressed into clinical phase I trials determining wound healing and postoperative outcomes in chronic rhinosinusitis patients (ACTRN12618000577213). These first in human studies are about to be completed and preliminary data indicate positive patient outcomes (manuscript in preparation).
A benefit of the Def-GaPP gel used in the clinical trial is that the treatment is instilled in the sinuses, precisely at the infection site. This topical approach delivers high concentrations of the antimicrobial compounds directly to the location where they are needed, thereby improving treatment efficacy and drug delivery to biofilms, while reducing the risk of systemic effects. This is in particular important for biofilm infections as biofilms are known to require up to 1000-fold higher drug concentrations to be effectively treated2 and oral drug delivery frequently fails to achieve these elevated drug concentrations without inducing side effects. Def-GaPP has potential to be used for a variety of topical applications, such as chronic rhinosinusitis, chronic wounds or during implant surgery either alone or as adjuvant therapy with antibiotics.
In addition, the surgical gel that was used as a carrier of Def-GaPP, exhibits strong wound healing properties and aids the prevention of adhesions and scarring tissue, a major complication of surgical procedures14. Def by itself also adds wound healing properties15 and preclinical validation is currently ongoing investigating Def-loaded gel for applications in laminectomy and abdominal surgery (manuscripts in preparation).
A critical point for the utilisation of Def-GaPP as a new treatment for infection control is the risk for resistance. On one hand the risk for resistance is low because if bacteria down-regulate mechanisms to limit the GaPP uptake, the uptake of iron and haem would be decreased as well16, being counterproductive for bacterial survival. On the other hand, bacteria could alter their nutrient preferences and switch to a lifestyle independent from haem acquisition, becoming less susceptible to Def-GaPP. More studies are warranted to understand the full potential of Def-GaPP to improve infection control.
Multi-pronged strategies
Quorum sensing is the cell-to-cell signalling in biofilms that enable bacteria to streamline their defences and coordinate their gene expression in a cell density dependent way17. Quorum sensing plays a vital role in biofilm formation and resistance, representing a suitable target for antimicrobial therapy. Literature described quorum sensing inhibitors, such as hamamelitannin, to increase the susceptibility of staphylococcal biofilms to antibiotics by disrupting the peptidoglycan synthesis and eDNA release18. Interestingly, these antibiofilm effects were potentiated when hamamelitannin was combined with Def-GaPP19. Multi-pronged strategies like this offer high potential to improve antimicrobial activity while reducing the risk for resistance. By combining drugs with different mechanisms of action, pathogens can be tackled from multiple sides for a potentially higher treatment efficacy. Such approaches could break down the biofilm matrix and effectively kill vulnerable bacteria, inhibit intercellular communication and destroy uncoordinated microbes, disperse bacteria and control the residual as well as the released bacteria. In addition, combination therapies could elevate the potency of conventional antibiotics. A multi-pronged approach of different technologies may bring the urgently needed help to fight the emerging threat of multidrug-resistant bacteria encountered in clinical practice. A plethora of novel antibiofilm strategies are available: the remaining challenge is to translate these ideas into pharmaceutical products for utilisation in every day practice.
Metals
Metals can also exhibit antimicrobial properties: in particular, silver has been known for positive health effects for centuries and found its way into modern healthcare, e.g. as silver-containing wound care products20. As a topical approach against staphylococcal biofilms, silver nanoparticles of different size and shape have been described21. Spherical, cubic and star-shaped colloidal silver nanoparticles were synthesised and characterised, determining safety and efficacy against S. aureus, MRSA and P. aeruginosa biofilms. Based on the absence of toxicity and elevated antibiofilm effects of spherical silver nanoparticles in vitro and in vivo21, a clinical phase I trial was carried out (ACTRN12616001558415). Spherical silver nanoparticles were used as a rinse for chronic sinus infections following sinus surgery. The study concluded that the colloidal silver rinse was safe to use, showed elevated antibacterial properties and similar improvement in symptoms and endoscopic scores as culture-directed oral antibiotics22.
Repurposing of drugs
Repurposing of drugs provides the opportunity to fast-track medical treatments. These compounds include drug candidates, abandoned drugs, approved drugs or withdrawn drugs that are re-used for an application for which they initially were not developed. This reduces the costs and speeds up the R&D process from pre-clinical validation to clinical trials and market approval, as the safety and pharmacology profile is already known and some administrative approvals are already in place3. An example is the use of excipients that are part of pharmaceutical formulations. While being part of commercial products, excipients such as ethylenediaminetetraacetic acid can show antibacterial effects and added as an adjuvant to the efficacy of therapies with and without antibiotic administration23.
Phage therapy
Whilst phage therapy was developed in the early 20th century in Eastern Europe, the discovery of penicillin and the golden age of antibiotic discovery in the 1950s and 1960s overshadowed the utilisation of phages24. However, with the rise of ‘superbugs’ the interest in phage therapy has awakened. Around the world phages have been revitalised and are being studied from in vitro experiments25 to clinical trials26. Successful patient outcomes increased the awareness of phage therapy as potential alternative or additional treatment to antibiotics26–29. Time will tell the promises and pitfalls of innovations from this rapidly advancing field.
Conclusion
New approaches not based on antibiotics have the potential to kill bacteria, biofilms and intracellular pathogens with a different mode of action, independent of established resistance. Moreover, some novel strategies make resistant bacteria susceptible again to antibiotics, maximising the efficacy of existing standard of care. To keep up with the pace of spreading antimicrobial resistance, the investment in R&D for innovative therapeutics and their rapid translation into clinical trials will be instrumental. Multi-pronged and alternative treatments, such as Trojan Horse approaches, quorum sensing inhibitors, metals and phage therapy, may hold promise to improve infection control.
Conflicts of interest
The author declares no conflicts of interest.
Acknowledgements
KR is recipient of a CJ Martin Biomedical Early Career Fellowship (NHMRC #1163634) by the National Health and Medical Research Council, Australia, and her research is supported by The Hospital Research Foundation, Woodville, South Australia.
References
[1] O’Neill, J. (2014) Antimicrobial resistance: tackling a crisis for the health and wealth of nations. Review on Antimicrobial Resistance.[2] Costerton, J.W. (1999) Bacterial biofilms: a common cause of persistent infections. Science 284, 1318–1322.
| Bacterial biofilms: a common cause of persistent infections.Crossref | GoogleScholarGoogle Scholar | 10334980PubMed |
[3] Richter, K. et al. (2017) Innovative approaches to treat Staphylococcus aureus biofilm-related infections. Essays Biochem. 61, 61–70.
| Innovative approaches to treat Staphylococcus aureus biofilm-related infections.Crossref | GoogleScholarGoogle Scholar | 28258230PubMed |
[4] Reniere, M.L. et al. (2007) Intracellular metalloporphyrin metabolism in Staphylococcus aureus. Biometals 20, 333–345.
| Intracellular metalloporphyrin metabolism in Staphylococcus aureus.Crossref | GoogleScholarGoogle Scholar | 17387580PubMed |
[5] Skaar, E.P. et al. (2004) Iron-source preference of Staphylococcus aureus infections. Science 305, 1626–1628.
| Iron-source preference of Staphylococcus aureus infections.Crossref | GoogleScholarGoogle Scholar | 15361626PubMed |
[6] Cassat, J.E. and Skaar, E.P. (2013) Iron in infection and immunity. Cell Host Microbe 13, 509–519.
| Iron in infection and immunity.Crossref | GoogleScholarGoogle Scholar | 23684303PubMed |
[7] Richter, K. et al. (2016) Mind ‘De GaPP’: in vitro efficacy of deferiprone and gallium-protoporphyrin against Staphylococcus aureus biofilms. Int. Forum Allergy Rhinol. 6, 737–743.
| Mind ‘De GaPP’: in vitro efficacy of deferiprone and gallium-protoporphyrin against Staphylococcus aureus biofilms.Crossref | GoogleScholarGoogle Scholar | 26919404PubMed |
[8] Richter, K. et al. (2017) A topical hydrogel with deferiprone and gallium-protoporphyrin targets bacterial iron metabolism and has antibiofilm activity. Antimicrob. Agents Chemother. 61, e00481-17.
| A topical hydrogel with deferiprone and gallium-protoporphyrin targets bacterial iron metabolism and has antibiofilm activity.Crossref | GoogleScholarGoogle Scholar | 28396543PubMed |
[9] Richter, K. et al. (2017) Deferiprone and gallium-protoporphyrin have the capacity to potentiate the activity of antibiotics in Staphylococcus aureus small colony variants. Front. Cell. Infect. Microbiol. 7, 280.
| Deferiprone and gallium-protoporphyrin have the capacity to potentiate the activity of antibiotics in Staphylococcus aureus small colony variants.Crossref | GoogleScholarGoogle Scholar | 28690982PubMed |
[10] Kelson, A.B. et al. (2013) Gallium-based anti-infectives: targeting microbial iron-uptake mechanisms. Curr. Opin. Pharmacol. 13, 707–716.
| Gallium-based anti-infectives: targeting microbial iron-uptake mechanisms.Crossref | GoogleScholarGoogle Scholar | 23876838PubMed |
[11] Gielen, M. and Tiekink, E.R. (2005) Metallotherapeutic drugs and metal-based diagnostic agents: the use of metals in medicine. John Wiley & Sons, Chichester, UK.
[12] Coraça-Huber, D.C. et al. (2018) Iron chelation destabilizes bacterial biofilms and potentiates the antimicrobial activity of antibiotics against coagulase-negative Staphylococci. Pathog. Dis. 76, fty052.
| Iron chelation destabilizes bacterial biofilms and potentiates the antimicrobial activity of antibiotics against coagulase-negative Staphylococci.Crossref | GoogleScholarGoogle Scholar | 29860413PubMed |
[13] Ooi, M.L. et al. (2018) Safety and efficacy of topical chitogel-deferiprone-gallium protoporphyrin in sheep model. Front. Microbiol. 9, 917.
| Safety and efficacy of topical chitogel-deferiprone-gallium protoporphyrin in sheep model.Crossref | GoogleScholarGoogle Scholar | 29867828PubMed |
[14] Valentine, R. et al. (2010) The efficacy of a novel chitosan gel on hemostasis and wound healing after endoscopic sinus surgery. Am. J. Rhinol. Allergy 24, 70–75.
| The efficacy of a novel chitosan gel on hemostasis and wound healing after endoscopic sinus surgery.Crossref | GoogleScholarGoogle Scholar | 20109331PubMed |
[15] Mohammadpour, M. et al. (2013) Wound healing by topical application of antioxidant iron chelators: kojic acid and deferiprone. Int. Wound J. 10, 260–264.
| Wound healing by topical application of antioxidant iron chelators: kojic acid and deferiprone.Crossref | GoogleScholarGoogle Scholar | 22621771PubMed |
[16] Costa, S.S. et al. (2013) Multidrug efflux pumps in Staphylococcus aureus: an update. Open Microbiol. J. 7, 59–71.
| Multidrug efflux pumps in Staphylococcus aureus: an update.Crossref | GoogleScholarGoogle Scholar | 23569469PubMed |
[17] Parsek, M.R. and Greenberg, E. (2005) Sociomicrobiology: the connections between quorum sensing and biofilms. Trends Microbiol. 13, 27–33.
| Sociomicrobiology: the connections between quorum sensing and biofilms.Crossref | GoogleScholarGoogle Scholar | 15639629PubMed |
[18] Brackman, G. et al. (2016) The quorum sensing inhibitor hamamelitannin increases antibiotic susceptibility of Staphylococcus aureus biofilms by affecting peptidoglycan biosynthesis and eDNA release. Sci. Rep. 6, 20321.
| The quorum sensing inhibitor hamamelitannin increases antibiotic susceptibility of Staphylococcus aureus biofilms by affecting peptidoglycan biosynthesis and eDNA release.Crossref | GoogleScholarGoogle Scholar | 26828772PubMed |
[19] Richter, K. (2017) Towards novel antibiofilm strategies. PhD thesis. The University of Adelaide, Adelaide, Australia. 1–211.
[20] Franci, G. et al. (2015) Silver nanoparticles as potential antibacterial agents. Molecules 20, 8856–8874.
| Silver nanoparticles as potential antibacterial agents.Crossref | GoogleScholarGoogle Scholar | 25993417PubMed |
[21] Richter, K. et al. (2017) Taking the silver bullet colloidal silver particles for the topical treatment of biofilm-related infections. ACS Appl. Mater. Interfaces 9, 21631–21638.
| Taking the silver bullet colloidal silver particles for the topical treatment of biofilm-related infections.Crossref | GoogleScholarGoogle Scholar | 28598149PubMed |
[22] Ooi, M.L. et al. (2018) Topical colloidal silver for the treatment of recalcitrant chronic rhinosinusitis. Front. Microbiol. 9, 720.
| Topical colloidal silver for the treatment of recalcitrant chronic rhinosinusitis.Crossref | GoogleScholarGoogle Scholar | 29696011PubMed |
[23] Cherian, L.M. et al. (2019) Effect of commercial nasal steroid preparation on bacterial growth. Int. Forum Allergy Rhinol. 9, 766–775.
| Effect of commercial nasal steroid preparation on bacterial growth.Crossref | GoogleScholarGoogle Scholar | 30748102PubMed |
[24] Sulakvelidze, A. et al. (2001) Bacteriophage therapy. Antimicrob. Agents Chemother. 45, 649–659.
| Bacteriophage therapy.Crossref | GoogleScholarGoogle Scholar | 11181338PubMed |
[25] Zhang, G. et al. (2018) Bacteriophage effectively kills multidrug resistant Staphylococcus aureus clinical isolates from chronic rhinosinusitis patients. Int. Forum Allergy Rhinol. 8, 406–414.
| Bacteriophage effectively kills multidrug resistant Staphylococcus aureus clinical isolates from chronic rhinosinusitis patients.Crossref | GoogleScholarGoogle Scholar | 29240296PubMed |
[26] Furfaro, L.L. et al. (2018) Bacteriophage therapy: clinical trials and regulatory hurdles. Front. Cell. Infect. Microbiol. 8, 376.
| Bacteriophage therapy: clinical trials and regulatory hurdles.Crossref | GoogleScholarGoogle Scholar | 30406049PubMed |
[27] Rhoads, D.D. et al. (2009) Bacteriophage therapy of venous leg ulcers in humans: results of a phase I safety trial. J. Wound Care 18, 237–243.
| Bacteriophage therapy of venous leg ulcers in humans: results of a phase I safety trial.Crossref | GoogleScholarGoogle Scholar | 19661847PubMed |
[28] Kutter, E. et al. (2010) Phage therapy in clinical practice: treatment of human infections. Curr. Pharm. Biotechnol. 11, 69–86.
| Phage therapy in clinical practice: treatment of human infections.Crossref | GoogleScholarGoogle Scholar | 20214609PubMed |
[29] Leitner, L. et al. (2017) Bacteriophages for treating urinary tract infections in patients undergoing transurethral resection of the prostate: a randomized, placebo-controlled, double-blind clinical trial. BMC Urol. 17, 90.
| Bacteriophages for treating urinary tract infections in patients undergoing transurethral resection of the prostate: a randomized, placebo-controlled, double-blind clinical trial.Crossref | GoogleScholarGoogle Scholar | 28950849PubMed |
Biography
Dr Katharina Richter is a biomedical researcher with global work stints in Germany, New Zealand, Switzerland, Denmark and Australia. A trained pharmacist she completed her PhD in medicine/applied microbiology in 2017 and founded her own research group in 2019 at the University of Adelaide. Her group has two priorities: (1) developing new treatments against antimicrobial-resistant biofilm infections to improve infection control; and (2) educating the society by effective science communication through public speaking, science outreach activities (like the Pint of Science festival) and leading STEM workshops at schools.