Antidepressants for treatment of depression in primary care: a systematic review and meta-analysis
Bruce Arroll 1 , Weng-yee Chin 2 , Waldron Martis 1 , Felicity Goodyear-Smith 1 , Vicki Mount 3 , Douglas Kingsford 4 , Stephen Humm 5 , Grant Blashki 6 , Stephen MacGillivray 71 Department of General Practice and Primary Health Care, University of Auckland, Auckland, New Zealand
2 Department of Family Medicine & Primary Care and Institute of Medical and Health Sciences Education, University of Hong Kong, Hong Kong
3 Auckland City Hospital, Grafton, Auckland, New Zealand
4 Chief Medical Information Officer & Executive Medical Director Interior Health Authority, Kelowna, British Columbia, Canada
5 Christchurch PsychMed, Christchurch, New Zealand
6 Department of General Practice, University of Melbourne, Melbourne, Australia
7 Evidence Synthesis Training and Research Group, Centre for Health and Related Research, University of Dundee, Dundee, Scotland
Correspondence to: Bruce Arroll, Department of General Practice and Primary Health Care, University of Auckland, Private Bag 92019, Auckland 1142, New Zealand. Email: bruce.arroll@auckland.ac.nz
Journal of Primary Health Care 8(4) 325-334 https://doi.org/10.1071/HC16008
Published: 21 December 2016
Journal Compilation © Royal New Zealand College of General Practitioners 2016.
This is an open access article licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Abstract
INTRODUCTION: Evidence for the effectiveness of drug treatment for depression in primary care settings remains limited, with little information on newer antidepressant classes.
AIM: To update an earlier Cochrane review on the effectiveness of antidepressants in primary care to include newer antidepressant classes, and to examine the efficacy of individual agents.
METHODS: Selection criteria included antidepressant studies with a randomly assigned placebo group where half or more subjects were recruited from primary care. The Cochrane Collaboration Depression, Anxiety and Neurosis (CCDAN) group searched multiple databases to identify eligible studies. Data extraction was performed independently by two reviewers. Data were analysed using Revman version 5.3.5.
RESULTS: In total, 17 papers and 22 comparisons were included for analysis. Significant benefits in terms of response were found for tricyclic antidepressants (TCA) with a relative risk (RR) = 1.23 (95% CI, 1.01–1.48), and serotonin selective reuptake inhibitors (SSRI) with a RR = 1.33 (95% CI, 1.20–1.48). Mianserin was effective for continuous outcomes. Numbers needed to treat (NNT) for TCA = 8.5; SSRI = 6.5; and venlafaxine = 6. Most studies were industry-funded and of a brief duration (≤ 8 weeks). There was evidence of publication bias. There were no studies comparing newer antidepressants against placebo.
CONCLUSION: Antidepressants such as TCA, SSRI, SNRI (serotonin–norepinephrine reuptake inhibitor) and NaSSA (noradrenergic and specific serotonergic antidepressant) classes appear to be effective in primary care when compared with placebo. However, in view of the potential for publication bias and that only four studies were not funded by industry, caution is needed when considering their use in primary care.
KEYWORDS: Antidepressant agents; primary health care; placebos; clinical trial; meta-analysis; general practice
| WHAT GAP THIS FILLS |
| What is already known: Much of the evidence on drug treatment of depression has been based on studies conducted in secondary and tertiary settings where the spectrum and course of illness tends to be more severe and complicated than depression encountered in primary care, raising questions about the effectiveness of antidepressants in patients being treated for depression in primary care settings. An earlier Cochrane review examined the effectiveness of TCA and SSRI in primary care settings and found both to be effective. |
| What this study adds: This study updates the Cochrane review by including newer antidepressant classes and calculating NNTs for individual drugs where data were available. There was evidence to support the effectiveness of TCAs and SSRIs when compared to placebo, and evidence of efficacy for SNRIs and NaSSA. NNT for TCA and SSRI classes and for specific agents (venlafaxine, amitriptyline, sertraline, escitalopram) were calculated. Publication bias and high levels of industry funding has also been reported and it is suggested that clinicians take this in to account when considering using antidepressant medication for depression in primary care. |
Introduction
Depression is a leading cause for global disease burden. In many places around the world, depression is mainly managed in primary care settings where the prevalence of depressive disorders has been estimated to range from 10 to 20%.1,2 Despite this, much of the evidence for the effectiveness of drug treatments used for depression have been based on studies conducted on patients treated in secondary and tertiary settings. Observational studies have found the naturalistic course of depressive disorders encountered in primary care tends to be less severe and less complicated than those seen in specialist settings, with many primary care patients going into remission without treatment.3 As a result, there is ongoing debate about the effectiveness of antidepressant therapy for the management of primary care depression amid concerns that previous evidence may have been based on biased reporting or false-positive results.4,5 In summary, the evidence for benefit with antidepressants is mainly in cases of severe depression, and yet most depression in general practice is mild to moderate.
This current review was conducted primarily to update and extend an earlier Cochrane review on antidepressants use in primary care, which was limited to the evidence for the effectiveness of tricyclic (TCA) and selective serotonin reuptake inhibitors (SSRI) against placebo.6 As newer antidepressant drug classes have become available in recent years, this review update was conducted to allow a more complete review of current papers and to examine the effectiveness of specific agents within each drug class. Recent reviews have suggested that some drugs may be more effective than others, raising the possibility that the benefits of antidepressants may not necessarily be a class effect.7 Knowledge of the NNT for individual agents would be helpful in informing clinicians on antidepressant choice when managing depression in primary care settings. The aim of this study was to assess the effectiveness of all classes of antidepressants versus placebo in primary care patients.
Methods
Using a population, intervention, control and outcome (PICO) format, inclusion criteria for study selection were:
-
Population. Primary care studies that examined the outcomes of antidepressant treatment of patients with depression where there was a placebo comparison group. ‘Primary care studies’ were defined as ≥ 50% of the study sample being subjects who have been recruited from primary care settings. ‘Patients with depression’ were defined as subjects diagnosed with depression by a primary care clinician or by diagnostic inventory or criteria.
-
Intervention. Any class of antidepressant medication. The treating clinician could be from primary or secondary care, as we were interested in the drug/placebo difference and any non-drug skill would apply equally to all arms of the trials.
-
Comparison. Placebo.
-
Outcomes. Response or remission to treatment for dichotomous outcomes, and the Hamilton Depression Rating Scale (HAM-D) or the Montgomery Asberg Rating Scale (MADRS) for continuous outcomes.
The search was conducted by the Cochrane Collaboration Depression, Anxiety and Neurosis (CCDAN) Group and included studies from the earlier Cochrane review and a CCDAN-Clinical Trials Registry update search (to examine records added to the registry from January 2007 to October 2015). The CCDAN search includes the databases of MEDLINE (1950–), EMBASE (1974–) and PsycINFO (1967–), and the Cochrane Central Register of Controlled Trials (CENTRAL) to include review-specific searches of additional databases. Reports of trials were also sourced from international trial registers via the World Health Organization’s trials portal (ICTRP), ClinicalTrials.gov, and drug companies. Finally, hand-searching of key journals, conference proceedings and other (non-Cochrane) systematic reviews and meta-analyses were performed. This search was updated in October 2015. Search terms used were for antidepressants versus placebo in primary care. The search is one page long, but in summary includes multiple terms for mood conditions (eg depression, dysthymia), generic names of all antidepressant medication, placebo, drug classes (eg SSRI, TCA, etc.) and primary care settings including general practice and ambulatory care. The search included all languages and references lists of papers were searched. We also wrote to authors of papers to see if they knew of any relevant papers we did not have. The final search was done in October 2015 and the full search results are available from the authors. Three authors (BA, WM and SH) selected the studies from the abstracts. Full papers were obtained where there was disagreement or uncertainty. Data were extracted from the selected papers in duplicate by two authors and recorded on a standardised data extraction form (Appendix 1). Data recorded included the PICO, funding sources, adverse events and those leading to withdrawal and the reviewer’s judgement on risk of bias. The principal summary measure used was relative risk (RR) and effect sizes were translated into NNTs to enable easier interpretation by clinicians. We measured standard mean differences for continuous outcomes and RRs, and NNTs for dichotomous outcomes to ensure a significant finding on a continuous outcome was also significant on a dichotomous outcome. Where standard deviations (s.d.) were not reported, we used the highest value of s.d. from other studies in that class, including the highest s.d. for the active drug and the highest s.d. for the placebo. We also measured withdrawal for any reason (WFAR) to quantify the dropout rates. The Cochrane RevMan software (version 5.3.5) was used to perform statistical analyses using a random effects model as the most conservative option. The PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) checklist (see Supplementary Material available at journal’s website) was followed.8
Results
The previous Cochrane review reported on 15 papers.6 The literature search identified 151 papers from 01 January 2007 to 31 December 2013 (Figure 1). In addition, a search before 2007 excluding known studies found five potential studies, none of which met the inclusion criteria. Two papers were removed because one was an abstract9 and the other was an early publication of the same study10 that was superseded by the full paper for the study.11 A paper from the earlier review was removed because the authors decided it did not meet the participant entry criteria.12
Two new papers came from the Linde (2015)13 review.14,15 An additional search from 01 January 2014 to 23 October 2015 found two additional studies.18,19 In summary, four new papers were found; one was excluded from our earlier review and a duplicate was removed. The final review included 17 papers with 10 TCA, nine SSRI, one venlafaxine and two mianserin comparisons with placebo.
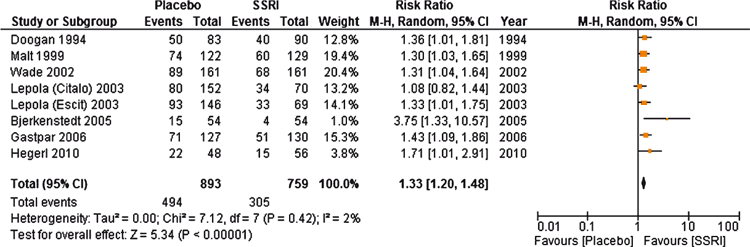
Table 1 shows the characteristics of each of the studies by their drug class categories. Both TCAs and SSRIs were significantly effective compared with placebo for dichotomous scores of response/remission (Figures 2 and 3). The RR for benefit was 1.23 (95% CI, 1.01–1.48) for TCAs, and 1.33 (95% CI, 1.20–1.48) for SSRIs. By using the pooled RR, the NNT for TCA (based on the median control event rate [MCER]) was 8.5, with a range of 7 to 16. The NNT for SSRI (based on the [MCER] was 6.5, with a range of 9 to 42. We are reporting a range of NNTs based on the range of control event rates, and it is not a confidence interval. Results for continuous outcomes were also significant for the TCA standard mean difference (SMD) = –0.26 (95% CI, –0.5 to –0.02) and for the SSRI group SMD = –0.27 (95% CI, –0.38 to –0.16). For participants on low doses of TCAs (≤ 100 mg per day), the SMD was –0.27 (95% CI –0.38 to –0.16). A SMD of 0.5 is considered a moderate effect size, so that SMDs reported here would be small. For studies longer than 8 weeks, the results were not statistically significant for TCAs but they were for SSRIs. There was significant heterogeneity for the TCA drugs (I2 = 77%) but not for the SSRI (I2 = 0).

|
|
There was limited but sufficient data to calculate the NNTs for some individual antidepressants (Table 2). This was possible for amitriptyline, sertraline, escitalopram and venlafaxine. We could not report a NNT for imipramine or citalopram, as their pooled estimate was significant on a fixed-effects analysis but not the random-effects analysis. There was insufficient data for dothiepin and paroxetine. We only had continuous data for mianserin, with a SMD of –0.37 (95% CI, –0.62 to –0.13) for the two studies, so while statistically significant, did not allow a NNT to be calculated.

|
The median withdrawal rate for TCAs was 19% and 14% for the SSRIs. The WFAR was not statistically significant for either TCA or SSRI. In a post-hoc sensitivity analysis, where those who withdrew were considered to have not improved, the RRs for TCAs and SSRIs remained non-significant.
Risk of bias
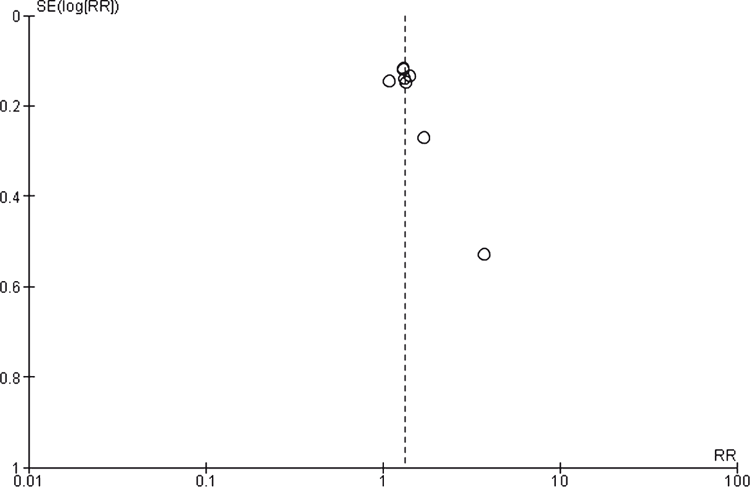
Most studies used a blinded active drug and placebo, but methods of randomisation or concealment were often not reported. Formal measures of bias would categorise most trials as having an unclear risk of bias; however, this may be unfair given most studies are old and the requirement for more rigid criteria for reporting have only been implemented in recent years. Of greater concern was industry involvement in 13 of the 17 studies; funnel plots indicated there were some small studies with small effect sizes missing for both tricyclics and SSRIs (Appendix 2 and 3).
Discussion
Summary
Our results suggest antidepressants are effective when compared with placebo for depression in primary care. There were four broad medication groups, including TCA, SSRI, SNRI and NaSSA, and all had evidence of efficacy. This includes what we have called heterogeneous depression, which was usually described as patients with a primary care clinical diagnosis of depression. In other studies, it included a diagnosis by structured criteria such as the Diagnostic and Statistical Manual of Mental Disorders, 4th edition.17 This was often augmented by a threshold from a depression inventory such as the Hamilton Depression Scale (mainly in the older papers), and the Montgomery Asberg Depression Rating Scale (in the more recent papers). The severity of depression and the effectiveness of antidepressants is a contentious issue, owing to concern about publication bias. A meta-analysis of 35 randomised controlled trials obtained from the Food and Drug Administration (which included unpublished data) found antidepressants were clearly effective only in patients with severe levels of depression (Hamilton Depression Rating Scale scores of ≥ 28).4 In this current review, the majority of participants were in the mild-to-moderate level of severity. While we were able to pool data and show amitriptyline, sertraline and escitalopram were effective in two or more studies, caution is needed in suggesting that these medications should be chosen over others in their classes, given that the choice to study a particular medication is industry-driven.
Strengths and limitations
There were several strengths to this current review. It contains only studies conducted with patients recruited from primary care settings. All medications were compared with placebo and/or with other medications where two medications were being studied. Authors were contacted to see if there were any relevant unpublished articles. This review contains two more papers than the 2015 review by Linde et al.13 and is the most comprehensive record of current papers.18,19 A key limitation of the review is the high rate of withdrawal from the evaluated studies and the high level of industry involvement. The funnel plot suggests there are some missing small studies with smaller effects for both the TCA and the SSRIs. The funnel plot analyses were limited by the small number of studies in each class. High withdrawal rates observed in the studies is problematic, but mimics what happens in everyday clinical practice. Sensitivity analysis performed, assuming all withdrawals did not respond, did not materially change the effect measures. The types of adverse effects have been reported elsewhere.6
Comparison with existing literature
There were notable absences of studies conducted in primary care on agents such as mirtazapine, bupropion and duloxetine, fluvoxamine, milnacipran and reboxetine, all of which have been studied in secondary care settings. Our review reports data in 22 drug versus placebo combinations. This is considerably less than a 30-year review of drug versus placebo studies, which included 121 comparisons.22 Their review included 19 different medications, while our review included 11. There were 17 studies comparing fluoxetine versus placebo, while our review had only one. The most recent study of an antidepressant versus placebo in primary care was in 2010,18 and, while the authors had disclosed involvement with pharmaceutical companies, the study did not seem to be industry-funded. The last medication versus medication study in primary care was published in 2012, and this was not funded by a pharmaceutical company.23 A recent network meta-analysis looked at both placebo-controlled and other anti-depressant-controlled trials.13 While this included newer medications, all trials were drug-versus-drug comparisons, which we feel is important due to the controversy of efficacy in mild-to-moderate depression, which predominates in primary care. Another systematic review of anti-depressants found these medications were not effective for mild and sub-threshold depression.24 This finding reinforces our view there should be a placebo group at least in the short-term.
Implications for practice
There appears to be some evidence to claim that antidepressants are effective for patients in primary care with depression. There was clear evidence for amitriptyline, mianserin, sertraline, escitalopram, venlafaxine, as individual drugs. There is almost certainly a commercial reason as to why particular medications were more studied, and the presence of evidence does not necessarily imply they are better than those with little or no evidence. Of more concern is the evidence of publication bias, as 13 of the 17 studies were funded by industry. We urge caution in their use. While antidepressant medications may be effective in primary care, our review does not answer the question of when they should be used, who should get them or how long primary care clinicians should wait to initiate prescribing.
Implications for research
There is an absence of evidence for the newer antidepressant medications versus placebo in primary care, many of which have been extensively studied in secondary care. This situation needs to be remedied. There also seems to be a trend towards medication-to-medication comparisons without a placebo arm, which may be problematic, as there is a risk that two medications may appear similar in efficacy, but neither is better than placebo. This is important in primary care, as the range of depressive illnesses encountered are mainly in the mild-to-moderate spectrum where antidepressants are generally less effective. Research is needed, which is free from industry funding and of sufficient power and duration, to determine effectiveness over a longer timeframe.
FUNDING
We wish to thank the University of Auckland Summer Student Research programme for funding Waldron Martis.
COMPETING INTERESTS
None.
ACKNOWLEDGEMENT
We would like to thank Sarah Dawson from CCDAN for assisting us with the literature search.
References
[1] Gilbody S, Gask L. Depressive disorders in primary care: a review. In: Herrman H, Maj M, Sartorius N, eds. Depressive Disorders. Hoboken, NJ: John Wiley & Sons, Ltd; 2009. p. 271–318.[2] Mitchell AJ, Vaze A, Rao S. Clinical diagnosis of depression in primary care: a meta-analysis. Lancet. 2009; 374 609–19.
| Clinical diagnosis of depression in primary care: a meta-analysis.Crossref | GoogleScholarGoogle Scholar |
[3] Schwenk TL, Coyne JC, Fechner-Bates S. Differences between detected and undetected patients in primary care and depressed psychiatric patients. Gen Hosp Psychiatry. 1996; 18 407–15.
| Differences between detected and undetected patients in primary care and depressed psychiatric patients.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK2s%2Fpt1answ%3D%3D&md5=3feb04a51f2945f752eb3b027ff17e12CAS |
[4] Kirsch I, Deacon BJ, Huedo-Medina TB, Scoboria A, Moore TJ, Johnson BT. Initial severity and antidepressant benefits: a meta-analysis of data submitted to the Food and Drug Administration. PLoS Med. 2008; 5 e45
| Initial severity and antidepressant benefits: a meta-analysis of data submitted to the Food and Drug Administration.Crossref | GoogleScholarGoogle Scholar |
[5] Ioannidis JP. Why most published research findings are false. PLoS Med. 2005; 2 e124
| Why most published research findings are false.Crossref | GoogleScholarGoogle Scholar |
[6] Arroll B, Elley CR, Fishman T, et al. Antidepressants versus placebo for depression in primary care. Cochrane Database Syst Rev. 2009; 2009 CD007954
[7] Cipriani A, Furukawa T, Salanti G, et al. Comparative efficacy and acceptability of 12 new-generation antidepressants: a multiple-treatments meta-analysis. Lancet 2009; 373 746–58.
| Comparative efficacy and acceptability of 12 new-generation antidepressants: a multiple-treatments meta-analysis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXisVOrtr8%3D&md5=300eefb7554eb35794db4198bb2de18cCAS |
[8] Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009; 339 b2535
| Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement.Crossref | GoogleScholarGoogle Scholar |
[9] Lepola U, Loft H, Reines EH. Escitalopram is efficacious and well tolerated for the treatment of depression in primary care. Annual Meeting of the American Medical Association, New Orleans. 2001.
[10] Montgomery SA, Loft H, Sanchez C, Reines EH, Papp M. Escitalopram (s enantiomer of Citalopram): clinical efficacy and onset of action predicted from a rat model. Pharmacol Toxicol. 2001; 88 282–6.
| Escitalopram (s enantiomer of Citalopram): clinical efficacy and onset of action predicted from a rat model.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXktVWmtbc%3D&md5=6c522abf955cf59b9fc749a0fdaf38a9CAS |
[11] Lepola UM, Loft H, Reines EH. Escitalopram (10–20 mg/day) is effective and well tolerated in a placebo controlled study in depression in primary care. Int Clin Psychopharmacol. 2003; 18 211–7.
| Escitalopram (10–20 mg/day) is effective and well tolerated in a placebo controlled study in depression in primary care.Crossref | GoogleScholarGoogle Scholar |
[12] Feighner JP. Mechanism of action of antidepressant medications. J Clin Psychiatry. 1999; 60(Suppl 4) 4–11.
[13] Linde K, Kriston L, Rucker G, et al. Efficacy and acceptability of pharmacological treatments for depressive disorders in primary care: systematic review and network meta-analysis. Ann Fam Med. 2015; 13 69–79.
| Efficacy and acceptability of pharmacological treatments for depressive disorders in primary care: systematic review and network meta-analysis.Crossref | GoogleScholarGoogle Scholar |
[14] Bjerkenstedt L, Edman GV, Alken RG, Mannel M. Hypericum extract LI 160 and fluoxetine in mild to moderate depression. A randomized, placebo-controlled multi-center study in outpatients. Eur Arch Psychiatry Clin Neurosci. 2005; 255 40–7.
| Hypericum extract LI 160 and fluoxetine in mild to moderate depression. A randomized, placebo-controlled multi-center study in outpatients.Crossref | GoogleScholarGoogle Scholar |
[15] Gastpar M, Singer A, Zeller K. Comparative efficacy and safety of a once-daily dosage of hypericum extract STW3-VI and citalopram in patients with moderate depression: a double- blind, randomised, multicentre, placebo-controlled study. Pharmacopsychiatry. 2006; 39 66–75.
| Comparative efficacy and safety of a once-daily dosage of hypericum extract STW3-VI and citalopram in patients with moderate depression: a double- blind, randomised, multicentre, placebo-controlled study.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XjvFOgtbc%3D&md5=7ce275ab1cca305d7559c2a87669ceeaCAS |
[16] Lecrubier Y, Bourin M, Moon CA, et al. Efficacy of venlafaxine in depressive illness. Acta Psychiatr Scand. 1997; 95 485–93.
| Efficacy of venlafaxine in depressive illness.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2sXkvVGjsLg%3D&md5=b8e610827bc99d9ae29c26a4abee3b34CAS |
[17] American Psychiatric Association. Diagnostic and statistical manual of mental disorders (4th edition) DSM-IV-TR. Washington, DC: APA; 2000.
[18] Hegerl U, Hautzinger M, Mergl R, et al. Effects of pharmacotherapy and psychotherapy in depressed primary-care patients: a randomized, controlled trial including a patients’ choice arm. Int J Neuropsychopharmacol. 2010; 13 31–44.
| Effects of pharmacotherapy and psychotherapy in depressed primary-care patients: a randomized, controlled trial including a patients’ choice arm.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXovVartw%3D%3D&md5=d2a33662ae5b36161c38bb56ebe5f180CAS |
[19] Miller SM, Naylor GJ, Murtagh M, Winslow G. A double-blind comparison of paroxetine and placebo in the treatment of depressed patients in a psychiatric outpatient clinic. Acta Psychiatr Scand. 1989; 80(Suppl 350) 143–4.
| A double-blind comparison of paroxetine and placebo in the treatment of depressed patients in a psychiatric outpatient clinic.Crossref | GoogleScholarGoogle Scholar |
[20] Thompson C, Thompson CM. The prescribing of antidepressants in general practice. II A placebo controlled trial of low-dose dothiepin. Hum Psychopharmacol. 1989; 4 191–204.
| The prescribing of antidepressants in general practice. II A placebo controlled trial of low-dose dothiepin.Crossref | GoogleScholarGoogle Scholar |
[21] Thomson J, Rankin H, Ashcroft GW, Yates CM, McQueen JK, Cummings SW. The treatment of depression in general practice: a comparison of L-tryptophan, amitriptyline and a combination of L-tryptophan and amitriptyline with placebo. Pscyhol Med. 1982; 12 741–51.
| The treatment of depression in general practice: a comparison of L-tryptophan, amitriptyline and a combination of L-tryptophan and amitriptyline with placebo.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaL3s7hvVWltA%3D%3D&md5=a642aec278e2db6db360832ce2b5dfabCAS |
[22] Undurraga J, Baldessarini RJ. Randomized, placebo-controlled trials of antidepressants for acute major depression: thirty-year meta-analytic review. Neuropsychopharmacology. 2012; 37 851–64.
| Randomized, placebo-controlled trials of antidepressants for acute major depression: thirty-year meta-analytic review.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XitlKitbg%3D&md5=91b5535de7087d0a1845bc878d0ab2acCAS |
[23] Wiles NJ, Mulligan J, Peters TJ, et al. Severity of depression and response to antidepressants: GENPOD randomised controlled trial. Br J Psychiatry. 2012; 200 130–6.
| Severity of depression and response to antidepressants: GENPOD randomised controlled trial.Crossref | GoogleScholarGoogle Scholar |
[24] Cameron IM, Reid IC, MacGillivray SA. Efficacy and tolerability of antidepressants for sub-threshold depression and for mild major depressive disorder. J Affect Disord. 2014; 166 48–58.
| Efficacy and tolerability of antidepressants for sub-threshold depression and for mild major depressive disorder.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXhtFWnu7jI&md5=fafb27fd05760bd99e63f35ee7061badCAS |
[25] Barge-Schaapveld DQCM, Nicholson NA. Effects of antidepressant treatment on the quality of daily life: an experience sampling study. J Clin Psychiatry. 2002; 63 477–85.
| Effects of antidepressant treatment on the quality of daily life: an experience sampling study.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XlsFSrsrg%3D&md5=26cfbd50be903665412dbdc8675671eaCAS |
[26] Blashki TG, Mowbray R, Davies B. Controlled trials of amitriptyline in general practice. BMJ. 1971; 1 133–8.
| Controlled trials of amitriptyline in general practice.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaE3M%2FnvFGluw%3D%3D&md5=f0920cd3ad3e113a7a5fdf3a6f81c482CAS |
[27] Doogan DP, Langdon CJ. A double-blind, placebo controlled comparison of sertraline and dothiepin in the treatment of major depression in general practice. Int Clin Psychopharmacol. 1994; 9 95–100.
| A double-blind, placebo controlled comparison of sertraline and dothiepin in the treatment of major depression in general practice.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK2czjsVGmug%3D%3D&md5=f75841fb866b175edf83085e1aba7976CAS |
[28] Hollyman JA, Freeling P, Paykell ES, Bhat A, Sedgwick P. Double-blind placebo-controlled trial of amitriptyline among depressed patients in general practice. J R Coll Gen Pract. 1988; 38 393–7.
| 1:STN:280:DyaL1Mzot1CgsQ%3D%3D&md5=1fb19ef046d0fa6e3aa433fc2c9f6427CAS |
[29] Mynors-Wallis LM, Gath DH, Lloyd-thomas AR, Tomlinson D. Randomised controlled trial comparing problem solving treatment with amitriptyline and placebo for major depression in primary care. BMJ. 1995; 310 441–5.
| Randomised controlled trial comparing problem solving treatment with amitriptyline and placebo for major depression in primary care.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK2M7nvV2huw%3D%3D&md5=5e715dbd793a8e85a2bd387ffe183573CAS |
[30] Philipp M, Kohnen R, Hiller KO. Hypercium extract versus imipramine or placebo in patients with moderate depression: randomised multicentre study of treatment for eight weeks. BMJ. 1999; 319 1534–8.
| Hypercium extract versus imipramine or placebo in patients with moderate depression: randomised multicentre study of treatment for eight weeks.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD3c%2FlvFCmtg%3D%3D&md5=dc373d0c0d72c1c4853fdcb8a01fb6e0CAS |
[31] Malt UF, Robak OH, Madsbu H-P, Bakke O, Loeb M. The Norwegian naturalistic treatment study of depression in general practice (NORDEP)-1: randomised double blind study. BMJ. 1999; 318 1180–4.
| The Norwegian naturalistic treatment study of depression in general practice (NORDEP)-1: randomised double blind study.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK1M3jvVKmsQ%3D%3D&md5=b9597db8936d8e509ad7ce63c6022dd6CAS |
[32] Wade A, Lemming OM, Hedegaard KB. Escitalopram 10 mg/day is effective and well tolerated in a placebo-controlled study in depression in primary care. Int Clin Psychopharmacol. 2002; 17 95–102.
| Escitalopram 10 mg/day is effective and well tolerated in a placebo-controlled study in depression in primary care.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD383ktlantg%3D%3D&md5=4d907631837816bb59d33472d9baffceCAS |
[33] Brink CW, vd Krogt JP, Dunbar GC, Behagel LH. A controlled clinical trial of mianserin and placebo in the treatment of depression in general practice. J Drug Ther Res. 1984; 9 513–7.

|

|
|




