‘I felt so empowered, respected and shame free.’ Let’s test for HPV participants’ experience of HPV primary screening
Sally B. Rose 1 * , Lynn McBain 1 , Susan M. Garrett
1 * , Lynn McBain 1 , Susan M. Garrett  1 , Rebecca Bell 2 , Carrie Innes 2 , Sarah Te Whaiti 2 , Alexandria Tino 2 , Peter Sykes 2
1 , Rebecca Bell 2 , Carrie Innes 2 , Sarah Te Whaiti 2 , Alexandria Tino 2 , Peter Sykes 2
1
2
Abstract
Aotearoa New Zealand’s National Screening Unit (NSU) moved to use of human papillomavirus (HPV) primary screening in November 2023.
This study aimed to evaluate participants’ views on favourable and unfavourable elements of HPV primary screening and to seek suggestions for potential improvements.
Primary care participants in a multi-region HPV primary screening implementation study were invited to complete an online follow-up survey in September 2023. This paper reports on qualitatively analysed responses to open-ended questions asking participants what they liked, disliked or thought could be improved for future screening participants.
Of 2361 invitations sent, 2302 were delivered, 969 people consented to participate and 921 were included in analyses (40%, 921/2302). Respondents were 24–71 years of age, from three regions, different ethnic groups and included under-screened participants. Most had chosen to self-test (92%) and 28.9% self-tested at home. Three quarters shared comments about what they liked, with themes related to ability to self-test, avoiding cervical tests, choice, communication and support. Twenty percent described unfavourable aspects, with themes related to inadequate information, self-testing issues, inappropriate physical space and process and programme-related factors. Seven key recommendations were identified from suggestions about potential improvements for future screening participants.
Survey participants’ experience of HPV primary screening was overwhelmingly positive, with choice of a self-test a clear benefit for most. Inadequate information or communication contributed to suboptimal experiences for some. Participant recommendations highlight practical steps screen-takers (and the NSU) could take to ensure screening participants receive a well-informed, affirming experience that supports ongoing participation in cervical screening.
Keywords: cervical cancer, cervical screening, human papillomavirus (HPV), patient experience, qualitative, self-sampling, self-test, survey.
| WHAT GAP THIS FILLS |
| What is already known: Access to human papillomavirus (HPV) self-testing improves participation in cervical screening among people who are un- or under-screened. Primary care clinicians involved in the early implementation of HPV primary screening in Aotearoa New Zealand support the change in primary screening modality. |
| What this study adds: The ability to self-test, clear clinician communication and support were important contributors to a good screening experience, while inadequately communicated information impacted negatively on multiple aspects of screening. Participants identified a range of practical suggestions for primary care providers to support access and acceptability among future screening participants. |
Introduction
Population-based cervical screening programmes have traditionally utilised cervical cytology to identify early pre-cancerous changes on the cervix. With early detection and treatment, invasive cervical cancer is almost entirely preventable,1 yet around 170 people are diagnosed and 60 people die from cervical cancer each year in Aotearoa New Zealand (NZ).2 The national cervical screening programme has been unable to achieve equitable screening coverage and clinical outcomes for the screening eligible population. The screening programme fails to reach some people for reasons including health service factors (cost, transport and other access barriers, negative past interactions) cancer fears, embarrassment and discomfort.3,4 Māori, Pacific and people living in higher deprivation have significantly lower cervical screening coverage, and higher cancer incidence and mortality than European/other ethnic groups and people living in less deprived areas.5
The National Screening Unit (NSU) moved away from cytology-based cervical screening to human papillomavirus (HPV) primary screening in September 2023. People are now offered the option of a self-collected HPV vaginal swab (self-test), or a clinician-taken cervical sample (cervical test). HPV primary screening offers an important opportunity for improved detection and prevention of cervical cancer, and reduction of long-standing inequities in screening coverage.4,6,7 More than 90% of all cervical cancers are caused by persistent infection with high risk subtypes of HPV – a common virus that is sexually transmitted.8 Testing for the presence of high-risk HPV types can, with high sensitivity, identify individuals at risk of having or developing pre-cancerous cervical cell changes. With appropriate clinical follow-up of individuals with HPV, any abnormal cell changes can be effectively treated.7 A crucial advantage of HPV testing is that it can be carried out with equal sensitivity and specificity on self-collected vaginal or clinician-taken cervical samples,9 and self-tests can be carried out at home or outside of clinical settings. This removes many of the psychological, emotional and physical barriers associated with clinician-taken cytology tests.10–12 Many people find self-tests convenient, easier and more comfortable than cervical tests.10,11,13–15 Research in NZ has already shown that self-tests are highly acceptable and associated with higher screening participation among Māori, Pacific and Asian people who have never screened or are under-screened.16,17
A number of high-income countries with organised cervical screening programmes adopted HPV primary screening some years ago; most utilise cervical tests as the primary method, offering self-tests only to under-screened individuals.18 The NZ NSU is among the first to routinely offer self-tests to all screening-aged asymptomatic individuals with no prior history of cervical abnormality. Prior to initiation of HPV primary screening, the NSU funded a multi-region study (‘Let’s test for HPV’) to inform implementation in primary care. Participating clinicians were positive about the new screening pathway and observed their patients’ enthusiasm for self-testing.19 The current study involved a follow-up survey of implementation study participants to understand their experiences with HPV primary screening. This paper evaluates participants’ views on favourable and unfavourable elements of HPV primary screening and describes their suggestions for improvements.
Method
Let’s test for HPV study participants
Participants in Let’s test for HPV (‘main study’) included 3308 people enrolled at 1 of 17 primary care practices who were due or overdue for cervical screening between August 2022 and February 2023. Participating practices were located in three regions: Canterbury (5 practices), Wellington (7) and Whanganui (5). Participants were offered the choice of an HPV self-test or clinician-taken HPV cervical test with reflex cytology if HPV was detected. For a full description of Let’s test for HPV methodology see https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=383849, and for information provided to participants see https://blogs.otago.ac.nz/hpv.
The main study was approved in 2022 (2022 Southern Health and Disability Ethics Committee [HDEC] FULL 12546). This follow-up survey was approved as an amendment (Southern HDEC 2023 AM 12546, 11 September 2023).
Survey recruitment
Main study participants who consented to be contacted for follow-up research (2394/3308, 72%) and provided an email address or mobile number (2361/2394, 99%) were eligible for survey participation. The first invitation was sent in September 2023, with three reminders sent in the following 5 weeks. Paper surveys and completion by phone were also available. On clicking the survey link, potential participants were presented with the information sheet and were required to check a ‘consent to participate’ checkbox to proceed.
Data collection and analysis
The secure Qualtrics survey platform was used to collect fully anonymised data. Survey questions were customised for the current study but drew on relevant local13,15,16,20 and international12,14,21–24 work to inform some response options. The final draft incorporated feedback from members of the Māori Steering Committee and Pacific Advisory Group (convened to provide support to the main study). This paper presents the qualitative analysis of three open-ended questions: (1) What, if any, parts of the new HPV test/cervical screening process worked well for you? (2) What, if any, parts of the new HPV test/cervical screening process did you find difficult or dislike? (3) How might your GP practice improve the HPV test/cervical screening process for you and others in the future? Free text comments shared in response to all other questions with an ‘other, please state’ option were reviewed, and content included if it described likes, dislikes or suggested improvements. The main quantitative survey results are being reported separately.
Responses to open-ended questions ranged from single words to several sentences. Data were analysed using an inductive thematic approach,25 led by the first author (SR) who was not part of the Let’s test for HPV study team. SR is a non-clinical researcher with over 20 years of experience in health services research and use of mixed-methods approaches. During data familiarisation, SR read all comments several times in the context of the full dataset. Comments were then extracted with participant descriptors (age-band, ethnicity, screening method, result and study region) and stored in an Excel file for initial coding. Coding was an iterative and emergent process that involved generating, reviewing and refining topic headings. Topics were grouped into sub-themes and themes in Word document tables for co-author review. Quotes were selected to illustrate the range of views and ideas shared by participants for each theme, including ideas that were common to many participants as well as some that were unique to fewer individuals. Ethnicity and a 10-year age-band are shown alongside quotes to reflect the diversity of participants who shared comments (region is omitted to preserve participant anonymity).
Results
Of 2361 invitations sent, 2302 were delivered and 969 people ticked ‘consent to participate’ and began the survey (42.1% partial completion rate, 969/2302). Most respondents answered all questions (92.7%, 898/969). Partially completed surveys were reviewed and an additional 23/101 partially complete surveys were included (if respondents had reached the question asking for reflection on receipt of results), giving a total of 921 respondents (40% response rate).
Table 1 presents the demographic characteristics of survey respondents. The age range was 24–71 years (median 49 years), 19% were Māori (24% in the main study), 14% were never or under-screened (30% in the main study), 92% chose to self-test (95% in the main study), with 29% self-testing at home (23% in the main study). Ten percent of survey participants returned an HPV detected result (13% in the main study). There was good engagement with open-ended survey questions, with 99% of participants responding to at least one of the questions.
| CharacteristicsA | Total participants (n = 921) | |||
|---|---|---|---|---|
| n | % | 95% CI | ||
| Age-band (years) | ||||
| <30 | 82 | 8.9 | (7.1–10.9) | |
| 30–39 | 183 | 19.9 | (17.3–22.6) | |
| 40–49 | 204 | 22.1 | (19.5–25.0) | |
| 50–59 | 251 | 27.3 | (24.4–30.3) | |
| 60 and over | 193 | 21.0 | (18.4–23.7) | |
| Region of residence | ||||
| Whanganui | 276 | 30.0 | (27.0–33.0) | |
| Wellington (including Kāpiti Coast) | 383 | 41.6 | (38.4–44.8) | |
| Canterbury | 260 | 28.2 | (25.3–31.3) | |
| Ethnicity (prioritised) | ||||
| Māori | 176 | 19.1 | (16.6–21.8) | |
| Pacific | 20 | 2.2 | (1.3–3.3) | |
| NZ and other European | 632 | 68.6 | (65.5–71.6) | |
| Asian | 62 | 6.7 | (5.2–8.5) | |
| Middle eastern, Latin American, African (MELAA) | 11 | 1.2 | (0.6–2.1) | |
| Other | 16 | 1.7 | (1.0–2.8) | |
| GenderB | ||||
| Female | 913 | 99.1 | (98.3–99.6) | |
| Male, non-binary, Takatāpui, gender diverse | 7 | 0.8 | (0.3–1.6) | |
| Language(s) spokenC | ||||
| English | 914 | 99.2 | (98.4–99.7) | |
| Māori | 40 | 4.3 | (3.1–5.9) | |
| NZ sign | 6 | 0.7 | (0.2–1.4) | |
| Samoan | 3 | 0.3 | (0.1–0.9) | |
| Other languages | 86 | 9.3 | (7.5–11.4) | |
| Cervical screening history | ||||
| Screened within 5 years | 730 | 79.3 | (76.5–81.8) | |
| Screened more than 5 years ago ‘under-screened’ | 103 | 11.2 | (9.2–13.4) | |
| Screened but can’t recall when | 54 | 5.9 | (4.4–7.6) | |
| Never screened ‘un-screened’D | 30 | 3.3 | (2.2–4.6) | |
| Unsure | 2 | 0.2 | (0–0.8) | |
| If screened, ever had abnormal cytologyE | 298 | 32.4 | (29.3–35.5) | |
| Screening method in Let’s test for HPV | ||||
| HPV self-test (clinic) | 585 | 63.5 | (60.3–66.6) | |
| HPV self-test (home) | 266 | 28.9 | (26.0–31.9) | |
| Clinician taken cervical test | 70 | 7.6 | (6.0–9.5) | |
Overarching themes
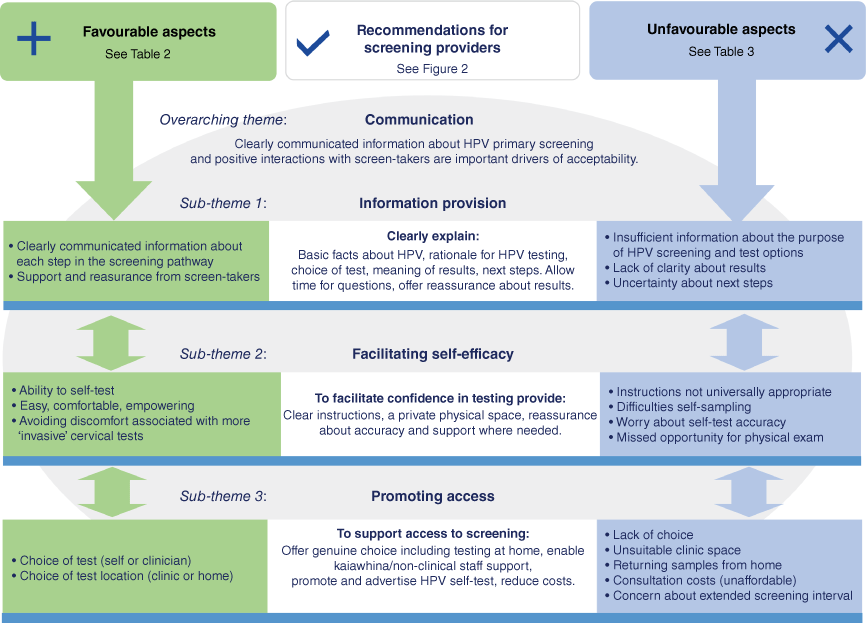
Fig. 1 draws together the primary themes identified across all comments, with communication identified as an overarching theme that impacted on multiple aspects of participants’ experience. Three overlapping sub-themes described factors that supported or detracted from screening experiences: information provision, facilitating self-efficacy and promoting access.
Participants appreciated clearly communicated information about HPV testing, and clinician support and reassurance throughout the process – factors that were key to supporting self-efficacy in sample collection and confidence in the new screening pathway. Conversely, poor communication and inadequate information provision impacted negatively patient perceptions at each stage of the screening pathway. Lack of clarity about the rationale for HPV testing, insufficient instruction on self-sampling and receipt of results without explanatory information contributed to uncertainty and worry about the effectiveness, accuracy and implications of their cervical screen. These themes and sub-themes are described in more detail below and in Tables 2 and 3.
| Themes and sub-themes | Selected participant quotes A | |
|---|---|---|
| 1. Ability to self-test | I felt so empowered, respected and, shame free!!! (Māori, 60+) | |
| It removes the embarrassment, it gives women control over their swab process. (European, 40–49) | ||
| Privacy, having control over my own body so not being ‘done to you’! (European, 60+) | ||
| Efficient, do it yourself, easy and quick results. (Māori, 40–49) | ||
| Being able to do it at home in private instead of the traditional smear was great and means I won’t procrastinate in the future. (European, 40–49) | ||
| The privacy and independent feeling of doing the swab alone. (Asian, 50–59) | ||
| I’d like to self-test from now on, so easy, less stress and fuss. (Māori, 40–49) | ||
2. Avoiding cervical tests (smears)
| Definitely faster than a smear, privacy and feeling of taking control of my health and dignity. A lifetime of smears has given me a range of experiences, rarely good or encouraging enthusiasm for the process. The swabs are a definite winner from me as a consumer. (Māori, 60+) | |
| Less painful, less embarrassing. (Asian, 40–49) | ||
| It was a chill experience - it was definitely less uncomfortable than going straight to a smear. (Māori, <30) | ||
| I had tried to have a cervical smear test taken the usual way via speculum by a nurse - however, as I am post-menopausal it was very painful and uncomfortable and I couldn’t complete it in this way. I was relieved that there was an alternative method of testing. (European, 60+) | ||
| I am an abuse victim and wouldn’t have smear tests. This test was a game changer for me. (European, 50–59) | ||
| Not having to get a smear with people I know, being from a small community. (European, 40–49) | ||
| 3. Choice supports access | I was pleased to be given the opportunity to be in control of my choice. I really think this is a great choice for each individual to make. (European, 60+) | |
| I liked that the smear (which I always find invasive and uncomfortable) was presented as an option that would be resorted to only if my first test came back positive. (Māori, <30) | ||
| The ease and improved testing being for the virus not the cellular changes. (Māori, 30–39) | ||
| Was able to do it at home, I wouldn’t have had a smear otherwise. (European, 40–49) | ||
| I live a long way from my GP surgery so no cost for travelling. (European, 50–59) | ||
| I’ve been so late getting smears as I work out of town. This is a game changer! (European, 40–49) | ||
| Our GP is very busy. Getting times to see any doctor is hard when living rurally. This is a very good option for rural women. (European, 30–39) | ||
| 4. Communication and support | The nurse was incredibly understanding and supportive and explained everything to me and the research that is being conducted behind it. I would not have had a cervical smear ever if I wasn’t able to do this self-test. (European, <30) | |
| I felt so reassured throughout the whole process and am super grateful for that as it can/was quite an anxious time. (Māori, 40–49) | ||
| I thought the process was done well, I have learned a lot about the infection and how it can progress and treatments. (Pacific, <30) | ||
| I remember being quite well informed at the time and there were lots of opportunities for questions. I liked the face to face with my nurse. (Māori, 40–49) | ||
| I have extensive sexual trauma so it was very hard to do, but I felt supported by the nurse who recommended it and explained things to me, and it felt less intrusive than the cervical smear. (European, <30) |
| Themes and sub-themes | Selected participant quotes A | |
|---|---|---|
| 1. Inadequate information | I wasn’t, and still am not, sure about the efficacy of it and being able to detect future changes in cells. (Māori, 50–59) | |
| I just hope they are valid and in a few years aren’t going to be found that people slipped through the cracks if they didn’t do it properly. (European, 40–49) | ||
| The nurse was rushed, I didn’t feel like I was given adequate information about the cervical smear and self-test option. (European, <30) | ||
| Had to talk the nurse into letting me take it home. She was adamant I should get it done at the practice even after telling me the self-test was an option. I was more comfortable doing it at home and wasn’t happy about having to insist I take it home. (Māori, 30–39) | ||
| I wanted to test for abnormal cells but did the self-test for convenience. I felt embarrassed to ask for a smear when the self-test was an option. Now I am worried I wouldn’t know if I was at risk. (European, <30) | ||
| I received minimal information about my result so had to Google it. There are so many sites, I wasn’t sure which site to trust. I was very worried. (European, 40–49) | ||
| Got results but was unsure of next step. (European, 50–59) | ||
| 2. Self-sampling challenges and concerns | Pamphlet needs larger font size so it’s easier to read the instructions. Simpler clearer diagrams, more cross section type less human. (European, 60+) | |
| I am a visual person but instructions were told to me so it was difficult to remember everything. (Māori, 30–39) | ||
| Lack of instruction on finding cervix and what if I don’t find the cervix. (European, 30–39) | ||
| Wasn’t quite sure whether I’ve done it correctly and whether that’ll affect the results. (Asian, 30–39) | ||
| The stick was not strong enough and would bend making it more difficult to insert. (European, 50–59) | ||
| I experienced extreme discomfort for three days post-self swab. I was unable to use the toilet without searing pain for those days. (Māori, <30) | ||
| I worry that self-testing will mean more women with lichens sclerosis/prolapses or other problems, may not be identified and go untreated. (European, 60+) | ||
| Compared to the traditional test it’s an improvement, but with the smear a medical professional can check for other problems e.g. discolouration or other lumps and bumps that you can’t see for yourself. (European, 50–59) | ||
| 3. Physical space | Having to do it at the practice, it’s a decent drive. (European, <30) | |
| I thought I was picking it up to take home but got there and had to do it on the spot and had small children who came into the toilet stall with me. (Māori, 40–49) | ||
| I understood I could do the test at home, but was told I was expected to do it at the surgery and in the toilet. I didn’t like this. (Pacific, 60+) | ||
| It was embarrassing and uncomfortable to only have a curtain between myself and other patients while trying to read and understand how to do it. (European, 60+) | ||
| There was nowhere to put the kit while I was getting ready to do the swab. I ended up putting it on the toilet lid while trying not to let the swab touch anything. A clean table or shelf is definitely needed. (European, 50–59) | ||
| The fiddliness of having to carefully remove the swab from the tube, placing the tube somewhere safely and placing it back together with my pants down. (Māori, 30–39) | ||
| 4. Programme/process issues | Pre-printed labels would help as hard to write on tube. (European, 40–49) | |
| Remembering to do it! then posting it. (European, 50–59) | ||
| Sending the sample in the mail, just lacked confidence in NZ post. (Other/not stated, 30–39) | ||
| Waiting for the test results were longer than a smear test. (European, 40–49) | ||
| Wording had confused me initially said ‘high-risk HPV’ THEN ‘not detected’! (European, 50–59) | ||
| I don’t know why I had to pay for a doctors appointment, felt it should have been free. (European, 30–39) | ||
| I had to pay a full fee for doing this myself - ideally should be funded, I don’t know how people will afford to do this. (European, 40–49) | ||
| I don’t feel secure with screening only done every 5 years. (European, 40–49) | ||
| Should the test be more often? (Māori, 60+) |
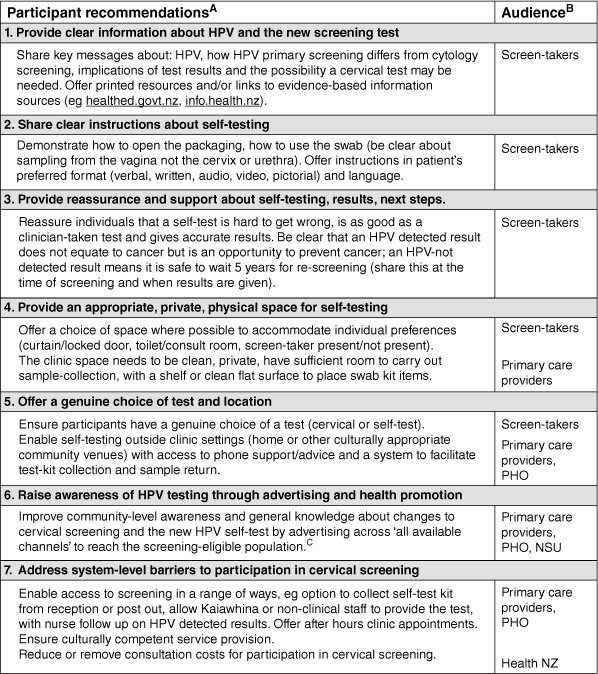
Comments shared in response to being asked what screen-takers could do to improve the screening experience for future participants addressed aspects of screening that they themselves were unhappy with, uncertain about or found difficult. Suggestions were collated to generate recommendations for screening providers that are described in Fig. 2 (and summarised in Fig. 1).
Participant recommendations for screening providers to support access, acceptability and ongoing participation in HPV primary screening. ASee Supplementary Table S1 for supporting data. Recommendations include commonly suggested ideas and those mentioned by fewer individuals. More frequent suggestions are presented at the top of the table, while less common suggestions appear lower down. BPrimary care providers: Clinics providing cervical screening including general practice, Kaupapa Māori providers, Pacific-led providers and other community health providers; PHO, Primary Health Organisation; NSU, National Screening Unit; Health NZ, Health New Zealand Te Whatu Ora (funder of the National Cervical Screening Programme). CSee Toolkit available at: https://www.tewhatuora.govt.nz/assets/For-the-health-sector/NSU/For-Health-professionals/Cervical-Screening-Programme/Maori-and-All-Aotearoa-Campaign-Provider-toolkit-3_5-October-2023.pdf.
Favourable aspects of HPV primary screening
Three quarters of participants shared something they liked about the HPV primary screening experience (680/921). Comments related to four themes: (i) ability to self-test, (ii) avoiding cervical tests (smears), (iii) choice supports access, (iv) communication and clinician support. These themes are further described in Table 2 with selected participant quotes. Twenty percent of people liked all aspects of the new screening pathway, sharing comments such as:
All of it. Super easy. Great info. Quick and less painless than with the duck contraption that normally is used. (Māori, 40–49)
Everything was perfect – such a wonderful change from the old way which I always found incredibly painful and therefore I put it off. (European, age unknown)
Gratitude for the introduction of self-testing emerged as a clear theme, as did ‘avoiding cervical tests.’ Many appreciated being given a choice of test and test location, describing the ways in which this supported their ability to access screening. Clearly communicated information, clinician support and reassurance were also valued. Just under half of participants shared a favourable comment about their experience with self-testing. Frequently used descriptors used by participants across all demographic groups were that it was ‘easy’, ‘convenient’, ‘private’, ‘quick’ and ‘painless’. Many people framed their comment in a way that compared the self-test to traditional cervical screening, describing it as ‘less invasive’, ‘less painful’ ‘less time consuming’ and ‘less intrusive’. People disclosed personal information about the reasons they had delayed or avoided screening in the past. Some noted that had self-testing been available earlier, they would have taken part in screening. Taken together, comments reflected overall appreciation of a more private, comfortable and empowering screening experience, that supported willingness to participate in future.
Unfavourable aspects of HPV primary screening
Around a fifth of participants identified something they disliked or felt did not work well for them during the screening process (200/921). Most of this group also shared comments about what they liked (16 people only identified something they disliked). There were fewer comments overall about things that did not work well for people, but a wider range of issues were identified. This reflects the wide-ranging needs and preferences of screening-eligible individuals. We identified four themes in our analysis of participant comments: (i) insufficient information, (ii) self-testing challenges and concerns, (iii) physical environment and (iv) process/programme issues. Themes and sub-themes are described in Table 3 with quotes chosen to reflect the main themes.
Inadequate or poorly communicated information was the strongest theme, with comments about information or knowledge gaps across all aspects of the screening pathway. Lack of clarity about how the HPV test differed to the old test was commonly reported. Some people felt they lacked choice about test options or felt pressured into choosing the self-test. Uncertainty about how to perform the self-test, worry about accuracy and issues related to the physical space provided at the clinic were frequently cited among participant’s dislikes. Privacy was an issue for some who felt exposed behind the curtain at the clinic, or the lack of a locked door. Cleanliness of toilet cubicles (and fear of contamination) was raised, and other practical considerations such as insufficient space and somewhere to put swab components when completing sample collection.
Missed opportunities to diagnose genital conditions was noted as a drawback of self-testing by a number of participants. Other process or programme-related drawbacks included challenges of returning samples from home, wait time for results, consultation costs and timing of screening interval. A small group of people reported that there was nothing they liked about the new test (13/200), some of whom preferred the ‘classic’ method of cervical cytology. Among those who disliked the self-test, there was evidence of misunderstanding (e.g. belief that the swab needed to reach the cervix) and a related lack of confidence in the process – again reflecting the impact of gaps in communication.
Participant recommendations for screen-takers
About one-third of participants suggested something their primary care provider could do better or differently to support future participation in screening (299/921). We drew together seven key recommendations from these data that are presented in Fig. 2. Collectively, suggestions describe ways that screen-takers could deliver an acceptable and affirming screening experience. Information about HPV and the option of a new method of screening (self-test) must be well communicated to support knowledge and understanding of the new process. Participants wanted more clearly communicated instructions about self-testing (accessible in a range of formats) and the choice of a clean, private space at the clinic or ability to self-test at home. The importance of widely advertising the new screening approach was highlighted, and a range of access barriers identified that would facilitate participation if addressed. Supplementary Table S1 includes illustrative quotes from which recommendations were drawn.
Discussion
The HPV primary screening experience was well-received by most participants. In addition to widespread gratitude for the option to self-test, participants appreciated clearly communicated information, clinician support and reassurance throughout the screening process. Good communication and information provision were key to supporting self-efficacy in sample collection and overall confidence in the new screening pathway. Conversely, gaps in communication negatively impacted patient perceptions of the process including confusion about the purpose of HPV primary screening, uncertainty about accuracy of results and effectiveness of their screen. Positive reflections of the screening experience outweighed negative views by around four to one, but a wider range of negative issues were raised. Participants shared practical suggestions about how to improve the screening experience for future participants, suggesting ways to support access and improve acceptability.
As expected, the ability to self-test was viewed as a clear benefit of HPV primary screening which aligns with past local research involving un-screened and under-screened Māori, Pacific and Asian participants.13,15 Self-testing was viewed as easy, private, comfortable and empowering, and many reflected on negative attributes of cervical tests that they were happy to avoid. Importantly, people conveyed that access to self-testing meant they would not hesitate to screen or delay screening as they had in the past. Similar to past research,14,15,20 a small group experienced challenges during or after self-testing including pain, discomfort and bleeding. In some instances, discomfort appeared to reflect misinterpretation or inadequate instruction about self-sample collection (e.g. where to swab, how far in to insert the swab). Our inability to prompt for further information from people who did not find self-testing easy means it is not clear where the issues lay nor whether they discussed these with their screen-taker. It is important that screen-takers are aware that a small proportion of individuals might experience pain, discomfort or bleeding that may warrant further review to rule out clinical causes or issues related to technique. Participants should be encouraged to report difficult or uncomfortable experiences to their screen-taker.
Although most people chose to self-test, they appreciated being given a choice. While in the minority, some people preferred the cervical test even when not clinically indicated. Several people felt they were not given a genuine choice and felt pressured by their screen-taker to choose the self-test. A draw-back of self-testing identified by some participants was the missed opportunity for a clinician to (visually) detect any genital pathology. This finding highlights the importance of discussing HPV testing options, offering genuine choice and sensitivity when taking a clinical history for people who may feel reticent about disclosing genital concerns or symptoms. Concern about missed opportunities for a gynaecological exam and screening for sexually transmitted infections and domestic violence have also been raised by healthcare providers in NZ19 and elsewhere,26,27 particularly for people who self-test at home and may not have a face-to-face interaction with a healthcare provider.
Feedback from individuals who self-tested at the clinic emphasised the importance of having a well set-up physical space. They expressed a range of different needs and preferences regarding the clinic environment. Privacy, cleanliness, sufficient space and screen-taker support (if desired) were deemed important. Some found toilet cubicles too cramped, had difficulty managing the self-test kit components and identified the need for a shelf or flat surface to place sample collection items. Where possible, individual preferences should be accommodated to ensure everyone feels safe and comfortable when self-testing at the practice. Although not raised in this survey, spaces must also be accessible for people with physical disabilities. For some, self-testing at home was preferred due to privacy, convenience and accessibility as it overcame barriers related to transport, time and cost. Ideally, the screening programme will be able to accommodate self-testing outside of clinic settings, given the strong preference never- and under-screened groups have expressed for this option.13,15
The importance of a good interaction with supportive screen-takers was a clear theme. Those who felt uncertainty about their ability to do the test correctly had not been equipped with sufficient information, support or reassurance. Similarly, lack of reassurance on receipt of test results led to widely-held concerns about HPV test accuracy, implications for follow-up and health outcomes. Past local research and international studies involving indigenous communities have also reported low confidence among participants in their ability to perform self-testing and related concerns that HPV test results may not be accurate.15,28
Views on the extension of the screening interval from 3 to 5 years were not specifically sought, but a few participants raised concerns about this. The 5-year screening interval has been identified as a point of contention elsewhere, for example in Australia, the proposed move to HPV primary screening in 2017 met with strong community opposition,29 and in Canada (where HPV primary screening is not yet available) many primary care trial participants were apprehensive about the impact of an extended screening interval.30 These concerns stem from low knowledge about HPV primary screening – which has been identified as an important driver of HPV test acceptance.31 Evidence from systematic reviews shows that a single negative experience with cervical screening (e.g. pain or discomfort, poor communication or interaction with a healthcare provider) can act as a barrier to future engagement with screening.32,33 Understanding and addressing the barriers and concerns identified by participants in this work will help to maximise the likelihood that every screening participant has a good screening experience.
Our survey findings also highlight the need for education and health promotion campaigns to raise awareness about changes to cervical screening – a conclusion also drawn from previous local research.13 Key messages that did not appear to be universally understood by survey participants included: (i) HPV testing is a better screening tool than the cytology test because it looks for the virus that can cause cell changes; (ii) a self-test is as reliable as a clinician-taken sample; (iii) if HPV is not detected the risk of developing pre-cancerous cell changes is very low so it is safe to extend the screening interval to 5 years; and (iv) if HPV is detected, it provides an important opportunity for monitoring to prevent the development of cancer.
Strengths and limitations
Inclusion of open-ended questions in our online survey enabled a large group of screening participants to share views on HPV primary screening in their own words but did not allow for in-depth exploration of the issues raised. Although some participant recommendations will be obvious to screen-takers and already part of programme delivery, they highlight elements of the screening pathway that participants themselves think important to get right for future participants. Paper surveys and phone completion were offered as alternatives to online completion, but our methodology favoured those with available time and connectivity to participate. Our sample includes a majority of European participants, and although 19% were Māori, this falls below the 24% participation by Māori in the main study. Despite the under-representation of Māori, Pacific and Asian participants, our analysis includes voices from both majority and minority demographic groups. People who had a less favourable experience with HPV primary screening or have less access to health care are likely to have been under-represented among survey respondents.
Implications
The introduction of HPV primary screening with the option to self-test is an important opportunity to reach people who might not otherwise engage in cervical screening. Participants shared practical advice about ways screen-takers could better support their varied needs. Addressing the barriers faced by a minority of participants and facilitating home self-testing will be crucial for supporting equitable access and participation in screening. While participating screen-takers were educated about HPV primary screening prior to the implementation study, their knowledge and delivery are likely to improve with time, and some of the issues identified here will resolve. Since this work was undertaken, resources and training have been developed for all screen-takers.34 It is essential that screen-takers access this education so they are well informed and equipped to effectively deliver HPV primary screening. A wide range of consumer resources35,36 and promotional campaign materials37 have also been developed, designed to be inclusive and accessible in different languages and formats. It is important that screen-takers and primary care providers link their patients with these resources.
Data availability
All survey data are being reported in full. Raw data are not being made publicly available. We did not seek participant permission to share data nor receive ethics approval to do so. Any queries about the content of the dataset can be directed to sally.rose@otago.ac.nz.
Conflicts of interest
SR and SG have no conflicts of interest to declare. LM, RB, CI and PS received funding from Te Whatu Ora Health New Zealand to conduct the main study. STW received funding from the main study fund as Chair of the Māori Steering Committee and AT received funding from the main study fund as Chair of the Pacific Advisory group. Te Whatu Ora Health New Zealand did not have any input into the study design, collection, analysis or interpretation of data, nor in the writing of the manuscript or decision to submit the article for publication.
Declaration of funding
This research was supported by a 2023 GRACI General Research Grant (Reference No: COOC2301_07), funds awarded in 2022 by Te Whatu Ora Health New Zealand (Contract No. 374890/00) for the Let’s test for HPV Implementation trial and University of Otago funding.
Acknowledgements
The authors thank all those participants who took the time to share their screening experience by completing a survey. Thanks also to Tania Batley from the Māori Steering Committee for input into the survey draft, Debra Smith for inviting feedback from participating practice nurses and Janine Nip who helped prepare study documents for ethics committee review and locality assessment. The authors also acknowledge the work of the Let’s test for HPV study investigators and team members (John McMenamin, Ben Hudson Beverley Lawton, Melanie Gibson) as well as staff at the 17 participating practices.
References
1 World Health Organization. Eliminating cervical cancer. 2022. Available at https://www.who.int/health-topics/cervical-cancer#tab=tab_2 [accessed 28 May 2024].
2 Te Whatu Ora – Health New Zealand. Historical cancer data, Cancer: Historical summary 1948–2020. Wellington: Te Whatu Ora – Health New Zealand; 2023. Available at https://www.tewhatuora.govt.nz/our-health-system/data-and-statistics/historical-cancer/#cancer-historical-summary-19482020 [accessed 28 May 2024].
3 Best Practice Advocacy Centre New Zealand (BPAC NZ). Cervical smears – achieving equity. 2009. Available at https://bpac.org.nz/bpj/2009/september/docs/bpj23_csmears_pages46-55.pdf [accessed 1 May 2024].
4 Bartholomew K, Lawton B, Sherman SM, et al. Recommendations for implementing HPV self-testing in Aotearoa. N Z Med J 2021; 134(1535): 11-6.
| Google Scholar | PubMed |
5 Te Whatu Ora – Health New Zealand. National Cervical Screening Programme and HPV Primary Screening. Coverage and PHO reports. Wellington: Te Whatu Ora – Health New Zealand. Available at https://tewhatuora.shinyapps.io/nsu-ncsp-coverage/ [accessed 20 September 2024].
6 Costa S, Verberckmoes B, Castle PE, et al. Offering HPV self-sampling kits: an updated meta-analysis of the effectiveness of strategies to increase participation in cervical cancer screening. Br J Cancer 2023; 128(5): 805-13.
| Crossref | Google Scholar | PubMed |
7 Koliopoulos G, Nyaga VN, Santesso N, et al. Cytology versus HPV testing for cervical cancer screening in the general population. Cochrane Database Syst Rev [8] 2017; CD008587.
| Crossref | Google Scholar | PubMed |
8 Okunade KS. Human papillomavirus and cervical cancer. J Obstet Gynaecol 2020; 40(5): 602-8.
| Crossref | Google Scholar | PubMed |
9 Arbyn M, Smith SB, Temin S, et al. Detecting cervical precancer and reaching underscreened women by using HPV testing on self samples: updated meta-analyses. BMJ 2018; 363: k4823.
| Crossref | Google Scholar | PubMed |
10 Nelson EJ, Maynard BR, Loux T, et al. The acceptability of self-sampled screening for HPV DNA: a systematic review and meta-analysis. Sex Transm Infect 2017; 93(1): 56-61.
| Crossref | Google Scholar | PubMed |
11 Nishimura H, Yeh PT, Oguntade H, et al. HPV self-sampling for cervical cancer screening: a systematic review of values and preferences. BMJ Glob Health 2021; 6(5): e003743.
| Crossref | Google Scholar | PubMed |
12 Creagh NS, Zammit C, Brotherton JM, et al. The experience of under-screened and never-screened participants using clinician-supported self-collection cervical screening within the Australian National Cervical Screening Program. Womens Health 2022; 18: 17455065221075905.
| Crossref | Google Scholar | PubMed |
13 Adcock A, Stevenson K, Cram F, et al. He Tapu Te Whare Tangata (sacred house of humanity): Under-screened Māori women talk about HPV self-testing cervical screening clinical pathways. Int J Gynecol Obstet 2021; 155(2): 275-81.
| Crossref | Google Scholar |
14 Sultana F, Mullins R, English DR, et al. Women’s experience with home-based self-sampling for human papillomavirus testing. BMC Cancer 2015; 15: 849.
| Crossref | Google Scholar | PubMed |
15 Sherman SM, Brewer N, Bartholomew K, et al. Human papillomavirus self-testing among unscreened and under-screened Māori, Pasifika and Asian women in Aotearoa New Zealand: A preference survey among responders and interviews with clinical-trial nonresponders. Health Expect 2022; 25(6): 2914-23.
| Crossref | Google Scholar | PubMed |
16 MacDonald EJ, Geller S, Sibanda N, et al. Reaching under-screened/never-screened indigenous peoples with human papilloma virus self-testing: A community-based cluster randomised controlled trial. Aust NZ J Obstet Gynaecol 2021; 61(1): 135-41.
| Crossref | Google Scholar |
17 Brewer N, Bartholomew K, Grant J, et al. Acceptability of human papillomavirus (HPV) self-sampling among never- and under-screened Indigenous and other minority women: a randomised three-arm community trial in Aotearoa New Zealand. Lancet Reg Health West Pac 2021; 16: 100265.
| Crossref | Google Scholar | PubMed |
18 Serrano B, Ibáñez R, Robles C, et al. Worldwide use of HPV self-sampling for cervical cancer screening. Prev Med 2022; 154: 106900.
| Crossref | Google Scholar |
19 Borchowsky K, Rush M, Mullally T, et al. Primary care experiences in the ‘Let’s test for HPV’ study: a qualitative analysis. J Prim Health Care 2023; 15(2): 147-54.
| Crossref | Google Scholar | PubMed |
20 Bromhead C, Wihongi H, Sherman SM, et al. Human Papillomavirus (HPV) Self-Sampling among Never-and Under-Screened Indigenous Māori, Pacific and Asian Women in Aotearoa New Zealand: A Feasibility Study. Int J Environ Res Public Health 2021; 18(19): 10050.
| Crossref | Google Scholar | PubMed |
21 O’Connor M, Costello L, Murphy J, et al. ‘I don’t care whether it’s HPV or ABC, I just want to know if I have cancer.’ Factors influencing women’s emotional responses to undergoing human papillomavirus testing in routine management in cervical screening: a qualitative study. BJOG 2014; 121(11): 1421-9.
| Crossref | Google Scholar | PubMed |
22 Tiro JA, Betts AC, Kimbel K, et al. Understanding Patients’ Perspectives and Information Needs Following a Positive Home Human Papillomavirus Self-Sampling Kit Result. J Womens Health 2019; 28(3): 384-92.
| Crossref | Google Scholar | PubMed |
23 Dodd RH, Mac OA, McCaffery KJ. Women’s experiences of the renewed National Cervical Screening Program in Australia 12 months following implementation: a qualitative study. BMJ Open 2020; 10(7): e039041.
| Crossref | Google Scholar | PubMed |
24 McBride E, Tatar O, Rosberger Z, et al. Emotional response to testing positive for human papillomavirus at cervical cancer screening: a mixed method systematic review with meta-analysis. Health Psychol Rev 2021; 15(3): 395-429.
| Crossref | Google Scholar | PubMed |
25 Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol 2006; 3(2): 77-101.
| Crossref | Google Scholar |
26 Zelli J, Hum S, Lofters A, et al. Clinician acceptability of self-collected human papillomavirus swabs as a primary cervical cancer screening method. Can Fam Physician 2022; 68(2): e31-8.
| Crossref | Google Scholar | PubMed |
27 Mao C, Kulasingam SL, Whitham HK, et al. Clinician and Patient Acceptability of Self-Collected Human Papillomavirus Testing for Cervical Cancer Screening. J Womens Health (2002) 2017; 26(6): 609-15.
| Crossref | Google Scholar | PubMed |
28 Styffe C, Tratt E, Macdonald ME, et al. HPV Self-sampling in Indigenous Communities: A Scoping Review. J Immigr Minor Health 2020; 22(4): 852-9.
| Crossref | Google Scholar | PubMed |
29 Obermair HM, Dodd RH, Bonner C, et al. It has saved thousands of lives, so why change it?’ Content analysis of objections to cervical screening programme changes in Australia. BMJ Open 2018; 8(2): e019171.
| Crossref | Google Scholar | PubMed |
30 Smith LW, Racey CS, Gondara L, et al. Women’s acceptability of and experience with primary human papillomavirus testing for cervix screening: HPV FOCAL trial cross-sectional online survey results. BMJ Open 2021; 11(10): e052084.
| Crossref | Google Scholar | PubMed |
31 Tatar O, Thompson E, Naz A, et al. Factors associated with human papillomavirus (HPV) test acceptability in primary screening for cervical cancer: A mixed methods research synthesis. Prev Med 2018; 116: 40-50.
| Crossref | Google Scholar |
32 Chorley AJ, Marlow LA, Forster AS, et al. Experiences of cervical screening and barriers to participation in the context of an organised programme: a systematic review and thematic synthesis. Psychooncology 2017; 26(2): 161-72.
| Crossref | Google Scholar | PubMed |
33 Wearn A, Shepherd L. Determinants of routine cervical screening participation in underserved women: a qualitative systematic review. Psychol Health 2024; 39(2): 145-70.
| Crossref | Google Scholar | PubMed |
34 Te Whatu Ora Health New Zealand. National Cervical Screening Programme and HPV Primary Screening. Learning resources. 2023. Available at https://www.tewhatuora.govt.nz/health-services-and-programmes/ncsp-hpv-screening/learning-resources/ [accessed 29 August 2024].
35 Te Whatu Ora Health New Zealand Health Information and Services. Cervical screening. 2024. Available at https://info.health.nz/keeping-healthy/cancer-screening/cervical-screening [accessed 29 August 2024].
36 HealthEd. Cervical screening resources. 2023. Available at https://healthed.govt.nz/collections/topic-cervical-screening [accessed 29 August 2024].
37 Te Whatu Ora Health New Zealand National Cervical Screening Programme. Provider toolkit, A guide to using the HPV Cervical Screening Māori and all Aotearoa campaign resources. 2023. Available at https://www.tewhatuora.govt.nz/assets/For-the-health-sector/NSU/For-Health-professionals/Cervical-Screening-Programme/Maori-and-All-Aotearoa-Campaign-Provider-toolkit-3_5-October-2023.pdf [accessed 29 August 2024].