Australian Journal of Chemistry
Volume 68 Number 6 2015
CH14499Nitrogen-Containing Ionic Liquids: Biodegradation Studies and Utility in Base-Mediated Reactions
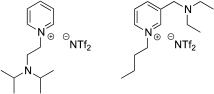
Several ILs were prepared in order to study the susceptibility of various cores and substituents to biodegradability using the ‘CO2 headspace’ test (ISO 14593, OECD 310). Several of the ILs contained tertiary amine substituents and were tested as solvents and reagents for several base mediated processes including Suzuki–Miyaura, Sonogashira, Knoevenagel, and Morita–Baylis–Hilman reactions.
CH14446Control of Gold Nanostructure Morphology by Variation of Temperature and Reagent Ratios in the Turkevich Reaction
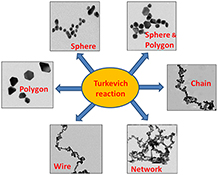
Gold nanosystems of various morphologies such as spheres, polygons, chains, wires, and networks were synthesised by controlling the reaction temperature and reagent ratios in the Turkevich reaction.
CH14370Ionic Liquid-Promoted Synthesis and Cholinesterase Inhibitory Activity of Highly Functionalized Spiropyrrolidines
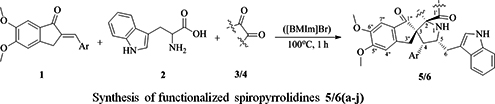
A library of novel, highly functionalized spiropyrrolidines has been synthesized stereoselectively for the first time in ionic liquid medium employing [3+2] cycloaddition of a series of 2-arylmethylidene-5,6-dimethoxy-2,3-dihydro-1H-inden-1-ones with an unexplored azomethine ylide derived from acenaphthenequinone or isatin and tryptophan. These compounds were evaluated in vitro for their acetylcholinesterase (AChE) and butyrylcholinesterase (BChE) inhibitory activities.
CH14328Synthesis and Bioevaluation of Novel Oxa-Caged Garcinia Xanthones as Anti-Tumour Agents

We prepared seven novel oxa-caged Garcinia xanthones that were for the first time varied at the C-2 position of B ring and at the C-21/22 or C-23 position of the prenyl group in the caged scaffold. Their bioactivities against cancer cell lines were reported. Their structure–activity relationships were also discussed.
CH14364Fabrication of High Efficiency Dye-Sensitised Solar Cell with Zirconia-Doped TiO2 Nanoparticle and Nanowire Composite Photoanode Film
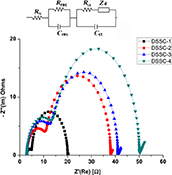
Dye-sensitised solar cells (DSSCs) were fabricated using zirconia-doped TiO2 nanoparticle and nanowire composite photoanode sensitised with coumarin NKX-2700 dye and quasi-solid state electrolyte sandwiched together with cobalt sulfide-coated counter electrode and Hafnium oxide (HfO2) as a blocking layer. Enhanced efficiency (η) of 9.93 % was obtained.
CH14442Group Position-Dependent Structurally Diverse Coordination Compounds Based on Isomeric Ligands
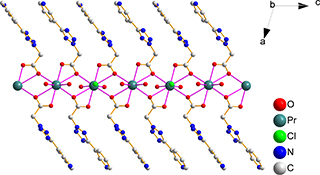
Two pairs of DyIII and PrIII coordination compounds based on isomeric ligands Htzpya and Hpytza were prepared whose structure variations were controlled by the different positions of the carboxylate group. The luminescent properties were investigated at room temperature in the solid state.
CH14383Difunctionalized N-Confused Porphyrins: Synthesis, Fluorescence, and Electrochemical Studies

The synthesis, fluorescence, and electrochemical studies of cis-A2B2-type and outer N-methyl-type difunctionalized N-confused porphyrins (NCPs) are reported. These NCPs showed blue-shifted emission maxima with smaller Stokes shift values and N-methyl NCP showed easier oxidations when compared with other difunctionalized NCPs.
CH14470Distinct 3D CdII Coordination Polymers with 1,2-Naphthalenedicarboxylate Regulated by Dipyridyl Co-Ligands with Different Spacers

The construction of two distinct three-dimensional CdII coordination polymers has been successfully achieved based on 1,2-naphthalenedicarboxylate, and their diverse structures can be well regulated by incorporating different dipyridyl co-ligands with C–C or C=C spacers.
CH14386A Simple and Efficient Two-Step Synthesis of 1,2,3-Triiodoarenes via Consecutive C–H Iodination/ipso-Iododecarboxylation Strategy: A Potential Application towards ortho-Diiodoarenes by Regioselective Metal–Iodine Exchange Reaction
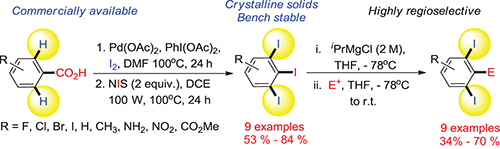
A general and efficient method for the conversion of benzoic acids to 1,2,3-triiodoarenes via a two-step synthesis is reported. Commercially available benzoic acids were used which can be performed on multi-gram scales with good yields. A potential application of the target compounds for novel regioselective metal-iodine exchange was also demonstrated.
CH14400Synthesis and Thermal Stability of New Polynitrostilbenes
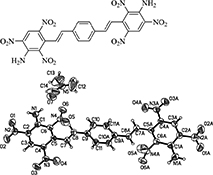
In recent years, safe, reliable, and stable explosives at elevated temperatures have attracted much attention. Polynitrostilbenes could be the intermediates of potential energetic explosives. The influence of the amino group and conjugated double bonds on the thermal stability of the polynitrostilbenes was studied.
CH14332A Green Approach for Copper-Free Sonogashira Reaction of Aryl Halides with Phenylacetylene in the Presence of Nano-Pd/Phosphorylated Silica (SDPP/Pd0)

One of the most straightforward methods for the preparation of aryl alkynes and conjugated enynes is the palladium-catalyzed coupling of terminal alkynes with aryl or alkenyl halides called the Sonogashira reaction. In this work, the efficiency of nano Pd(0)/SDPP (silicadiphenyl phosphinite) as a heterogeneous catalyst was examined in the Sonogashira reaction which shows the excellent efficiency, stability, and recyclability of the catalyst with different aryl halides.
CH14399Modelling of Transition State in Grignard Reaction of Rigid N-(Aryl)imino-Acenapthenone (Ar-BIAO): A Combined Experimental and Computational Study
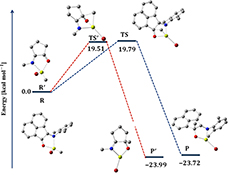
We present a combined synthetic and computational study on the addition of Grignard reagents RMgBr/RMgI (R = Me, Et) to various sterically rigid N-(aryl)imino-acenapthenone (Ar-BIAO) compounds (Ar = 2,6-iPr2C6H3 (1), 2,6-Me2C6H3 (2), and 2,4,6-Me3C6H2 (3) ligands).
CH14490Ethanolic RAFT Dispersion Polymerization of 2-(Naphthalen-2-yloxy)ethyl Methacrylate and 2-Phenoxyethyl Methacrylate with Poly[2-(dimethylamino)ethyl Methacrylate] Macro-Chain Transfer Agents
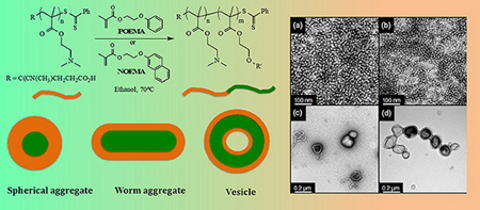
The ethanolic reversible addition-fragmentation chain transfer dispersion polymerization of two aryloxy methacrylates is described employing a poly[2-(dimethylamino)ethyl methacrylate] macro-CTA (chain transfer agent). Though copolymerizations are not well controlled, the formulations do give soft matter nanoparticles exhibiting a range of self-assembled morphologies.
CH14495Host–Guest Inclusion System of Scutellarin with Polyamine-β-Cyclodextrin: Preparation, Characterisation, and Anti-cancer Activity

The inclusion-complexation behaviour of scutellarin (SCU) with the four polyamine-modified β-cyclodextrins has been investigated in both solution and the solid state. The results showed that the hosts were able to solubilise SCU to higher levels than parent β-CD (9.0 mg mL–1). Besides, the anti-tumour activity of SCU was obviously increased after formation of inclusion complexes.
CH14159Three Coordination Polymers Built by Methoxyphenyl Imidazole Dicarboxylate: Solvothermal Syntheses, Crystal Structures, and Properties
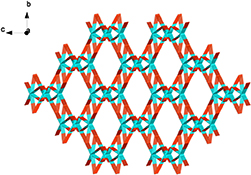
Three polymers [Sr(p-H2MOPhIDC)2]n (1) (p-H3MOPhIDC = 2-p-methoxyphenyl-1H-imidazole-4,5-dicarboxylic acid), {[Cd2(p-HMOPhIDC)2(4,4′-bipy)] H2O}n (4,4′-bipy = 4,4′-bipyridine) (2), and [Zn(p-HMOPhIDC)(4,4′-bipy)]n (3) have been synthesized under solvothermal conditions and characterized by single-crystal X-ray diffraction. The thermal and solid-state photoluminescence properties of the polymers have also been investigated.
H2O}n (4,4′-bipy = 4,4′-bipyridine) (2), and [Zn(p-HMOPhIDC)(4,4′-bipy)]n (3) have been synthesized under solvothermal conditions and characterized by single-crystal X-ray diffraction. The thermal and solid-state photoluminescence properties of the polymers have also been investigated.
CH14418Nickel Oxide Nanoparticle-Assembled Microspheres with a High Rate Capability for Lithium Storage
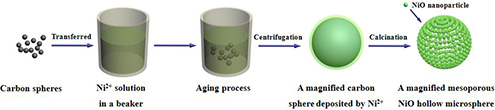
The conventional fabrication procedures for hollow structures generally involve using the hydrothermal method. Here, our results provide a novel and general route to prepare hollow NiO microspheres by aging processes only. Moreover, this method could be extended to prepare other hollow metal oxides.
CH14422Cu(hfac)2 Complexes with Nitronyl Ketones Structurally Mimicking Nitronyl Nitroxides in Breathing Crystals
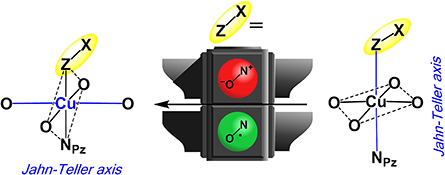
Breathing crystals based on complexes of Cu(hfac)2 (hfac = hexafluoroacetylacetonate) with nitronyl nitroxides demonstrate a variety of structural rearrangements and accompanying magnetic phenomena. We report on the synthesis of diamagnetic analogues of nitronyl nitroxides that are able to isostructurally substitute the latter in copper-nitroxide systems. Such substitution leads to the disappearance of the rearrangements characteristic of breathing crystals.
CH14492Catalytic Olefin Hydroalkoxylation by Nano Particles of Pollucite
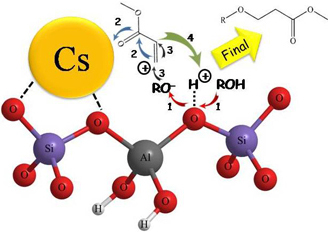
The catalytic hydroalkoxylation reactions of α,β-unsaturated esters, nitriles, and ethers with aliphatic and aromatic alcohols over pollucite as the stable heterogeneous catalyst in different conditions were studied. X‐ray diffraction, volumetric nitrogen adsorption surface area analysis, and CO2 temperature‐programmed desorption were used for characterizing the prepared pollucite.
CH14516Carbon-Supported Spinel Nanoparticle MnCo2O4 as a Cathode Catalyst towards Oxygen Reduction Reaction in Dual-Chamber Microbial Fuel Cell
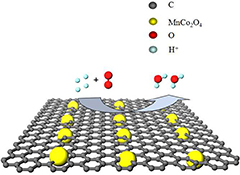
Pt/C is widely used for oxygen reduction reaction (ORR) in microbial fuel cells (MFCs). However, its high cost, scarcity, and poisoning sensitivity hinder further commercialization of MFCs. Carbon-supported spinel MnCo2O4 shows comparable ORR catalytic activity to Pt/C. It can be a promising alternative to Pt/C in commercialising MFCs due to its low cost, easy availability, high stability, high efficiency, and low toxicity.
CH14505A Straightforward and Practical Approach to Chiral Inducer: (2R,3R)-1,4-Dimethoxy-1,1,4,4-tetraphenylbutane-2,3-diol
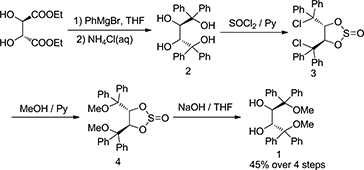
Chiral inducer (2R,3R)-1,4-dimethoxy-1,1,4,4-tetraphenylbutane-2,3-diol 1 was synthesised based on highly selective 2,3-cyclosulfitation of (2R,3R)-1,1,4,4-tetraphenylbutanetetraol 2. (2R,3R)-2 was reacted with excess thionyl chloride to afford (4R,5R)-4,5-bis(diphenyl- chloromethyl)-1,3,2-dioxathiolane-2-oxide 3, which was sequentially reacted with MeOH to afford (4R,5R)-4,5-bis(methoxy- diphenylmethyl)-1,3,2-di-oxathiolane-2-oxide 4. And finally the thionyl group was removed to give (2R,3R)-1 in excellent yield.
CH14485Molecular Docking and Spectroscopic Study on the Interaction of Serum Albumin with Iron(III) Diamine Sarcophagine

Interactions of iron(III) DiAmsar with HSA and BSA are reported. Stern–Volmer quenching constants at different temperatures were calculated using fluorescence quenching. Molecular docking was performed to explore possible binding sites and assess the microenvironment around the bound iron(III) DiAmsar.