Blistered skin in a 30-year-old woman
Michael Tran 1 3 , Vallapan Thiruvilangam 21 Church St Medical Practice, Newtown, NSW 2042, Australia; and Western Sydney University, NSW, Australia.
2 Douglass Hanly Moir Pathology, Sydney, NSW, Australia.
3 Corresponding author. Email: Michael.tran.gp@gmail.com
Journal of Primary Health Care 11(4) 380-383 https://doi.org/10.1071/HC19086
Published: 18 December 2019
Journal Compilation © Royal New Zealand College of General Practitioners 2019 This is an open access article licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License
Case details
A woman aged 30 years presented with a 1-year history of blistering skin lesions over her trunk, arms and legs (Figures 1 and 2). The lesions began as an erythematous macule and then transformed into a series of tense blisters with an annular appearance after the blistering resolved. These lesions had occurred abruptly. The lesions were intensely pruritic and took a few days to resolve and then a further few months to heal. Eruptions of inflammation and blistering tended to occur in similar areas in a relapsing and remitting pattern. There was no significant antecedent or co-morbid pathology. She had been otherwise well.
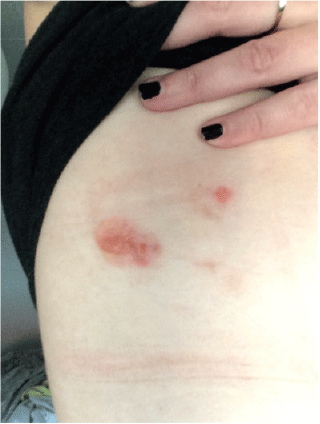
|
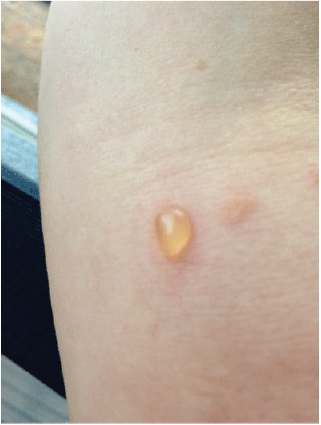
|
Investigation and diagnosis
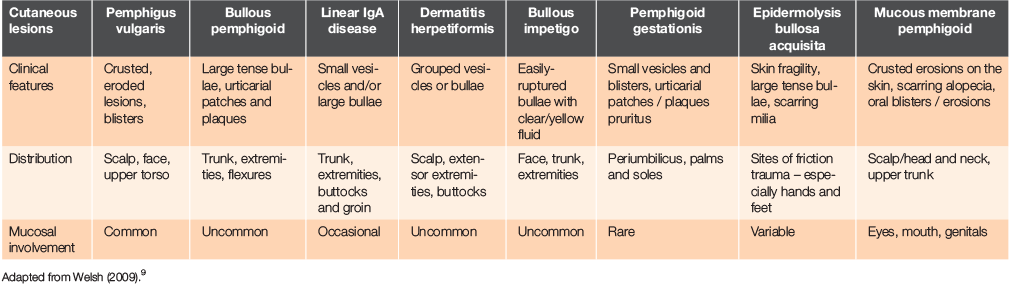
The differential for a blistering skin disorder is broad (Table 1) and includes a drug eruption, pemphigus vulgaris, bullous pemphigoid, dermatitis herpetiformis, bullous impetigo and linear immunoglobulin A (IgA) disease.
|
To confirm a diagnosis for blistering disorders of the skin, a paired biopsy is often required. This includes histopathology with haematoxylin and eosin staining of the blistered skin, as well as peri-lesional punch biopsy. Peri-lesional skin is clinically normal-appearing skin adjacent to a lesion which is sent as a fresh specimen (on saline-moistened gauze or Michel’s transport medium). This will often demonstrate the underlying pathology on direct immunofluorescence.1 Best results are usually obtained within 48 h of collection. It is recommended that the receiving laboratory be informed of the specimen’s arrival so that prompt processing can occur.
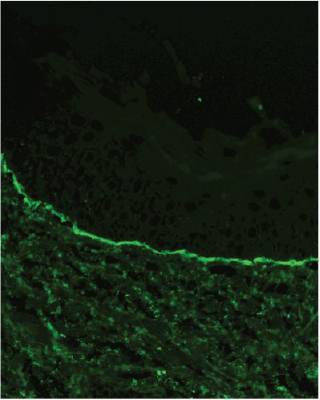
In this case, the demonstration of linear deposits of IgA along the basement membrane zone via direct immunofluorescence of the non-lesional skin (Figure 3) confirmed a diagnosis of linear IgA bullous dermatosis. IgA deposition is present in nearly 80% of cases. The remainder may have IgG, IgM or C3 deposition.2
|
Histopathology of the blistered skin with haematoxylin and eosin staining alone will only demonstrate a subepidermal blister with neutrophil-predominant dermal infiltrate and this can closely resemble dermatitis herpetiformis.
Linear IgA bullous dermatosis
The presentation in this case was consistent with linear IgA bullous dermatosis (LABD). This is a rare, idiopathic or drug-induced autoimmune blistering disease that can occur in both children aged 6 months to 10 years and in adults, usually aged >60 years.3
| Key points of linear IgA bullous dermatosis | |
| |
LABD often presents with lesions on both the skin and mucous membranes. Sub-epidermal blisters are typically tense, rather than flaccid as may be seen in pemphigus vulgaris. In children, blisters often form at the periphery of resolving lesions, resulting in an annular appearance. The distribution of lesions often involves the trunk, peri-oral region, genitalia, hands and feet.
In adults, similar areas of involvement as in children are seen, as well as over the extensor extremities and buttocks. Annular lesions demonstrating peripheral vesiculation develop less frequently than in children. Pruritus is common, can be intense and result in excoriation.4
Mucosal surfaces such as the eyes, nasal cavity and pharynx may also be affected in up to 80% of patients and present primarily as erosions or ulcers.
Although the binding of IgA antibodies to the basement membrane zone is a feature of LABD, the mechanism of lesion formation is not well understood. Both humoral and cellular immune responses are thought to be involved. Tissue injury resulting from an antibody-induced local inflammatory response with release of proteolytic enzymes by neutrophils and other inflammatory cells may contribute.3
The inciting factor for disease development often remains unknown. Some medications such as vancomycin, antibiotics and non-steroidal anti-inflammatory drugs may contribute. Genetic factors may also contribute, though further research is required.5 LABD has been documented to occur in the context of ulcerative colitis, haematologic malignancies and other autoimmune disorders including psoriasis and systemic lupus erythematosus.
Treatment
Dapsone, also known as diaminodipheynl sulfone, is an immunomodulatory antibiotic that is considered the first-line treatment for LABD. As an oral preparation, it is well tolerated by most patients. It is commenced at a low dose and titrated upwards over several weeks. Clinical response is often rapid and present within days.6 The medication needs to be administered cautiously as side-effects can include haemolysis, methaemoglobinaemia, agranulocytosis and peripheral motor neuropathy. Dapsone should be avoided in patients with glucose-6-phosphate (G6PD) deficiency as the risk for severe haemolytic anaemia is elevated.7
Topical corticosteroids are sometimes used as adjunctive therapy. Severe and refractory disease may require the use of immunosuppressive agents including systemic glucocorticoids or glucocorticoid-sparing agents such as mycophenolate, cyclophosphamide and cyclosporine.6
Drug-induced LABD typically resolves with withdrawal of the offending agent.
Given the potential risks associated with treatment, a dermatologist or immunologist should be involved in the care of patients with LABD. Patients with signs or symptoms of ocular disease should be referred to an ophthalmologist.8
Patient progress
The patient was commenced on a combination of oral prednisone and dapsone with good effect and resolution of symptoms. Treatment is ongoing with low-dose dapsone to manage occasional recurrences of the disease.
Competing interests
The authors declare no competing interests.
Funding
This research did not receive any specific funding.
References
[1] Stevenson P, Rodins K. Improving diagnostic accuracy of skin biopsies. Aust J Gen Pract. 2018; 47 216–20.| 29621863PubMed |
[2] Patterson JW, Hosler GA. Linear IgA bullous dermatosis. In: Weedon’s Skin Pathology, 4th edn. JW Patterson (ed.). Philadelphia: Churchill, Livingstone: Elsevier; 2016. Ch. 6, p. 186–205.
[3] Fortuna G, Marinkovich MP. Linear immunoglobulin A bullous dermatosis. Clin Dermatol. 2012; 30 38–50.
| Linear immunoglobulin A bullous dermatosis.Crossref | GoogleScholarGoogle Scholar | 22137225PubMed |
[4] Venning VA. Linear IgA disease: clinical presentation, diagnosis, and pathogenesis. Dermatol Clin. 2011; 29 453–8.
| Linear IgA disease: clinical presentation, diagnosis, and pathogenesis.Crossref | GoogleScholarGoogle Scholar | 21605811PubMed |
[5] Fortuna G, Salas-Alanis JC, Guidetti E, Marinkovich MP. A critical reappraisal of the current data on drug-induced linear immunoglobulin A bullous dermatosis: a real and separate nosological entity? J Am Acad Dermatol. 2012; 66 988–94.
| A critical reappraisal of the current data on drug-induced linear immunoglobulin A bullous dermatosis: a real and separate nosological entity?Crossref | GoogleScholarGoogle Scholar | 22169257PubMed |
[6] Chorzelski TP, Jabłońska S, Maciejowska E. Linear IgA bullous dermatosis of adults. Clin Dermatol. 1991; 9 383–92.
| Linear IgA bullous dermatosis of adults.Crossref | GoogleScholarGoogle Scholar | 1806226PubMed |
[7] Ng SY, Venning VV. Management of linear IgA disease. Dermatol Clin. 2011; 29 629–30.
| Management of linear IgA disease.Crossref | GoogleScholarGoogle Scholar | 21925008PubMed |
[8] Wojnarowska F, Marsden RA, Bhogal B, Black MM. Chronic bullous disease of childhood, childhood cicatricial pemphigoid, and linear IgA disease of adults. A comparative study demonstrating clinical and immunopathologic overlap. J Am Acad Dermatol. 1988; 19 792–805.
| Chronic bullous disease of childhood, childhood cicatricial pemphigoid, and linear IgA disease of adults. A comparative study demonstrating clinical and immunopathologic overlap.Crossref | GoogleScholarGoogle Scholar | 3056993PubMed |
[9] Welsh B. Blistering skin conditions. Aust Fam Physician. 2009; 38 484–90.
| 19575066PubMed |


