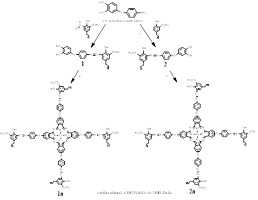
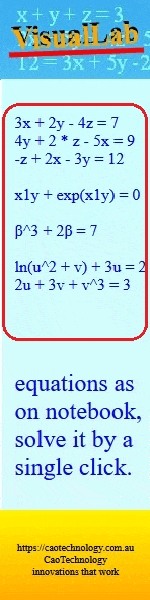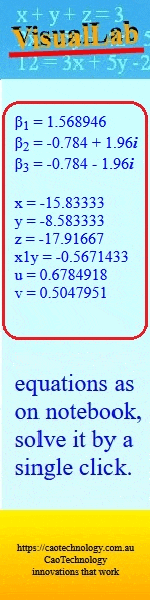
A new zinc(II) phthalocyanine bearing an eugenol Schiff base was synthesised and characterised, and its UV/Vis spectral properties were compared with those of its phthalocyanine analogue containing an eugenol azo dye at different pH. It was found that the azo dye-containing phthalocyanine exhibited more pronounced pH-dependent spectral changes than the Schiff base-substituted phthalocyanine. (Image credit: Günay Kaya Kantar.)

Australian Journal of Chemistry
Volume 77 Number 6 2024
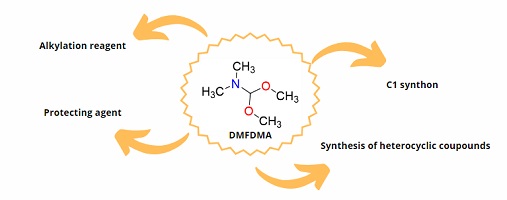
N,N-Dimethylformamide dimethyl acetal (DMFDMA) contains two important sites one that can act like an electrophile, the other a nucleophile. Because of its structure, DMFDMA can react with many different organic groups and is a versatile reagent used in gas chromatography, as a protecting group and as a C1 synthon, especially in the construction of heterocycles. (Image credit: N. F. Araujo.)
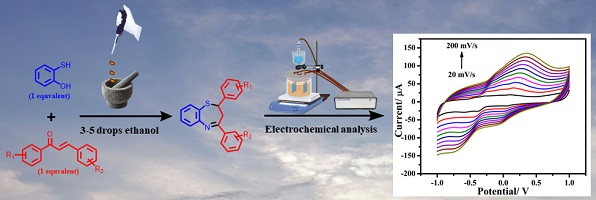
This article introduces a metal-free, liquid-assisted grinding method for synthesising 2,3-dihydro-1,5-benzothiazepine systems. This protocol offers the benefits of non-hazardous reaction conditions, quick reaction times, tolerance to various functional groups and easy product isolation. It utilises the cost-effective tools of a mortar and pestle. Electrochemical techniques are also employed to determine the electrochemical properties and diffusion coefficients of 2,3-dihydro-1,5-benzothiazepine derivatives that can be helpful in their application in the pharmaceutical industry. This innovative approach shows promise for synthesising therapeutic derivatives and their applications. (Image credit: Manjit Singh.)
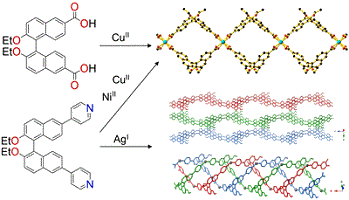
Chiral supramolecular compounds containing 1,1′-binapthyl are increasingly explored for chiroptical techniques including fluorescence-based sensing. Careful design of these compounds can yield systems with accessible voids and complimentary intermolecular interactions that can be tailored as a function of the 1,1′-binapthyl’s steric hinderance and hydrogen bonding ability. (Image credit: Carol Hua.)
This article belongs to the 10th Anniversary Collection of RACI and AAS Award papers
CH24031 Abstract | CH24031 Full Text | CH24031PDF (4.2 MB) | CH24031Supplementary Material (1.1 MB) Open Access Article