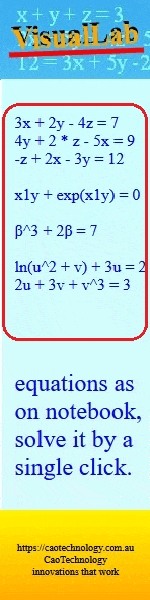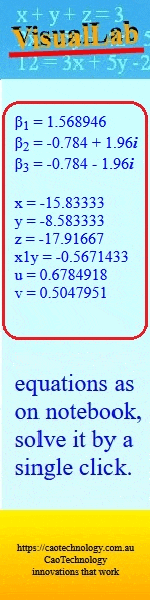
Australian Journal of Chemistry
Volume 69 Number 2 2016
RESEARCH FRONT: Natural Products Chemistry

Natural products chemistry in the 21st century is central to studies across multiple disciplines and branches within those disciplines. This research front contains a selection of papers that showcase current research in natural products chemistry.
CH15601Genome-Guided Discovery of Natural Products and Biosynthetic Pathways from Australia’s Untapped Microbial Megadiversity

The genetics underpinning the biosynthesis of microbial natural products is so well understood that some pathways and indeed their natural products may be predicted from DNA sequences alone. Mining of microbial genomes for biosynthesis genes is now seen as a viable platform for natural products discovery and this account overviews some of our research in this field.
CH15203New Cytotoxic Norditerpenes from the Australian Nudibranchs Goniobranchus Splendidus and Goniobranchus Daphne

Aplytandiene-3 and six new metabolites with ‘gracilin’-type carbon skeletons are isolated from Goniobranchus splendidus. The structure of gracilin G is revised and a C-6 configuration deduced by 1D and 2D NMR spectroscopy. Goniobranchus daphne yields a lactone with a rearranged spongionellin-type skeleton. Selected isolated metabolites are cytotoxic against a HeLa S3 cell line.
CH15456Antiproliferative Aporphine Alkaloids from Litsea glutinosa and Ethnopharmacological Relevance to Kuuku I’yu Traditional Medicine

In Australia, Litsea glutinosa is an important plant used in the traditional medicine system of the Kuuku I’yu (Northern Kaanju) people in the Cape York Peninsula, Queensland. Here, we report the cytotoxicity of crude extracts and four aporphine alkaloids isolated from the leaves of L. glutinosa against a panel of human cancer cell lines.
CH15488Kumbicins A–D: Bis-Indolyl Benzenoids and Benzoquinones from an Australian Soil Fungus, Aspergillus kumbius

A novel Australian fungus, Aspergillus kumbius FRR6049, yielded three new bis-indolyl benzenoids, kumbicins A–C, and a new bis-indolyl benzoquinone, kumbicin D.
CH15586The Use of the Toxic Plant Myoporum montanum in a Traditional Australian Aboriginal Medicine

Chemical investigation of a traditional Australian Aboriginal medicine revealed that the plant used was the toxic shrub, Myoporum montanum, but that the method of preparation plays a role in reducing the toxicity of the extract.
CH15476Identification of the Cat Attractants Isodihydronepetalactone and Isoiridomyrmecin from Acalypha indica

Acalypha indica has a long history of medicinal use and the roots of this plant are known to have a peculiar effect on cats. In this study, we identify two iridoids, (4R,4aR,7S,7aR)-isodihydronepetalactone (4) and (4R,4aS,7S,7aR)-isoiridomyrmecin (6), that are emitted by the roots of this plant and are likely to be responsible for this cat effect.
CH15194Host–Guest Inclusion System of Luteolin with Polyamine-β-cyclodextrin: Preparation, Characterisation, Anti-oxidant and Anti-cancer Activity

The water solubility, anti-oxidant activity and anti-cancer activity of LU were significantly increased in the inclusion complex with polyamine-β-cyclodextrin. The LU/CDs complex will be useful for its application as herbal medicine or healthcare product.
CH15278Carbon-Doped Hollow Titania with Tuneable Shell Architecture for Supercapacitors

Carbon-doped hollow anatase spheres, prepared in situ by directly carbonizing cationic polystyrene templates, exhibit outstanding capacitor performances compared to that of undoped P25.
CH15224Enzymatic Digestion of Keratin for Preparing a pH-Sensitive Biopolymer Hydrogel

Keratin has a wide variety of sources, is inexpensive and abundant in nature. This paper describes a simple and efficient enzymatic digestion method to pig hair keratin. A type of pH-sensitive keratin-based biopolymer hydrogel with excellent swelling properties and pH-sensitive drug release properties was prepared.
CH15286The Nucleophilic Addition of In Situ Generated Calcium Thiolate of Benzonitrile to the Sidewall of Single-Walled Carbon Nanotubes: A New and Direct Approach for Thioamidation

A new and efficient covalent thioamidation of single-walled carbon nanotubes (SWCNTs) consisting of a direct nucleophilic addition of calcium thiolate to the sidewalls of the tubes has been developed for the first time.
CH15315Controlled Delivery of Levothyroxine Using Porous Silicon as a Drug Nanocontainer

Capitalizing on the large loading capacity, biocompatibility and bioresorbability of porous silicon, films for the controlled delivery of the prohormone levothyroxine were fabricated. Amine functionalization of the films increased loading capacity by 50 % and sustained drug release under physiological conditions for 14 days. ELISA based release confirmed that levothyroxine was not deactivated or degraded.
CH15176Preparation of Uniform BiOI Nanoflowers with Visible Light-Induced Photocatalytic Activity

Uniform 3D hierarchical BiOI nanoflowers were developed via a facile solvothermal method. The obtained BiOI sample presents favourable recycling characteristics for mixed organic dye photodegradation, thereby presenting significant potential for practical wastewater treatment.
CH15163Synthesis and Characterization of Mesoporous Tin Oxide-Functionalized Reduced Graphene Oxide Nanoplatelets for Ultrasensitive Detection of Guaiacol in Red Wines

Mesoporous tin oxide-functionalized reduced graphene oxide (SnO2-rGO) is prepared through a simple hydrothermal method. A subsequent study reveals that SnO2-rGO film-modified electrode exhibits excellent sensitivity and reproducibility for the ultrasensitive detection of guaiacol in red wine samples.
CH15133One-Pot Synthesis of Substituted Piperidinones and 3,4-Dihydropyrimidinones Using a Highly Active and Recyclable Supported Ionic Liquid Phase Organocatalyst

This paper describes the synthesis of 1-ethyl-3-methylimidazolium ethyl sulfate [EMIM][EtSO4] and its application in supported form (SILPOC) for the synthesis of piperidinones and pyrimidinones.
CH15637Synthesis of Sulfonyldiazomethanes and Acetyldiazomethanes via an Alumina-Mediated Decarboxylation Strategy

Herein, we report a mild and simple method to synthesize sulfonyldiazomethanes and acetyldiazomethanes though neutral alumina-catalyzed decarboxylation of diazosulfonyl acetates.