Enlisting plants in the battle for new antibacterial compounds
Dane Lyddiard A and Ben W Greatrex BA Biomedical Science
School of Science and Technology
University of New England
Armidale, NSW 2351, Australia
Tel: +61 2 6773 4050
Fax: +61 2 6773 3267
Email: dlyddia2@une.edu.au
B Pharmacy
School of Rural Medicine
University of New England
Armidale, NSW 2351, Australia
Tel: +61 2 6773 2402
Fax: +61 2 6773 3267
Email: ben.greatrex@une.edu.au
Microbiology Australia 40(4) 169-172 https://doi.org/10.1071/MA19050
Published: 8 November 2019
A rise in antibacterial drug resistance comes at a time when our once reliable sources of antibacterial natural products, bacteria and fungi, are failing us. The search for new drugs to fight pathogens has led to a range of innovative approaches and includes screening organisms which have developed evolutionary adaptions to prevent bacterial attack. The discovery of antibacterial phytochemicals from plants can be achieved using an activity-guided platform involving biological and chemical pre-screening, compound isolation, structure elucidation, and the direct testing of isolated compounds. Challenges include the clean isolation of natural products, avoiding the rediscovery of known compounds, toxicity, and poor levels of activity.
For a good part of the 20th century, humans had the upper hand against bacterial pathogens thanks to the pioneering work of Alexander Fleming, René Dubos and Selman Waksman et al. who demonstrated the value of mining antibacterial natural products from bacteria and fungi. For a few decades this approach (which yielded the likes of penicillin, streptomycin and tetracycline) seemed to be an impenetrable fortress against bacterial pathogens, until the walls began to strain under the force of growing antimicrobial resistance and a dearth of new antibiotic classes1. The ‘old’ approach eventually failed to produce significantly new clinical agents against the background of known compounds1. Researchers have responded in a range of novel ways: running large compound libraries through high throughput screenings2, mining the natural products in previously unculturable organisms3, screening the chemicals hidden away on the shelves of chemistry labs4, disarming bacteria of their virulence factors5, and developing phage therapies6; each approach with its merits and limitations. Another approach is screening botanical natural products.
The plant world as a whole is estimated to produce over 100 000 secondary metabolites with low molecular mass, generally derived from isoprenoid, phenylpropanoid, alkaloid and fatty acid or polyketide pathways7. While plants and animals have some common antibacterial defences such as apoptosis of infected tissue, antibacterial peptides (purothionins from Triticum aestivum8 are a noteworthy plant-based example) and the targeted exploitation of reactive oxygen species, they do not produce antibodies, relying instead on a limited number of receptors to recognize pathogens along with a diverse armoury of small molecules with antibacterial activity9. Compounds with known specific antibacterial targets are not common in plants, although there are examples such as coumarins with comparable action to the DNA-gyrase inhibitor novobiocin9. While activity is usually weak, it is possible that plants target virulence rather than growth or that relatively weak antibacterial agents work in synergy with each other to create potent activity as seen with the antibacterial compound berberine from Berberis fremontii together with the multi drug resistance (MDR) pump inhibitor 5’-methoxyhydnocarpin9,10.
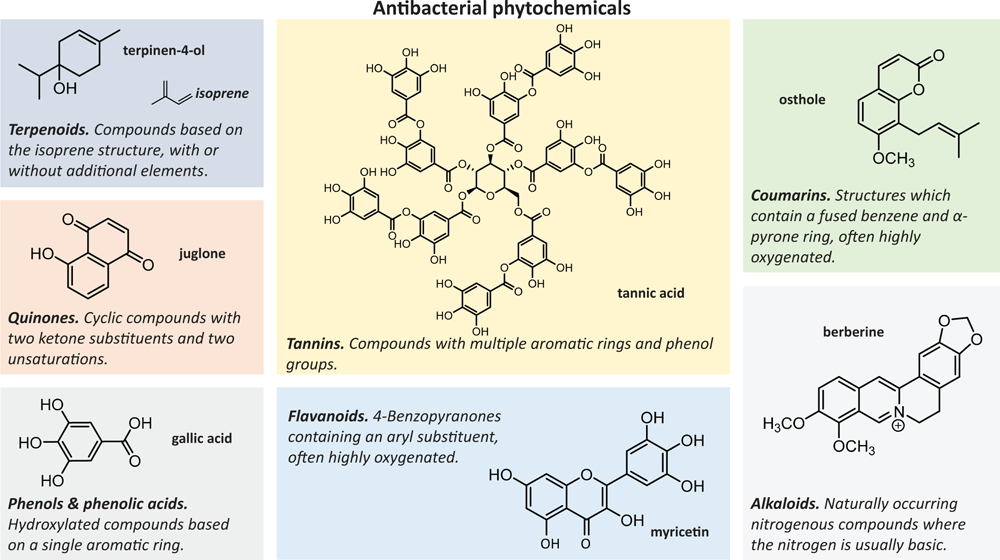
Important phytochemical groups include phenolics and polyphenols, quinones, coumarins, flavonoids, terpenoids and alkaloids11 (Figure 1). Phenolics and polyphenols include the simple phenols, phenolic acids and tannins. Antibacterial examples are found in the tea plant Camellia sinensis and include gallic acid, a phenolic acid which disrupts cell membranes12,13, and the tannin tannic acid which reduces Staphylococcus aureus biofilm formation14. A representative of the quinones is juglone found in the black walnut tree Juglans nigra15, while the coumarins include osthole found in Arracacia tolucensis var. multifida16. Flavonoids include myricetin, found in the sweet potato plant Ipomoea batatas and which appears to affect protein synthesis17,18.
Terpenoids are common phytochemicals based on the isoprene structure and include terpinen-4-ol, an antibacterial terpene found abundantly in Melaleuca alternifolia tea tree oil19, which evidence suggests leads to damage to the cell membrane and loss of cytoplasmic material20. Alkaloids are another common phytochemical group and include berberine (previously discussed) found in Coptis chinensis and Berberis fremontii. The mode of action of berberine may be through binding with double helical DNA21 and/or the inhibition of the bacterial division protein FtsZ22.
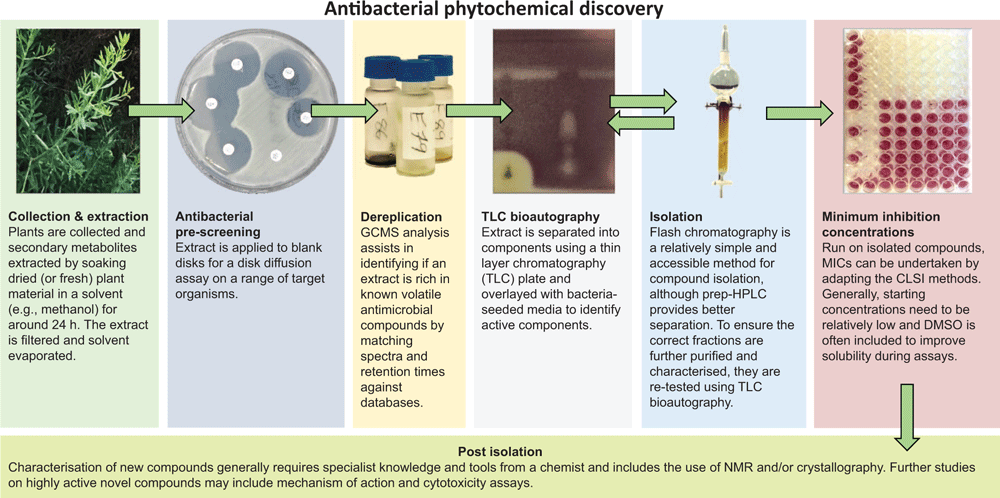
There are many ways to decipher a plant’s defences to find these antibacterial small molecules and these are accessible to a microbiologist who has support from a multidisciplinary team that includes a chemist and botanist. A simple schema developed in our lab is shown in Figure 2 and involves plant collection, secondary metabolite extraction, antimicrobial disk diffusion screen (adapted from the EUCAST23 method), GC-MS analysis coupled with databases (e.g. NIST Mass Spectral Libraries) for dereplication, TLC bioautography24,25, compound isolation by flash chromatography (or prep-HPLC) guided by testing fractions by TLC bioautography, elucidating new compound structures by NMR and/or crystallography and undertaking MICs on isolated compounds (adapting the CLSI methods26). Additionally, screening phytochemicals against specific virulence factors could uncover a trove of treasures, but there are diverse targets5 and each target requires a suitable assay: ultimately lots of work which may result in few if any hits. Inclusion in a compound library for high throughput screening is a possible solution. If good activity is seen during crude extract screening but is poor in the isolated compounds, combinations of compounds suspected to potentiate each other can be tested in a checkerboard assay27.
While the potential of antimicrobial phytochemicals is clear, there is a dearth of examples that have made it into the clinic. Many reasons for this exist including the differences in human and plant biology and physiology giving rise to toxicity concerns. An isolated compound with promising MIC activity needs to demonstrate low toxicity with preliminary tests such as in vitro cytotoxicity assays28 presenting a hurdle. Other factors for the lack of plant-based antibacterial agents include plants making diverse antimicrobial compounds but each with relatively poor activity9, and their production of a range of structurally similar compounds making isolation difficult and resource intensive. Compounding these problems, often the researcher spends time and resources to simply discover a known compound.
Attacking drug resistant bacteria from multiple fronts gives us the best chance for success. Screening phytochemicals as one of those approaches makes sense given the reliance of flora on secondary metabolites for antibacterial protection, and the incredible diversity of structures present across an enormous number of plant species. While plants have thus far generated few clinical candidates, successes in other anti-infective classes such as that of the antimalarial drug artemisinin from Artemisia annua29 allow for optimism. In Australia, only a limited number of researchers have looked at our unique flora as a potential solution and research has tended to focus on a limited number of genera, notably, Acacia, Melaleuca, Eucalyptus and Eremophila, leaving most species still to be screened.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgements
Our research has been supported by an Australian Government Research Training Program (RTP) Scholarship.
References
[1] Blaskovich, M.A. (2019) The diminished antimicrobial pipeline. Microbiol. Aust. 40, 92–96.[2] Payne, D.J. et al. (2007) Drugs for bad bugs: confronting the challenges of antibacterial discovery. Nat. Rev. Drug Discov. 6, 29–40.
| Drugs for bad bugs: confronting the challenges of antibacterial discovery.Crossref | GoogleScholarGoogle Scholar | 17159923PubMed |
[3] Ling, L.L. et al. (2015) A new antibiotic kills pathogens without detectable resistance. Nature 517, 455–459.
| A new antibiotic kills pathogens without detectable resistance.Crossref | GoogleScholarGoogle Scholar | 25561178PubMed |
[4] Blaskovich, M.A. et al. (2015) Helping chemists discover new antibiotics. ACS Infect. Dis. 1, 285–287.
| Helping chemists discover new antibiotics.Crossref | GoogleScholarGoogle Scholar | 27622818PubMed |
[5] Dickey, S.W. et al. (2017) Different drugs for bad bugs: antivirulence strategies in the age of antibiotic resistance. Nat. Rev. Drug Discov. 16, 457–471.
| Different drugs for bad bugs: antivirulence strategies in the age of antibiotic resistance.Crossref | GoogleScholarGoogle Scholar | 28337021PubMed |
[6] Dedrick, R.M. et al. (2019) Engineered bacteriophages for treatment of a patient with a disseminated drug-resistant Mycobacterium abscessus. Nat. Med. 25, 730–733.
| Engineered bacteriophages for treatment of a patient with a disseminated drug-resistant Mycobacterium abscessus.Crossref | GoogleScholarGoogle Scholar | 31068712PubMed |
[7] Dixon, R.A. (2001) Natural products and plant disease resistance. Nature 411, 843–847.
| Natural products and plant disease resistance.Crossref | GoogleScholarGoogle Scholar | 11459067PubMed |
[8] De Caleya, R.F. et al. (1972) Susceptibility of phytopathogenic bacteria to wheat purothionins in vitro. Appl. Microbiol. 23, 998–1000.
[9] Lewis, K. and Ausubel, F.M. (2006) Prospects for plant-derived antibacterials. Nat. Biotechnol. 24, 1504–1507.
| Prospects for plant-derived antibacterials.Crossref | GoogleScholarGoogle Scholar | 17160050PubMed |
[10] Stermitz, F.R. et al. (2000) Synergy in a medicinal plant: antimicrobial action of berberine potentiated by 5ʹ-methoxyhydnocarpin, a multidrug pump inhibitor. Proc. Natl. Acad. Sci. USA 97, 1433–1437.
| Synergy in a medicinal plant: antimicrobial action of berberine potentiated by 5ʹ-methoxyhydnocarpin, a multidrug pump inhibitor.Crossref | GoogleScholarGoogle Scholar | 10677479PubMed |
[11] Cowan, M.M. (1999) Plant products as antimicrobial agents. Clin. Microbiol. Rev. 12, 564–582.
| Plant products as antimicrobial agents.Crossref | GoogleScholarGoogle Scholar | 10515903PubMed |
[12] Borges, A. et al. (2013) Antibacterial activity and mode of action of ferulic and gallic acids against pathogenic bacteria. Microb. Drug Resist. 19, 256–265.
| Antibacterial activity and mode of action of ferulic and gallic acids against pathogenic bacteria.Crossref | GoogleScholarGoogle Scholar | 23480526PubMed |
[13] Zuo, Y. (2002) Simultaneous determination of catechins, caffeine and gallic acids in green, Oolong, black and pu-erh teas using HPLC with a photodiode array detector. Talanta 57, 307–316.
| Simultaneous determination of catechins, caffeine and gallic acids in green, Oolong, black and pu-erh teas using HPLC with a photodiode array detector.Crossref | GoogleScholarGoogle Scholar | 18968631PubMed |
[14] Payne, D.E. et al. (2013) Tannic acid inhibits Staphylococcus aureus surface colonization in an IsaA-dependent manner. Infect. Immun. 81, 496–504.
| Tannic acid inhibits Staphylococcus aureus surface colonization in an IsaA-dependent manner.Crossref | GoogleScholarGoogle Scholar | 23208606PubMed |
[15] Clark, A.M. et al. (1990) Antimicrobial activity of juglone. Phytother. Res. 4, 11–14.
| Antimicrobial activity of juglone.Crossref | GoogleScholarGoogle Scholar |
[16] Figueroa, M. et al. (2007) Constituents, biological activities and quality control parameters of the crude extract and essential oil from Arracacia tolucensis var. multifida. J. Ethnopharmacol. 113, 125–131.
| Constituents, biological activities and quality control parameters of the crude extract and essential oil from Arracacia tolucensis var. multifida.Crossref | GoogleScholarGoogle Scholar | 17582715PubMed |
[17] Xu, H.-X. and Lee, S.F. (2001) Activity of plant flavonoids against antibiotic-resistant bacteria. Phytother. Res. 15, 39–43.
| Activity of plant flavonoids against antibiotic-resistant bacteria.Crossref | GoogleScholarGoogle Scholar | 11180521PubMed |
[18] Ojong, P.B. et al. (2008) Variation of flavonoid content among sweetpotato accessions. J. Am. Soc. Hortic. Sci. 133, 819–824.
| Variation of flavonoid content among sweetpotato accessions.Crossref | GoogleScholarGoogle Scholar |
[19] Carson, C.F. and Riley, T.V. (1995) Antimicrobial activity of the major components of the essential oil of Melaleuca alternifolia. J. Appl. Bacteriol. 78, 264–269.
| Antimicrobial activity of the major components of the essential oil of Melaleuca alternifolia.Crossref | GoogleScholarGoogle Scholar | 7730203PubMed |
[20] Carson, C.F. et al. (2002) Mechanism of action of Melaleuca alternifolia (tea tree) oil on Staphylococcus aureus determined by time-kill, lysis, leakage, and salt tolerance assays and electron microscopy. Antimicrob. Agents Chemother. 46, 1914–1920.
| Mechanism of action of Melaleuca alternifolia (tea tree) oil on Staphylococcus aureus determined by time-kill, lysis, leakage, and salt tolerance assays and electron microscopy.Crossref | GoogleScholarGoogle Scholar | 12019108PubMed |
[21] Li, W.-Y. et al. (1998) Spectroscopic and binding properties of berberine to DNA and its application to DNA detection. Spectrosc. Lett. 31, 1287–1298.
| Spectroscopic and binding properties of berberine to DNA and its application to DNA detection.Crossref | GoogleScholarGoogle Scholar |
[22] Boberek, J.M. et al. (2010) Genetic evidence for inhibition of bacterial division protein FtsZ by berberine. PLoS One 5, e13745.
| Genetic evidence for inhibition of bacterial division protein FtsZ by berberine.Crossref | GoogleScholarGoogle Scholar | 21060782PubMed |
[23] Matuschek, E. et al. (2014) Development of the EUCAST disk diffusion antimicrobial susceptibility testing method and its implementation in routine microbiology laboratories. Clin. Microbiol. Infect. 20, O255–O266.
| Development of the EUCAST disk diffusion antimicrobial susceptibility testing method and its implementation in routine microbiology laboratories.Crossref | GoogleScholarGoogle Scholar | 24131428PubMed |
[24] Hamburger, M.O. and Cordell, G.A. (1987) A direct bioautographic TLC assay for compounds possessing antibacterial activity. J. Nat. Prod. 50, 19–22.
| A direct bioautographic TLC assay for compounds possessing antibacterial activity.Crossref | GoogleScholarGoogle Scholar | 3110376PubMed |
[25] Lyddiard, D. and Greatrex, B.W. (2018) Serrulatic acid diastereomers identified from an antibacterial survey of Eremophila. Fitoterapia 126, 29–34.
| Serrulatic acid diastereomers identified from an antibacterial survey of Eremophila.Crossref | GoogleScholarGoogle Scholar | 29154865PubMed |
[26] Clinical and Laboratory Standards Institute (2012) Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically; Approved Standard – Ninth edition. CLSI document M07-A9, Wayne, PA, USA.
[27] Orhan, G. et al. (2005) Synergy tests by E test and checkerboard methods of antimicrobial combinations against Brucella melitensis. J. Clin. Microbiol. 43, 140–143.
| Synergy tests by E test and checkerboard methods of antimicrobial combinations against Brucella melitensis.Crossref | GoogleScholarGoogle Scholar | 15634962PubMed |
[28] Tolosa, L. et al. (2015) General cytotoxicity assessment by means of the MTT assay. Methods Mol. Biol. 1250, 333–348.
| General cytotoxicity assessment by means of the MTT assay.Crossref | GoogleScholarGoogle Scholar | 26272156PubMed |
[29] Tu, Y. (2011) The discovery of artemisinin (qinghaosu) and gifts from Chinese medicine. Nat. Med. 17, 1217–1220.
| The discovery of artemisinin (qinghaosu) and gifts from Chinese medicine.Crossref | GoogleScholarGoogle Scholar | 21989013PubMed |
Biographies
Dane Lyddiard is a PhD candidate at the University of New England. His research interests include the phytochemistry of Australian native plants and its application to antimicrobial compound discovery.
Dr Ben Greatrex is a synthetic organic chemist in the Pharmacy discipline at the University of New England. His research interests include the discovery of bioactive natural products in Australian native plant species, and the synthesis of advanced functional materials and pharmaceuticals from waste lignocellulosic biomass.