Methods to assess fire-induced tree mortality: review of fire behaviour proxy and real fire experiments
Alistair M. S. Smith A B * , Raquel Partelli-Feltrin C , Aaron M. Sparks B , James G. Moberly D , Henry D. Adams E , Dylan W. Schwilk F , Wade T. Tinkham
C , Aaron M. Sparks B , James G. Moberly D , Henry D. Adams E , Dylan W. Schwilk F , Wade T. Tinkham  G , John R. Kok H , David R. Wilson I , Alex Thompson J , Andrew T. Hudak K , Chad M. Hoffman L , James A. Lutz M , Alexander S. Blanco A , Mark A. Cochrane N , Robert L. Kremens O , Joseph Dahlen P , Grant L. Harley A , Scott W. Rainsford A , Li Huang A , Douglas D. Hardman A , Luigi Boschetti B and Daniel M. Johnson P
G , John R. Kok H , David R. Wilson I , Alex Thompson J , Andrew T. Hudak K , Chad M. Hoffman L , James A. Lutz M , Alexander S. Blanco A , Mark A. Cochrane N , Robert L. Kremens O , Joseph Dahlen P , Grant L. Harley A , Scott W. Rainsford A , Li Huang A , Douglas D. Hardman A , Luigi Boschetti B and Daniel M. Johnson P
A
B
C
D
E
F
G
H
I
J
K
L
M
N
O
P
Abstract
The increased interest in why and how trees die from fire has led to several syntheses of the potential mechanisms of fire-induced tree mortality. However, these generally neglect to consider experimental methods used to simulate fire behaviour conditions.
To describe, evaluate the appropriateness of and provide a historical timeline of the different approaches that have been used to simulate fire behaviour in fire-induced tree mortality studies.
We conducted a historical review of the different actual and fire proxy methods that have been used to further our understanding of fire-induced tree mortality.
Most studies that assess the mechanisms of fire-induced tree mortality in laboratory settings make use of fire proxies instead of real fires and use cut branches instead of live plants.
Further research should assess mechanisms of fire-induced tree mortality using live plants in paired combustion laboratory and landscape fire experiments.
Keywords: behaviour, cambium, fire-induced mortality, intensity, phloem, physiology, severity, tree mortality, xylem.
Introduction
The mechanisms responsible for fire-induced tree mortality have received considerable attention and numerous hypotheses of extreme heat-induced tree mortality have been proposed with varied degrees of support (Sachs 1875; Hare 1961; Levitt 1972; Michaletz and Johnson 2007; Wahid et al. 2007; Bita and Gerats 2013; Hood et al. 2018; O’Brien et al. 2018; Bär et al. 2019; Kleynhans et al. 2021; Dickman et al. 2023; Hudiburg et al. 2023; Partelli-Feltrin et al. 2021, 2023; West et al. 2023). Following Anderegg et al. (2012), we define tree mortality as the permanent cessation of critical physiological processes that allow the whole tree to function, such as the regeneration of meristematic shoots, hydraulic transport, resprouting, respiration and photosynthesis. Fire-induced tree mortality is likely complex and determined by multiple inter-related mechanisms, where the magnitude and timing of any given process will be impacted by the presence or absence of evolutionary adaptations, abiotic and biotic stressors, age and life cycle of the plant, and the degree and frequency of fire exposure (Agee 1993; Whelan 2002; Smith et al. 2018). Improved understanding of dynamic forest changes following wildfires is needed to reduce uncertainties of carbon stocks and fluxes in fire effects and fire-enabled Earth system models (Hanan et al. 2022; Shuman et al. 2022). This knowledge is also critical for advancing climate–vegetation models given the observed changes in global fire regimes (Archibald et al. 2013), the feedback between fire and forests in the global carbon cycle (Smith et al. 2014; Stenzel et al. 2019) and the potential role of forest management in moderating anthropogenic climate change (Bastin et al. 2019). Wildfires and prescribed fires can cause both immediate and delayed (months to years) tree mortality (Ryan and Reinhardt 1988), and many tree species have developed evolutionary traits that allow them to survive multiple fires (Dieterich and Swetnam 1984; Niklasson and Drakenberg 2001; Lombardo et al. 2009).
Although many syntheses exist that describe potential mechanisms of fire-induced tree mortality (Dickinson and Johnson 2004; Hood et al. 2018; O’Brien et al. 2018; Dickman et al. 2023; Hudiburg et al. 2023), less attention has been given to understanding the fire behaviour experiments used to assess the different potential mechanisms. For instance, use of fire proxies is widespread, but research to assess mechanisms connecting quantitative measures of heat transfer from actual fires to physiological impacts on trees, termed pyro-ecophysiology (Smith et al. 2017; Jolly and Johnson 2018), remains relatively sparse (e.g. Battipaglia et al. 2016; Smith et al. 2016; Steady et al. 2019; Partelli-Feltrin et al. 2021, 2023; Niccoli et al. 2023; Reed and Hood 2024). Notably, Varner et al. (2021) highlighted that a key question in wildland fire science is the need to assess the relative advantages and disadvantages of different fire proxy methods, especially given their predominance in providing evidence in support of potential mechanisms of fire-induced tree mortality. Therefore, the objectives of the current study are to:
A historical look at the potential mechanisms of fire-induced tree mortality
Multiple syntheses exist that describe the different potential mechanisms of fire-induced tree mortality (Hare 1961; Michaletz and Johnson 2007; Butler and Dickinson 2010; Hood et al. 2018; O’Brien et al. 2018; Bär et al. 2019; Kleynhans et al. 2021; Dickman et al. 2023; Hudiburg et al. 2023; West et al. 2023). In the present paper, we do not seek to repeat these reviews but rather seek to provide a historical context to the different methods applied to simulate wildfire behaviour in studies to further understand fire-induced tree mortality.
A historical assessment of fire-induced effects on trees starts with earlier work to evaluate extreme heat impacts on plant tissues. From the late 19th to mid-20th century, extreme heat-induced plant mortality research focused on multiple potential direct and indirect physiological mechanisms (Sachs 1875; Levitt 1972; Stephan et al. 2010). In this early research, apparatuses involving either heated water baths or candles were widely used in both research and teaching environments to simulate elevated high temperature conditions on plants (Sachs 1875). However, it was recognised in an early textbook (Sachs 1875) and by later studies (Shirley 1936) that hot water was a poor surrogate for elevated heat in air and that higher temperatures and durations would be needed to cause the equivalent plant damage when exposed to hot air as compared with samples immersed in hot water (Shirley 1936). These studies documented that heat stress on plants could occur above 54°C, but that the threshold at which heat caused adverse effects varied considerably across species and was highly dependent on the duration that the plants were exposed to elevated temperatures (Levitt 1972). Another early observation was that as the heat duration increases, the temperatures required to induce mortality decrease, which has clear implications for fires with long residence times and low intensities (Belehradek 1935), such as fires burning in masticated fuel beds or deep organic soils (e.g. Kreye et al. 2014; French et al. 2020). An early theory of extreme heat-induced plant mortality was that increased temperatures led to the denaturation of protoplasmic proteins and nucleic acids that in turn led to irreversible coagulation and potential liquefaction of lipids (Peacocke and Walker 1962; Brock 1967; Stephan et al. 2010). Although little support has been provided for these hypotheses (Levitt 1972), they remain widely discussed in literature describing the potential impacts of elevated heat stress on plants under climate change (Stephan et al. 2010; Bita and Gerats 2013).
Importantly, heat stress temperatures do not mean temperatures that plants experience during wildland fires (i.e. peaks of 800–1100°C). As described in detail in several recent syntheses on cultivated plants, it is widely accepted that heat stress associated with sustained temperatures between 5 and 15°C above normal ambient conditions can impact several physiological processes including germination rates, plant physiology and the metabolism of plant cells (Wahid et al. 2007; Bita and Gerats 2013; Hassan et al. 2021; dos Santos et al. 2022). Notably, elevated temperatures do not necessarily cause top kill or even whole plant death. It has also been widely understood that the duration of elevated temperatures is an important factor, as high temperatures over short durations may produce different physiological responses than lower elevated temperatures over longer durations (Levitt 1972; Stephan et al. 2010). For instance, Kurtz (1958) documented that the seeds and seedlings of Prosopis spp. and Carnegiea gigantea remained viable after exposure to air temperatures exceeding 83°C for 4 and 7 days, respectively. Also, some dry plant tissues, seeds, wheat grains, mosses and lichens have been documented to exhibit heat-induced mortality temperatures thresholds of ~120–140°C for exposure durations upwards of 240 min (Beadle 1940; Watanabe 1953; Levitt 1972). In terms of mechanisms in living plants, high degrees of heat stress have been associated with numerous molecular and physiological processes. Molecular processes include autophagy (Yang and Bassham 2015), unfolded protein responses and redox homeostasis (Malini et al. 2020), and production of ‘heat-shock’ proteins that increase the plant’s tolerance to heat stress (Maestri et al. 2002; Sakamoto and Murata 2002; Bita and Gerats 2013; Li et al. 2021; dos Santos et al. 2022). Physiological processes impacted by heat stress can include rates of net assimilation (Wahid et al. 2007), water transport and use within the plant (Mazorra et al. 2002; Choat et al. 2018), decreasing transpiration due to closure of the stomata (dos Santos et al. 2022) and decreasing photosynthesis (Barnabás et al. 2008; Farooq et al. 2009).
The 1930s saw increased interest in studies seeking to statistically model the likelihood of fire-induced tree mortality (Butler and Dickinson 2010). An indirect mechanism of fire-induced tree mortality that was first proposed in the 1930s was that increases in the air temperature surrounding leaves could cause non-linear increases to the vapour pressure gradient and result in unsustainable water loss due to increases in leaf transpiration (Curtis 1936; Levitt 1972). Recently, this theory has been revisited through the hypothesis that these vapour pressure gradients may lead to the formation of irreversible emboli in the xylem conduits that lead to tree death (Kavanagh et al. 2010). However, the only study to assess this mechanism with fire-induced tree mortality did not evaluate emboli, but rather only measured water uptake on cut branches of Magnolia grandiflora in gasoline fires (Hoffmann et al. 2021). Although a compelling mechanism, given its physical basis, a major challenge in evaluating it is developing an approach that can be applied to living trees in real wildland fires.
The mid to latter half of the 20th century saw an increase in studies specifically focusing on predicting fire-induced tree mortality from pre-fire morphology such as bark thickness and post-fire morphology such as crown scorch, bole char and damage (Peterson 1985), followed by studies incorporating this information with other tree morphological features to develop logistic regressions to predict fire-induced tree mortality (Wyant et al. 1986; Ryan and Reinhardt 1988; Butler and Dickinson 2010; Woolley et al. 2012; Cansler et al. 2020). Many of these studies were conducted using trees burned in wildland fires (Ryan and Reinhardt 1988; Hood et al. 2007; Hood and Lutes 2017). Bark thickness remains a widely used metric to infer the critical time for the cambium to exceed ~60°C for 120 s, as these parameters have been widely assumed to cause cambium and tree death (Hare 1961). Although many studies continue to assume cambium death will occur under these conditions (e.g. Espinosa et al. 2021), others have expressed concerns regarding how long fires would need to be located beside mature trees to elevate internal cambium temperatures through conductive heat flux (van Mantgem and Schwartz 2003). Furthermore, as noted by Dickinson et al. (2004) and Dickinson and Johnson (2004), the simplification of the problem to these arbitrary cambium temperature and time thresholds likely limited research in exploring underlying temperature-dependent physiological mechanisms.
Around the start of the 21st century, the focus shifted back to the identification of potential physiological mechanisms of fire-induced tree mortality to improve mechanistic models (Dickinson et al. 2004; Jones et al. 2004, 2006; Butler and Dickinson 2010; Michaletz et al. 2012; Chatziefstratiou et al. 2013; Smith et al. 2017; Hood et al. 2018; O’Brien et al. 2018; Dickman et al. 2023; West et al. 2023). Since this shift, multiple mechanisms have been proposed, including (i) consumption of fine roots, leading to an inability for the fine root system to acquire enough water and soil nutrients to support canopy demand (Varner et al. 2009; O’Brien et al. 2010); (ii) heat altering plant oxygen supply, membrane function and enzymes leading to an increase in ethanol accumulation, impaired aerobic respiration and tree mortality (Kelsey and Westlind 2017); (iii) cambium damage through stem heating (Jones et al. 2004, 2006; Chatziefstratiou et al. 2013); (iv) damage to canopy, crowns and meristematic tissues (Smith et al. 2017; Bison et al. 2022), including the vapour pressure gradient hypotheses (Kavanagh et al. 2010); (v) favourable nutrient conditions before and after fires leading plants to grow other components at the expense of repairing damaged critical tissues (Jump et al. 2017; Smith et al. 2017; Sparks et al. 2023a); (vi) localised carbon starvation due to phloem failure causing eventual hydraulic failure (Partelli-Feltrin et al. 2023), (vii) insufficient plant sugars or soil nutrients preventing the rebuilding of photosynthetic machinery, meristematic tissues, or reproductive organs (Partelli-Feltrin et al. 2021, 2023); (viii) cambium death leading to an inability to repair phloem or xylem leading to delayed mortality (Partelli-Feltrin et al. 2023); and (ix) fire causing reductions in hydraulic conductivity due to irreversible emboli and irrevocable thermal softening to polymers in the xylem conduits (Michaletz et al. 2012; West et al. 2016; Lodge et al. 2018).
Although multiple syntheses support xylem-based hypotheses (Michaletz and Johnson 2007; Hood et al. 2018; Michaletz 2018; O’Brien et al. 2018; Bär et al. 2019; Kleynhans et al. 2021; Dickman et al. 2023; West et al. 2023), others have expressed doubts (Varner et al. 2021; Hudiburg et al. 2023). Importantly, studies that support xylem-based mechanisms predominately used proxies of fire behaviour applied to cut branches and did not use real fires applied to live trees to draw their conclusions. Additionally, no studies using real laboratory fires and prescribed fires in Pinus species have provided evidence to support the embolism or xylem deformation mechanisms (Battipaglia et al. 2016; Niccoli et al. 2023; Partelli-Feltrin et al. 2021, 2023). The laboratory-based studies each burned under well-watered conditions and assessed main stems, whereas Niccoli et al. (2023) assessed the impacts of a wildfire on the branches of Pinus pinaster in stands where available soil water capacities were <10%. This suggests that some conifers may exhibit different dominant mechanisms of fire-induced tree mortality (Partelli-Feltrin et al. 2021), that fire-affected Pinus species may be more dependent on disruption of photosynthesis and carbon transport than hydraulic failure (Partelli-Feltrin et al. 2023; Reed and Hood 2024), or that some of the fire proxy methods may produce experimental artifacts that are potentially not observed when real fires are used (Nolan et al. 2024). This uncertainty has led some studies since 2020 to revisit cambium, phloem and crown-based related hypotheses of fire-induced tree mortality (Partelli-Feltrin et al. 2021, 2023; Bison et al. 2022; Reed and Hood 2024).
Proxy methods to simulate fire behaviour
Fire behaviour proxies are to real fires as models are to reality, i.e. as George Box is often quoted as saying, ‘all models all wrong, some are useful’. The same is true with fire behaviour proxies. Fire proxies and actual fires differ in heat transfer modes, heat fluxes, total heat incident on the organisms, and likely morphological and physiological impacts. However, the promise of proxies to assess fire impacts on trees is compelling, as they allow a diverse array of researchers to assess the morphological and physiological impacts of wildland fires on trees without the limitations of working with real vegetation fires, such as safety, access, specialised instrumentation, environmental control and permitting, among others.
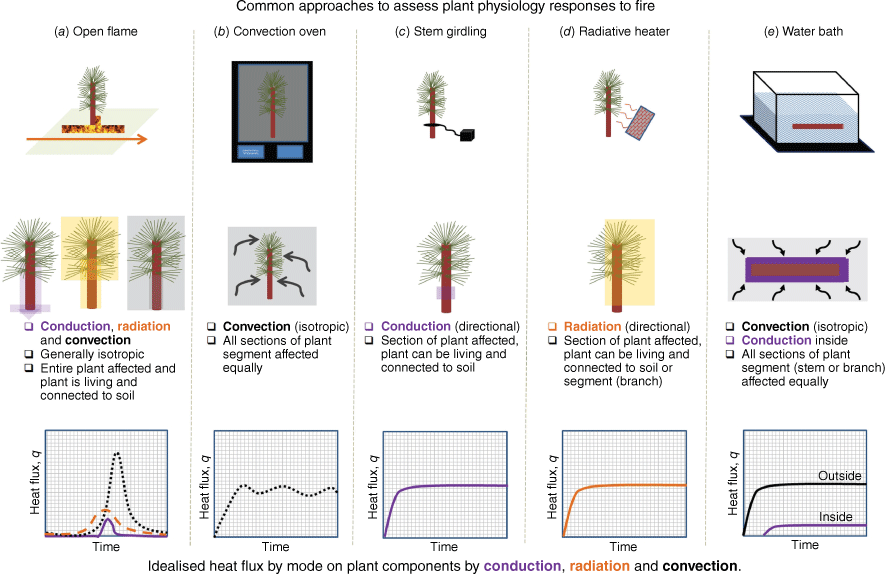
In some cases, fire proxies are associated with similar modes of heat transfer to fires (e.g. propane torches), but in others, they are limited by the modes of heat transfer they produce (e.g. convection ovens). Likewise, some fire proxies can be described in terms of heat (W) and energy (J), potentially enabling a more direct comparison with actual fire behaviour, whereas others can only provide information in terms of temperatures (K) and durations (s) (Fig. 1). A major challenge with proxy studies is that a lack of consistent experimental methods further limits inference of what could occur in real fires (see Tables 1–4). We acknowledge that we may not have included all studies that have used different fire behaviour proxy methods but contend that those that are included in this synthesis sufficiently illustrate the advantages and disadvantages of each approach.
Overview of fire proxy and actual fire methods to assess fire-induced tree mortality, with the general characteristics of heat transfer during the experiments shown: (a) actual fires, (b) convection ovens, (c) girdling and heating plates, (d) heaters, and (e) water baths.
| Species | Water bath temperature (°C) | Heating duration (min) | Cooling duration (min) | Part of tree | Reported change in PLC or xylem conductivity (k)? | Reported xylem cell wall deformation? | Citation | |
|---|---|---|---|---|---|---|---|---|
| Populus tremuloides | 43–65 | 0–10 | Rapid | Bark | n/a | n/a | Dickinson and Johnson (2004) | |
| Picea engelmannii | ||||||||
| Pseudotsuga menziesii | ||||||||
| Pinus contorta | ||||||||
| Acer rubrum | 70–80 | 5–10 | 18–24 h | Phloem tissue | n/a | n/a | Dickinson et al. (2004) | |
| Quercus prinus | ||||||||
| Psuedotsuga menziesii | ||||||||
| Pinus ponderosa | ||||||||
| Populus balsamifera | 65 | 5 | 5 | Branch | n/a | Yes | Michaletz et al. (2012) | |
| Populus balsamifera | 95 | 5 | 5 | Branch | n/a | Yes | ||
| Kiggelaria africana | 70 | 6 | n/a | Branch | Yes (PLC) | Yes | West et al. (2016) | |
| Kiggelaria africana | 100 | 6 | n/a | Branch | Yes (PLC) | Yes | ||
| Eucalyptus cladocalyx | 70 | 6 | n/a | Branch | No | No | Nel (2014) | |
| Eucalyptus cladocalyx | 100 | 6 | n/a | Branch | No | No | ||
| Picea abies | 90 | 60 | 20 | Branch | No (k) | Yes | Bär et al. (2018) | |
| Pinus sylvestris | 90 | 60 | 20 | Branch | No (k) | Yes | ||
| Fagus sylvatica | 90 | 60 | 20 | Branch | Yes (k) | Yes | ||
| Pinus palustris | 23 | 0 | 0 | Branch | n/a | n/a | Lodge et al. (2018) | |
| Pinus palustris | 41 | 5 | 0 | Branch | n/a | n/a | ||
| Pinus palustris | 54 | 5 | 0 | Branch | n/a | n/a | ||
| Eucalyptus obliqua | 40–70 | 1–5 | Cambium tissue | n/a | n/a | Achchige et al. (2021) | ||
| Eucalyptus radiata | ||||||||
| Eucalyptus ovata |
| Species | Oven temperature (°C) | Heating duration (min) | Part of tree | Observations | Citation | |
|---|---|---|---|---|---|---|
| Kiggelaria africana | 70 | 6 | Branch (foliated) | Plume-induced cavitation occurs | West et al. (2016) | |
| Kiggelaria africana | 100 | 6 | No xylem deformation | |||
| Eucalyptus cladocalyx | 70 | 6 | Branch (foliated) | Plume-induced cavitation occurs | Nel (2014) | |
| Eucalyptus cladocalyx | 100 | 6 | No xylem deformation | |||
| Sequoia sempervirens | 70 | 6, 15, 30, 45 and 60 | Branch (foliated) | Cambium viable after 15 min | Salladay and Pittermann (2023) | |
| Sequoia sempervirens | 100 | 6, 15, 30, 45 and 60 | Branch (foliated) | 60 min reduced hydraulic conductivity by 40% | ||
| 6, 15, 30, 45 and 60 | Branch (foliated) | Cambium dead after 6 min | ||||
| 45 min reduced hydraulic conductivity to zero |
| Species | Proxy type | Temperature (°C) | Duration | Part of tree | Observations | Citation | |
|---|---|---|---|---|---|---|---|
| Pinus palustris | Kerosene and Society of Automatic Engineers type 30 motor oil tied around tree | 537–832 | ~7 min | Live stem (0.3048 m above ground) | Primarily a methodology publication presenting an approach for a field-based fire behaviour proxy | Hare (1965a) | |
| Pinus elliottii | |||||||
| Quercus nigra | |||||||
| Taxodium distichum | |||||||
| Acer rubrum | |||||||
| 15 speciesA | Burning rope tied around tree | ~500 | ~2.5 min | Live stem | Strong relationship between bark thickness and cambium temperatures | Uhl and Kauffman (1990) | |
| 16 speciesB | Burning rope tied around tree | ~600 | Not reported | Live stem | Positive correlation between specific gravity and thermal conductivity | Hengst and Dawson (1993) | |
| Pinus halepensis | Electrical heating tape (500 W) | 250 | >1 min | Live stem | Near total cambium damage was needed to cause mortality after 5 months. Sap flow was significantly reduced in these trees after 1 week | Ducrey et al. (1996) | |
| 15 speciesC | Burning rope tied around tree | ~500 | 6 min | Live stem | Cambium temperature driven by bark thickness and less by bark moisture content or specific gravity | Pinard and Huffman (1997) | |
| Pseudotsuga menziesii | Copper heating pad | 400 | Varied | Stem | Lethal cambium temperatures observed after 10 min of exposure in 1 cm bark trees | Van Mantgem and Schwartz (2003) | |
| Calocedrus decurrens | |||||||
| Pinus ponderosa | Time to lethal temperatures increased logarithmically with bark thickness | ||||||
| Abies concolor | |||||||
| 8 speciesD | Type Lower Heat Platerod heater (400 W) | 70 | Until cambium reached 70°C | Branches | Depth of tissue necrosis increased with total heat flux on stem sections | Chatziefstratiou et al. (2013) | |
| Acacia nigrescens | Paraffin-soaked wick wrapped around stem and ignited | 2 min | Live stem | Cambium damage was low when bark was intact. Xylem damage became important for fire-induced mortality when bark was removed | Moncrieff et al. (2008) | ||
| Symplocos tinctoria | 12 V (10 W) thin film polyimide resistive heater | 90 | 3–5 min | Stem | Reduced conductivity after several weeks | Hoffmann et al. (2024) | |
| No xylem cellular deformation in scanning electron micrographs |
| Species | Proxy type and fire behaviour | Heating duration | Stage or part of tree | Observations | Citation | |
|---|---|---|---|---|---|---|
| 14 speciesA | Propane torch | Until cambium reached 60°C | Stem | Bark thickness drives fire resistance | Hare (1965b) | |
| Segment | ||||||
| Pinus strobus | Air heated by a Bunsen burner or electric resistance coil element. Temperature controlled by rheostat | 15 s to 10 min | Saplings | Dormant saplings experienced greater resistance to fire. No saplings died, but several became defoliated; 6 weeks after, Larix spp. exhibited significant numbers of new needles | Kayll (1968) | |
| Fagus grandifolia | ||||||
| Picea abies | ||||||
| Larix leptolepis | 45–117°C | |||||
| Larix decidua | ||||||
| Eucalyptus obliqua | 40–100°C | Varied | Seedlings | Mortality was a function of heating temperature and duration | Moore et al. (1977) | |
| Pinus taeda | 139–718°C | 240–360 s | Trees | Mortality was a function of base stem temperature and stem diameter | Greene (1983) | |
| Quercus nigra | 36–98 kJ s−1 m−1 | |||||
| Liquidambar styraciflua | ||||||
| 24 speciesB | Propane torch on 5 × 5 cm bark | 13 min | Trees | The presence of water in bark reduced cambium temperatures. Mortality driven by fire frequency and scorch height | Brando et al. (2012) | |
| Eucalyptus globulus | Cambium: blowlamp incident on metal plate 60–70°C | 5–7 min | Saplings | Changes in sap flow, cambium viability and lead stomatal conductance were related to the loss of physiological activity | Jimenez et al. (2012) | |
| Crowns: foliage heated with propane torch | ||||||
| Eucalyptus microcarpa | Torch: 750°C | 15 min | Stem | Bark thickness drives cambium heating. Water in the bark surface reduced heat pulses into cambium | Wesolowski et al. (2014) | |
| Eucalyptus leucoxylon | ||||||
| Eucalyptus tricarpa | Segment | |||||
| Athrotaxis cupressoides | Torch: 33 kW m−1 | 0, 15, 30, 45, 60 s | Seedlings | Top kill driven by duration of flame and seedling size | Prior et al. (2018) | |
| Eucalyptus coccifera | ||||||
| Eucalyptus delegatensis | Resprouting driven by species | |||||
| Leptospermum lanigerum | ||||||
| Pinus canariensis | Heated fan: 39°C plus 70°C | 1 h plus 5 min | Saplings | Loss of hydraulic conductivity had no impact on resprouting | Pita et al. (2023) | |
| Pinus pinea | ||||||
| Pinus oocarpa | ||||||
| Pinus pinaster |
Heat transfer in real fires
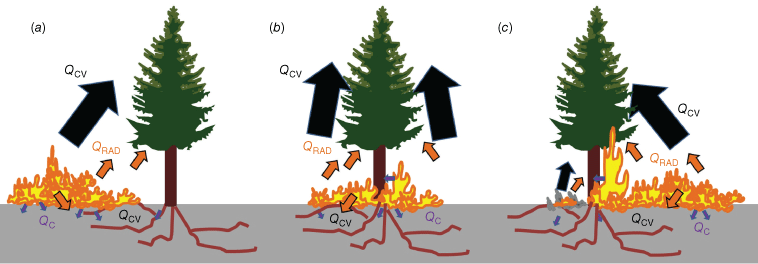
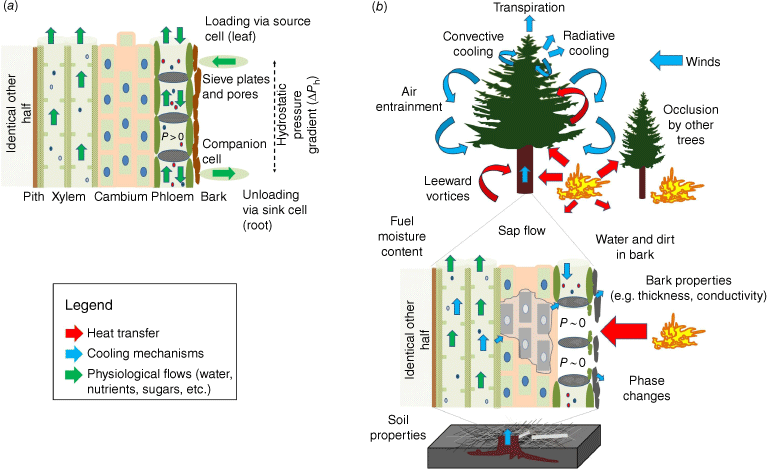
The heat transfer dynamics associated with wildland fire behaviour are widely described in the literature (e.g. Gutsell and Johnson 1996; Kremens et al. 2010; Michaletz et al. 2012; Wooster et al. 2021; Dickman et al. 2023). The most salient points are that plants are physiologically impacted by conduction (through plant tissues and soil), radiation (preheating) and convection (canopy, soils), but that these vary in magnitude with fire intensity and duration (Figs 1 and 2). Also, multiple cooling mechanisms exist in live trees exposed to wildland fires including internal water flow (e.g. sap flux, transpiration), air entrainment due to fire behaviour, intumescence in response to heat, wind, heat occlusion due to other plants, heat dissipation due to heat absorption by bark, material and water within bark, bark ablation, heat transmission through multiple layers of varying heat capacities, heat required for preheating and phase changes, among others (Gutsell and Johnson 1996; Potter and Andersen 2002; Jones et al. 2004, 2006; Chatziefstratiou et al. 2013; Smith et al. 2013; O’Brien et al. 2018; Dickman et al. 2023, Fig. 3b).
(a–c) Conceptual figure of heat transfer on trees during fires. Qcv denotes convective heat flux, Qc denotes conductive heat flux, and QRAD denotes radiative heat flux.
(a) Conceptual figure of undamaged xylem, cambium and phloem. (b) Conceptual figure of cooling mechanisms occurring within the fire–tree system. Note: tissues not to scale.
As fire approaches a tree (Fig. 2a), radiative and convective heat dries and preheats the fuel, driving out moisture and enabling phase changes. The rising temperatures lead to the thermal decomposition of the plant components (i.e. pyrolysis) and the production of volatile gases and charring of wood. Convective cooling and evaporation of fuel moisture delay ignition until hot gases overcome the cooling (Finney et al. 2015). Volatiles ignite at a sufficient temperature (~325°C), concentration and oxygen level (Drysdale 2011). As the fire interacts with the tree (Fig. 2b), heat is transferred to the foliage and stem by convection and radiation (Michaletz et al. 2012). Within the stem, heat is transferred internally through conduction; cooling mechanisms include heat dissipation through interactions with sap flow processes and losses due to transmission through multiple layers (e.g. bark, phloem, xylem: sapwood and heartwood) that can exhibit variable densities, thermal conductivities and heat capacities (Chatziefstratiou et al. 2013). Heat is also transferred belowground into the duff, litter and soil through conduction and convection (Massmann et al. 2010). Flames transfer heat to the soil through convection and radiation, whereas smouldering combustion transfers heat to the soil through conduction, convection and radiation (Kremens et al. 2010). As fire passes the tree, radiation and convection will continue to heat the stem and foliage (Fig. 2c), and heat will be dissipated to the environment through convection, radiation and phase changes (O’Brien et al. 2018; Dickman et al. 2023). In the absence of accumulated fuels around the base of the plant that can cause extended smouldering combustion (e.g. Kreye et al. 2014), the fire will pass the plant and maximum temperatures may only last for a few seconds.
Fire proxy: heated water baths
Heated water baths have been used to simulate exposure of plants to high temperature extremes in plant physiology research for over 150 years (Sachs 1875), and although many studies have stated that they do not represent the heat transfer conditions that trees would experience during wildland fires (Hood et al. 2018; Varner et al. 2021), results using them have nevertheless been widely used to infer what would happen during real wildland fires (Table 1). Notably, potential artifacts (i.e. where the choice of the experimental approach produces results that do not otherwise occur) have long been recognised as likely when using water baths to assess heat injury in plants (Gibson 1907; Nolan et al. 2024). Importantly, the heat transfer in water baths is very different from that in wildland fires as samples in water baths exhibit no cooling mechanisms and heat is predominantly transferred by convection, with conduction occurring as heat penetrates the sample (Balmer 2011, Fig. 1).
In water bath studies, the sample is usually a partially trimmed and cut branch, which may lead to differences in heat penetration as compared with using the main live stems with bark present in a real fire. However, no study has yet shown what water bath conditions (such as duration and temperatures) lead to amounts of heat equivalent to those transferred under real fire behaviour conditions or that the physiological impacts of heat from hot water baths on cut branches are an effective surrogate to describe the physiological impacts of heat from fires on live main stems (Varner et al. 2021). Importantly, branches are not main stems (i.e. trunks). Damage to a single branch will not kill a tree, but damage to a main stem can (Johnson et al. 2022). This difference has led to significant debate within the plant hydraulics community on the suitability of branches being used as proxies for entire trees (e.g. McCulloh et al. 2019; Johnson et al. 2022). Again, the promise of proxies is compelling as collecting branches is fairly simple (except in very tall trees) and less destructive than cutting down an entire tree to assess potential fire impacts on the main stem. Working with branches in a laboratory is also easier than conducting experiments on the live tree in situ. It is also easier to fit branches within experimental apparatus such as ovens.
As summarised in Table 1, in the studies that have used heated water baths as a proxy for actual fires, there is no clear or consistent methodology, further limiting cross-comparisons. When considering preparing samples for water bath treatment, multiple methodological differences are apparent. In Michaletz et al. (2012), in which air conductivity was used a proxy for hydraulic conductivity, the water bath sample preparation involved trimming and shaving branch segments. In Bӓr et al. (2018), the leaves remained attached during water immersion and, prior to vulnerability analysis, samples were debarked and recut multiple times under water. In Lodge et al. (2018), water bath sample preparation included removing all needles and bark from branch tips. In Nel (2014) and West et al. (2016), stems were used for the water bath and following the water bath treatment, shoots were debarked and defoliated under water by removing the lamina at its junction prior to subsequent measurements.
These water bath methodological differences are potentially important as they may lead to differences in heat penetration as compared with using the live stems with the entire bark present in a real fire, especially as the bark can contain surface water and live trees can transport water and sap to dissipate heat (Vines 1968). It is also difficult to cross-compare these water bath studies as no consistent parameters were used for heated water temperatures (45–95°C), immersion durations (5–60 min) and post-treatment cooling durations (2–30 min). The cooling duration is of particular importance if assessments of hydraulic conductivity are being made immediately following treatment, given the viscosity of liquids is usually related to the inverse of the temperature (Balmer 2011).
A significant challenge with heated water baths is that they likely cannot be used to assess non-xylem mechanisms of fire-induced tree mortality, such as those related to the inability of trees to assimilate carbon or transport stored carbon reserves in the phloem. Phloem tissue is found on the outside of the stem of a woody plant after its first year of growth, so any heat must pass through the phloem and cambium before the xylem can be affected (Fig. 3). Phloem is a delicate tissue that operates under positive pressure, which makes it more susceptible to flow disruption than xylem cells (Cayla et al. 2019). Therefore, any heat pulses from fires that cause substantial damage to xylem tissues would already have had a destructive impact on phloem and cambium tissues, severely limiting a tree’s ability to transport stored carbon resources to meet metabolic demand for tissue re-growth and repair post-fire (Partelli-Feltrin et al. 2023).
Fire proxy: ovens
Although ovens may anecdotally be thought of as a good fire behaviour proxy, they have infrequently been used to assess the mechanisms of fire-induced tree mortality (Table 2), likely owing to the challenges of what size of sample can be used within an oven chamber and how to limit contact of the tree components with the oven walls. In ovens, use of live plants of any size is not practicable given the impacts on soil and water dynamics and the mode of heat transfer that dominates depends on the type of oven. Nel (2014), later reported by West et al. (2016), used ovens at two temperatures (70 and 100°C) for 6 min to assess the impacts of fires on plume-induced xylem cavitation in 2-m sized branches of Eucalyptus cladocalyx and Kiggelaria africana. Nel (2014) reported in each species that deformation of xylem cells was not observed but that evidence of plume-based cavitation was present. In terms of the oven methodology, the end of the branches was wrapped in Parafilm™ and held upright by placing them in a beaker lined with polystyrene. Salladay and Pittermann (2023) also used ovens to expose Sequoia sempervirens branches to temperatures of between 70 and 100°C for between 6 and 60 min as a proxy to assess the impacts of fire heat plumes on cambium and xylem tissues. Although this subsequent study recognised that ovens cannot simulate fire, it still concluded that the approach could be used to improve understanding of how cambium and xylem respond to fire (Salladay and Pittermann 2023). Like Nel (2014), the approach involved placing branches vertically in the oven, without contact with the walls and where the cut ends were held in beakers lined with Styrofoam™ to limit the impacts of conduction. However, this study concluded that cambium more than xylem injury likely drives fire-induced tree mortality given the xylem remained viable long after the cambium tissues were no longer viable (Salladay and Pittermann 2023).
Fire proxy: heaters and stem wrapping methods
Heaters can cover a range of equipment including radiant heaters and resistive heaters (Table 3) that fundamentally operate under different (and limited) modes of heat transfer compared with real fires. Radiant heaters predominately transfer heat through radiation but can exhibit convective heat flows vertically above the heater. Resistive heaters are commonly used to enable heat transfer by conductive heat flux through contact with the bark or leaves with a plate, foil, or wrap. In Chatziefstratiou et al. (2013), which was conducted to parameterise and assess the stem heating model FIRESTEM 2D, rod heaters were positioned 5 cm away from an exposed 0.1 × 0.1 m section of bark, and the rest of the cut branch was wrapped in fire shelter material. A study using resistive heaters on Symplocos tinctoria saplings (~10 mm diameter, >1 m heights) observed internal stem temperatures exceeding 90°C after 86 s of sustained heating (Hoffmann et al. 2024). Although no xylem cell deformation was observed in this study, even with scanning electron micrographs (SEMs), the treatments did lead to delayed reductions in leaf conductivity and whole plant conductance (Hoffmann et al. 2024). Related to heaters are strips used to heat sections of the tree. Ducrey et al. (1996) used a heated electrical strip to assess cambium damage and observed that fires would need to wholly destroy the cambium to induce fire-induced tree mortality. In many respects, the work of Ducrey et al. (1996) highlights the limitations of looking for a sole mechanism to explain fire-induced tree mortality, as these results led future studies to dismiss the role of cambium injury processes (i.e. Kavanagh et al. 2010).
A variant of heating rings that have been widely applied in the assessment of fire-induced tree mortality mechanisms in forests is to wrap tree boles with paraffin-soaked wicks or ropes and ignite them to assess the impacts of fire on tree cambium (Uhl and Kauffman 1990; Hengst and Dawson 1993; Pinard and Huffman 1997; Moncrieff et al. 2008). Wick-based methodologies were proposed by Hare (1965a) as a repeatable field-based fire behaviour proxy. Pinard and Huffman (1997) applied this method to 16 tropical tree species and observed that durations to attain peak cambium temperatures (35–108°C) varied from 3 to 68 min. Moncrieff et al. (2008) used this fire proxy approach to assess the impacts of stem heating on Acacia nigrescens, where they allowed a paraffin-soaked wick that was wrapped around each stem to burn for a set duration. They calibrated the duration of the heating experiments by comparing water loss in aluminium cans during the proxy method and during real fires (Moncrieff et al. 2008). A challenge with this approach is that the thermal conductivity of aluminium (~236 W m−2 K−1) is several orders of magnitude higher than the typical thermal conductivity of tree bark (~0.08 W m−2 K−1).
Irrespective of any differences in the mode of heat transfer between these wick-based studies and real fires, an assessment of whether stem heating methods scale to larger trees is best considered through the study by van Mantgem and Schwartz (2003). In this study, they used a flexible copper heating pad affixed directly to the stem of young conifer trees (~5 cm diameter) across four species (Pseudotsuga menziesii, Calocedrus decurrens, Pinus ponderosa, Abies concolor). They demonstrated that although after 3 min outside bark temperatures reached 400°C, trees that had ~1 cm of bark would only attain lethal cambium temperatures (>60°C) after at least 10 min of constant exposure of bark temperatures exceeding 400°C, which they noted would likely only be achieved during sustained smouldering combustion of woody or other accumulated debris (van Mantgem and Schwartz 2003). They further noted that this time to attain lethal cambium temperatures increased logarithmically as bark thickness increased (van Mantgem and Schwartz 2003), casting serious doubt on stem heating as a significant driver of fire-induced tree mortality in mature conifers with thick bark.
Fire proxy: gas torches and forced heated air
Propane and other combustible gas torches have been used to assess fire effects and the mechanisms of fire-induced mortality, likely in large part owing to their producing similar visual appearance to flames in real wildland fires (Table 4). Propane burners were used by Prior et al. (2018) to assess the degree of top kill and resprouting of seedlings of four species in Australia. Robberecht and Defosse (1995) also applied a ring of propane burners to assess fire impact on two bunch grass species. Wesolowski et al. (2014) also conducted a study in Australia focused on Eucalyptus macrocarpa, Eucalyptus leucoxylon and Eucalyptus tricarpa. In this study, they used a propylene torch set at 750°C to heat bark for 900 s to simulate an extreme fire scenario. They observed that bark thickness affected cambium heating, but that water in the bark surface reduced heat pulses into the cambium (Wesolowski et al. 2014). A key study that evaluated fire-induced tree mortality using propane torches in a neotropical forest was Brando et al. (2012). In this study, they evaluated 24 different tree species where the presence of water in the bark acted to mitigate the impacts of cambium heating (Brando et al. 2012). Pita et al. (2023) applied heated air to 2-year-old saplings of four pine species to assess the role of needle water potential and osmotic potential on the survival of a resprouting pine species during fires. They observed that the loss of hydraulic conductance had no impact on the likelihood of resprouting (Pita et al. 2023)
Real fires: indoor and outdoor pyro-ecophysiology experiments
Research to assess the physiological mechanisms of fire-induced tree mortality in real fires includes the use of indoor laboratory and outdoor experiments during prescribed and wildfires. These types of experiments fall under the discipline of pyro-ecophysiology, which is the study of how fire, within its environment, mechanistically interacts with the physiology of an organism (Smith et al. 2017; Jolly and Johnson 2018). Rather than focusing on the mechanisms of fire-induced mortality, many landscape-scale studies have assessed regressions to predict mortality based on pre- and post-fire tree morphology whereas others have focused on the degree of top kill and resprouting (e.g. Wener and Franklin 2010; Grayson et al. 2017; Trouvé et al. 2021). For example, Wyant et al. (1986) assessed fire-induced mortality in a Pinus ponderosa and Pseudotsuga menziesii forest and after 22 months found that crown scorch and bole char were the most significant predictors. Ryan and Reinhardt (1988) evaluated data from 43 prescribed fires across the northwestern United States and determined that crown scorch was a significant predictor of fire-induced tree mortality. Given that the focus of these studies is assessing potential mechanisms of fire-induced mortality, we refer interested readers to the review by Woolley et al. (2012) for more details on logistic regression models to predict fire-induced tree mortality. Further, Cansler et al. (2020) provide information on the Fire and Tree Mortality database that includes details of empirical relationships derived from prescribed and wildland fires to predict fire-induced tree mortality in 142 tree species.
Indoor pyro-ecophysiology research has predominately followed a toxicological dose–response framework on containerised saplings (Table 5), where fire behaviour metrics associated with the energy incident on plants are dosage levels, and the morphological and physiological impacts on the plants are the responses (Smith et al. 2016; Smith et al. 2017; Jolly and Johnson 2018). Dose–response studies that subject trees to known doses of heat flux via surface fires have shown that post-fire physiology, morphology and mortality of several sapling species vary as a function of fire intensity measures such as fire radiative power (W m−2) and its temporal integral fire radiative energy (J m−2) (Smith et al. 2016, 2017; Sparks et al. 2016, 2017, 2023a, 2023b; Steady et al. 2019; Partelli-Feltrin et al. 2021, 2023; Wilson et al. 2022). These studies have shown that increasing maximum fire radiative power and its time integral of fire radiative energy results in decreased physiological function in terms of photosynthesis (Smith et al. 2017; Sparks et al. 2023b), chlorophyll fluorescence and phloem function (Smith et al. 2017; Partelli-Feltrin et al. 2023; Sparks et al. 2023b), decreased diameter and height growth (Smith et al. 2017; Steady et al. 2019), and increased probability of mortality in multiple conifer sapling species (Fig. 1d) (Smith et al. 2017; Steady et al. 2019; Sparks et al. 2023a). These studies with well-watered scenarios provided evidence to support cambium, phloem and crown damage processes as potential indicators of fire-induced tree mortality (Smith et al. 2017; Partelli-Feltrin et al. 2021, 2023; Sparks et al. 2023). These dose–response experiments also included studies where the fire dosage was held constant and different levels of water stress were applied to assess fire and drought interactions on post-fire recovery processes (Sparks et al. 2018a, 2024; Partelli-Feltrin et al. 2020; Wilson et al. 2022).
| Species | Part of tree | Observations | Citation | |
|---|---|---|---|---|
| Notholithocarpus densiflorus | Branch | Foliage consumption driven by foliar moisture content | Kuljian and Varner (2013) | |
| Pinus contorta | Live sapling | Spectral indices may be used to predict fire impacts on tree physiology | Sparks et al. (2016) | |
| Larix occidentalis | ||||
| Pinus contorta | Live sapling | Net photosynthesis and stomatal conductance following fires exhibit as dose response with fire radiative energy treatments. Chlorophyll fluorescence ‘false recovery’ trends may serve as an indicator of fire-induced mortality | Smith et al. (2017) | |
| Larix occidentalis | ||||
| Larix occidentalis | Live sapling | Severely water stressed plants had lower fire-induced mortality | Sparks et al. (2018a) | |
| Pinus ponderosa | Live sapling | Water stress increases vulnerability to fire-induced tree mortality | Partelli-Feltrin et al. (2020) | |
| Pinus ponderosa | Live sapling | Fires did not impact xylem hydraulic conductivity or xylem cell structure. Long-term new xylem growth in surviving plants showed deformations | Partelli-Feltrin et al. (2021) | |
| Pinus palustris | Species if very resistant to fires, where mortality and resprouting only occurs at very high fire intensity levels | Wilson et al. (2022) | ||
| Pinus ponderosa | Live sapling | Fires did not impact xylem hydraulic conductivity or xylem cell structure. Photosynthesis and whole plant/root NSCs decreased following fires | Partelli-Feltrin et al. (2023) | |
| Pseudotsuga menziesii | Live sapling | Provided more evidence to support that chlorophyll fluorescence ‘false recovery’ trends may serve as an indicator of fire-induced mortality. Also shows that spectral induces may be used to predict fire impacts on tree physiology | Sparks et al. (2023) | |
| Pinus monticola | Live sapling |
A major limitation of many planned landscape fire experiments is the inability to simulate wildfire intensity conditions. To overcome this, Trouvé et al. (2021) took advantage of the 2009 Black Saturday fires to evaluate the susceptibility of 10 Australian tree species to fire-induced mortality. This retrospective study, however, did not consider physiological drivers of fire-induced mortality. A non-exhaustive list of studies that have used real landscape fires to assess fire-induced tree mortality mechanisms is provided in Table 6. Although many studies take advantage of measurement opportunities on wildfires or planned fires, some conduct tree-level experiments. For example, in Brown and DeByle (1987), 1 m2 circular fuel beds were used around the base of trees to assess fire-induced mortality. A similar experimental set-up was used by Varner et al. (2009) to assess damage to roots. However, although they used a controlled experiment, Brown and DeByle (1987) still sought to develop logistic regression rather than a more refined physiological understanding of fire-induced tree mortality. Notably, several outdoor pyro-ecophysiology research studies have provided evidence to support cambium, phloem and crown damage processes as indicators of fire-induced tree mortality. Specifically, Varner et al. (2009) observed that changes in root non-structural carbohydrates (NSCs) occurred during high levels of bole heating and Reed and Hood (2024) observed that Pinus ponderosa fire-induced tree mortality was associated with reductions in phloem and stem NSCs as well as crown scorch, providing evidence supporting prior indoor pyro-ecophysiology research on saplings (i.e. Partelli-Feltrin et al. 2023).
| Species | Tree characteristics | Observations | Citation( | |
|---|---|---|---|---|
| Pseudotsuga menziesii | >13 cm DBH | Scorched volume, rather than scorched height, drove fire-induced tree mortality | Peterson (1985) | |
| Pinus contorta | ||||
| Abies lasiocarpa | ||||
| Thuja plicata | ||||
| Pinus contorta | ||||
| Populus tremuloides | 10–25 cm DBH | Mortality strongly related to degree of charring | Brown and DeByle (1987) | |
| Pinus halepensis | 2.7–58.1 cm DBH | Crown scorch and depth of charring were drivers of fire-induced tree mortality | Rigolot (2004) | |
| Pinus pinea | ||||
| Pinus sylvestris | ~30–45 years | Mortality driven by wind speed and flame height | Sidoroff et al. (2007) | |
| Pinus palustris | Mature trees | Changes in root NSC strongly correlated with duration of >60°C heat at 5 cm into soil | Varner et al. (2009) | |
| Pinus palustris | ~35 cm DBH | Crown scorch was unrelated to post-fire sap flow but was related to forest floor consumption, providing evidence for fine root damage | O’Brien et al. (2010) | |
| Eucalyptus miniata | Varied | Small eucalypts (<150 cm tall) were top killed but resprouted irrespective of fire season. Saplings (150–199 cm) exhibited mixed results and saplings >200 cm rarely exhibited top kill or mortality | Wener and Franklin (2010) | |
| Eucalyptus tetrodonta | ||||
| Corymbia porrecta | ||||
| Pinus pinea | 40 ± 11 cm DBH | No impact on xylem cells and no impact on resistance to hydraulic failure | Battipaglia et al. (2016) | |
| Pinus ponderosa | ~30 years old | Post-fire growth reduced for up to 8 years after the fire, where impact was proportional to the maximum fire radiative power on trees. Short-term (1 year) increase in resin ducts following fires of all intensities | Sparks et al. (2017) | |
| Acacia dealbata | 10–70 cm DBH | Top kill of non-eucalypt species was driven by fire intensity and not tree size | Trouvé et al. (2021) | |
| Acacia melanoxylon | 10–48 cm DBH | |||
| Atherosperma moschatum | 10–77 cm DBH | |||
| Nothofagus cunninghamii | 10–164 cm DBH | |||
| Olearia argophylla | 10–31 cm DBH | |||
| Eucalyptus obliqua | 10–112 cm DBH | Top kill of eucalypt species was driven by tree size and to a lesser degree fire intensity | ||
| Eucalyptus dives | 10–80 cm DBH | |||
| Eucalyptus radiata | 10–162 cm DBH | |||
| Eucalyptus viminalis | 19–100 cm DBH | |||
| Eucalyptus regnans | 12–376 cm DBH | |||
| Pinus pinaster | ~35 years old | Fires did not alter xylem hydraulics. Crown damage reduced growth and transpiration | Niccoli et al. (2023) | |
| Pinus ponderosa | 2–17 cm DBH | Trees that died exhibited a strong negative relationship between reductions in phloem and stem NSC and crown scorch | Reed and Hood (2024) |
Discussion
Potential research directions
Crown scorch and related metrics of crown and bole damage (bole scorch height, scorch height, percentage crown damaged, etc.) remain widely used to predict fire-induced tree mortality in a wide range of species (Ryan et al. 1994; Stephens and Finney 2002; McHugh and Kolb 2003; Fowler et al. 2010; Shearman et al. 2022). However, crown scorch estimates are known to have significant errors given they rely on subjective ocular estimates acquired post-fire (i.e. often determined without knowledge of the pre-fire condition of the tree crown) (Smith et al. 2016; Varner et al. 2021). These measures also lack scalability beyond the plot scale as current methods require ground-based assessment and the measures are inherently three-dimensional rather than two-dimensional measures of cover that are easier to scale. Furthermore, crown scorch and bole char models are also known to exhibit higher uncertainties when predicting delayed fire-induced mortality beyond 1 year post-fire, implying that other mechanisms, stressor interactions and/or feedback processes may influence long-term fire-induced tree mortality (Shearman et al. 2022). Therefore, regardless of the fire-induced tree mortality mechanisms of interest, studies should explore the potential integration of physiological traits as a first step to developing improved logistic or other regressions of fire-induced tree mortality that could be used with mechanistic fire–vegetation models. Future studies could assess whether a critical percentage threshold of damaged branches exists that leads to whole tree mortality. Using single branches as a subset of an entire damaged crown could then provide important insight into how whole tree processes respond to fire, for example assessing how the whole tree hydraulic system responds to compounded stressors such as when fires occur during extreme drought stress scenarios (Brodribb and Cochard 2009; Tonet et al. 2023).
One fire proxy approach that has merit for further investigation is the wick-based approach used by Moncrieff et al. (2008). This method could be refined: given the thermal conductivity of both the aluminium cans and the bark can be determined, with a uniform thickness, the convective and conductive components could be estimated to determine the heat transfer into the trees. Further research should investigate the development of an experimental approach to assess the potential formation of emboli due to vapour pressure gradients (Curtis 1936; Kavanagh et al. 2010; Hoffmann et al. 2024), given this remains a compelling mechanism of fire-induced tree mortality, but no study has yet proposed how to achieve this using living trees. Although it is feasible that some of the other fire-similar proxies such as propane torches and convection ovens may provide a reasonable approximation of fire convective heat flux, no study has presented data. As such, research should be conducted that compares experiments with live plants in real fires with the closest equivalent fire proxy approximation to assess whether potential experimental artefacts exist, and if none are observed, steps could then be taken to establish a repeatable and transferable fire-proxy methodology, where durations and temperatures could be assessed to simulate certain wildland fire conditions. In developing these experiments, it may not be possible to quantify or match the heat doses on the plants between the proxy and real fire cases. Therefore, an initial comparative assessment could evaluate extreme conditions (i.e. lethal doses) to objectively assess whether different morphological and physiological responses are observed between the proxy and real fire conditions.
Although considerable advances have been made in assessing how different tree species respond to fires in indoor and outdoor pyro-ecophysiology experiments, several questions warrant further investigation (Smith et al. 2017; Jolly and Johnson 2018; Sparks et al. 2018b, 2023a):
Do results from these containerised saplings extend to similar aged trees grown via natural regeneration?
Do the sapling-based results scale to mature trees?
To what extent does phenotypic plasticity impact the observed responses to fire?
Can we use pyro-ecophysiology to explore recovery when considering interactions between fire and other stressors beyond drought?
Can we use pyro-ecophysiology to inform selection of species or variants that exhibit greater fire or heat resistance?
Do the drivers of fire-induced mortality change when considering trees under increasing levels of concurrent severe drought and heat stress?
How do individual and coupled plant water and carbon processes within leaves, plants and communities regulate flammability and fire behaviour? and
Can we describe plant traits and strategies for fire using a pyrogeography framework by drawing on eco-evolutionary, spatial sciences and landscape genetic principles?
Future indoor and outdoor pyro-ecophysiology experiments could also explore the impacts of convective heat flux on tree crowns and bud necrosis (Bison et al. 2022), especially given crown damage-related metrics are excellent predictors of 1-year post fire-induced tree mortality (Ryan et al. 1988; Stephens and Finney 2002; McHugh and Kolb 2003; Fowler et al. 2010; Shearman et al 2022).
Many other potential mechanisms of fire-induced tree mortality remain fairly unexplored (Dickman et al. 2023) and pyro-ecophysiology research into these could also help tangential lines of inquiry such as research into potential impacts of elevated heat stress on trees due to changing climates. More research is needed to assess how trees, plants, cones and seeds physiologically respond to elevated temperatures, whether within or adjacent to fires, or under higher air temperature conditions associated with anthropogenic climate change. Studies should also explore whether fine root consumption from fires contributes to fire-induced tree mortality in shallow-rooted species and whether the impacts of fires on other belowground mechanisms contribute to fire-induced tree mortality, such as impacts to soils, mycorrhiza and morphological features that enable resprouting such as lignotubers (Adkins et al. 2020). Future indoor and outdoor pyro-ecophysiology experiments should explore the wide array of potential mechanisms, as improved knowledge of how fires kill trees could aid land management mitigation actions, such as decisions to wrap boles of trees or methods to reduce canopy damage.
The need for paired indoor and outdoor pyro-ecophysiology experiments
As highlighted by Van Wagner (1971) and Smith et al. (2016), there are advantages and disadvantages to both indoor and outdoor fire behaviour and pyro-ecophysiology experiments (Table 7). Van Wagner (1971) made clear that both indoor and outdoor fire experiments were needed, but that the two research approaches were often estranged. Outdoor experiments enable a scaling assessment of how fire impacts trees over a range of ages allow fire impacts to be monitored for extended periods of time, and allow an evaluation of interactions and feedback associated with weather, vegetation composition, soils and animals, among other ecosystem components (Battaglia et al. 2009). However, outdoor experiments are often limited by the ability to produce and safely control the upper limit of wildfire intensities (e.g. Canadian Crown Fire Experiment) and it can be difficult to accurately characterise the four-dimensional heat transfer and physiological processes (Van Wagner 1971). As a result, regression-based study sample sizes are usually large; given that they are determined by the number of individual trees within a landscape-scale fire, conversely, the sample sizes associated with exploring specific mechanisms at discrete levels of fire intensity are usually low owing to the challenges associated with instrumentation, size of tree to be investigated and monitoring resources. However, using planned landscape-scale fires to assess fire effects on multiple trees burning under a single prescription can lead to pseudoreplication errors and a lack of statistically significant replicates (Legendre 1993; Bataineh et al. 2006). Further, owing to increased complexity and high variance in outdoor experimental datasets, statistical over mechanistic approaches are often used to tease out generalities (Van Wagner 1971).
The main advantage of indoor experiments is the ability to conduct these and assess the physics of heat transfer and the physiological impacts through a series of repeatable and traceable treatments, replicates and controls (Smith et al. 2016). Another advantage of indoor pyro-ecophysiology experiments is the ability to assess each plant individually, minimising pseudoreplication errors and producing statistically significant results (Steady et al. 2019). During indoor fire experiments, the fuels can be well characterised and controlled, which is rarely possible in outdoor fire experiments without significant resources (Van Wagner 1971). Indoor pyro-ecophysiology experiments clearly limit inferences associated with local meteorological, microclimatic and soil conditions. Also, using containerised plants (i.e. those grown in pots) may not produce readily transferable results compared with plants grown via natural regeneration processes (seed dispersal, cones, resprouting, etc.), owing to differences in water and nutrient availability (Piper and Paula 2020). However, in many managed forests, this may be of lower concern given the widespread planting of containerised plants as part of restoration initiatives, due to a lack of regional seed sources (Thiffault et al. 2014; Chirico 2019).
As Van Wagner (1971) highlighted, a major limitation of outdoor fire experiments that does not arise in indoor fire experiments is how to decouple the variations associated with natural fuel complexes from the meteorological conditions at the time of each fire experiment. Several other experimental challenges can exist with outdoor fire experiments. Using opportunistic wildfires can lead to limited pre-fire data, limiting inferences (Smith et al. 2016). During planned fires, trees of interest are usually burned within the perimeter of a large fire, which can lead to pseudo-replication concerns. Equally, concerns can exist regarding how much heat is incident on a given tree of interest, owing to either nearby trees blocking heat or combusting and applying additional heat to the tree. A further challenge that has been difficult to assess in outdoor fire science experiments is the impact of interactions such as fire with drought, fertilisation, frost or other disturbances. Further complicating these questions is the nature of the interaction, including whether two or more stressors are concurrent (i.e. occurring at the same time) or consecutive (one followed by the other). Such differences can be more readily assessed using indoor pyro-ecophysiology fire experiments (e.g. Sparks et al. 2018b; Partelli-Feltrin et al. 2020; Wilson et al. 2022; Sparks et al. 2024), enabling potential mechanisms to be identified for future investigation during well-instrumented wildland fires such as those conducted under RxCadre (Ottmar et al. 2015), the Fire and Smoke Model Evaluation Experiment (FASMEE, Prichard et al. 2019), or FireSense field campaigns (Falkowski et al. 2024). Other outdoor pyro-ecophysiology experiments have included the Pine Integrated Network: Education, Mitigation, and Adaptation Project (PINEMAP), which conducted controlled experiments involving fire, drought and fertilisation with the goal of advancing process models of forest growth (e.g. Gonzalez-Benecke et al. 2016).
Conclusions
Even though the use of real fires is recommended in most cases, we acknowledge that some fire behaviour proxies have merit for further investigation to help understand the mechanisms of fire-induced tree mortality. Although some recent studies have suggested that future pyro-ecophysiology research should primarily focus on outdoor field experiments (e.g. Piper and Paula 2020; Hudiburg et al. 2023; Reed and Hood 2024), we assert both indoor and outdoor pyro-ecophysiology experiments are critically needed and are essential to advance wildland fire science (Van Wagner 1971; Smith et al. 2016; Shuman et al. 2022; Dickman et al. 2023). There is a clear trade-off between control and realism in these types of pyro-ecophysiology experiments, where indoor experiments maximise control over experimental treatments and outdoor experiments approach a higher degree of realism to wildfires in a field setting. Taking everything into account, indoor pyro-ecophysiology experiments enable investigations to isolate and narrow in on specific fire–vegetation system questions through repeatable experiments on a wide variety of topics that include, but are not limited to: assessing the different modes of heat transfer; evaluating the roles of rate of heat flux (duration and magnitude); testing physiological mechanisms of repair, death and recovery; and assessing the dynamic impacts of consecutive and concurrent stressors. In turn, outdoor pyro-ecophysiology experiments can assess how those results transfer to the natural environment, across spatial-temporal scales, species and genera. Future indoor and outdoor pyro-ecophysiology experiments should ideally be associated with each other at the planning stage, where comparative studies between containerised and naturally grown trees are conducted during field-based experiments. Such paired experiments could aid in the development of mechanistic fire effects sub-models of operational fire–atmosphere models (e.g. QUIC-FIRE) that seek to link the physics of fire heat transfer to the physiological impacts of fires on plants (Linn et al. 2020). The essentiality of paired indoor and outdoor fire behaviour experiments has been demonstrated in multiple other fire behaviour applications, such as increasing our understanding of the formation, dynamics and behaviour of fire whirls, fire scar formation from leeward vortices, ember generation, fire plumes and smoke transport, and the effectiveness of different mitigation strategies to limit fire ignition in the wildland–urban interface (Gutsell and Johnson 1996; Tohidi et al. 2018; Shuman et al. 2022; Dickman et al. 2023). In many of these examples, it is difficult to deliberately plan and safely implement wildland fires that can generate the fire behaviour conditions necessary to assess impacts of extreme fire behaviour events. Rather, indoor fire experiments can explore the potential mechanisms, their impacts, and through using dimensionless numbers (Froude, Reynolds, Prandtl, Rossby, etc.), help improve models to scale predictions to landscape scales (Gutsell and Johnson 1996; Tohidi et al. 2018).
In closing, we applaud the studies that have used fire proxies to further understand how extreme heat and fires impact the morphology and physiology of plants, as these studies initiated new conversations and lines of inquiry that undoubtedly have helped move wildland fire science forward. Yet we caution on the use of results from fire proxies to make conclusions about how plants will respond to actual wildland fires. To further advance wildland fire science, studies should ideally use indoor, outdoor, or paired pyro-ecophysiology experiments using real fires on live plants.
Conflicts of interest
Alistair Smith and Andrew Hudak are Associate Editors of International Journal of Wildland Fire. To mitigate this potential conflict of interest, they had no editor-level access to this paper during the peer review process. The other authors declare they have no conflicts of interest.
Declaration of funding
Funding for this work was provided by the USDA NIFA project 2023-67013-39411 and the National Science Foundation and the Idaho Established Program to Stimulate Competitive Research awards 2242769 and 2316126. H. D. A. was supported by the USDA NIFA McIntire Stennis Project 1019284. Partial funding for Sparks was provided by the USDA NIFA McIntire Stennis project IDAZ-ES-0609. A. M. S. was also supported by the NASA FireSense Implementation Team project under award 80NSSC24K1305.
Acknowledgements
The authors extend thanks to Professor Michael Knoblauch for comments and input on an earlier version of this article and to the former Editor-in-Chief, Dr Susan G. Conard for the candid advice that led us on the path to further developing this and related articles.
Author contributions
A. M. S. S., R. P. F., D. M. J., A. M. S., G. L. H., H. D. A. and J. G. M conceived the research and contributed equally to the paper. A. M. S. S., R. P. F., A. M. S., D. M. J. and G. L. H. wrote the manuscript with input from J. G. M., H. D. A., D. W. S., W. T. T., J. R. K., R. A. T., A. T. H., A. S. B., D. D. H., D. R. W., C. M. H., J. A. L., M. A. C., R. L. K., L. B., S. W. R., L. H. and J. D. All authors approved the final manuscript.
References
Achchige SYM, Volkova L, Weston CJ (2021) Effect of temperature and exposure time on cambium cell viability in vitro for Eucalyptus species. Forests 12, 445.
| Crossref | Google Scholar |
Adkins J, Docherty KM, Gutknecht JLM, Miesel JR (2020) How do soil microbial communities respond to fire in the intermediate term? Investigating direct and indirect effects associated with fire occurrence and burn severity. Science of The Total Environment 745, 140957.
| Crossref | Google Scholar | PubMed |
Anderegg WRL, Berry JA, Field CB (2012) Linking definitions, mechanisms, and modeling of drought-induced tree death. Trends in Plant Science 17(12), 693-700.
| Crossref | Google Scholar | PubMed |
Archibald S, Lehmann CE, Gómez-Dans JL, Bradstock RA (2013) Defining pyromes and global syndromes of fire regimes. Proceedings of the National Academy of Sciences 110(16), 6442-6447.
| Crossref | Google Scholar | PubMed |
Bӓr A, Nardini A, Mayr S (2018) Post-fire effects in xylem hydraulics of Picea abies, Pinus sylvestris and Fagus sylvatica. New Phytologist 217, 1484-1493.
| Crossref | Google Scholar | PubMed |
Bär A, Michaletz ST, Mayr S (2019) Fire effects on tree physiology. New Phytologist 223, 1728-1741.
| Crossref | Google Scholar | PubMed |
Barnabás B, Jäger K, Fehér A (2008) The effect of drought and heat stress on reproductive processes in cereals. Plant, Cell and Environment 31, 11-38.
| Crossref | Google Scholar | PubMed |
Bastin J-F, Finegold Y, Garcia C, Mollicone D, Rezende M, Routh D, Zohner CM, Crowther TW (2019) The global tree restoration potential. Science 365(6448), 76-79.
| Crossref | Google Scholar | PubMed |
Bataineh AL, Oswald BP, Bataineh M, Unger D, Hung I-K, Scognamillo D (2006) Spatial autocorrelation and pseudoreplication in fire ecology. Fire Ecology 2, 107-118.
| Crossref | Google Scholar |
Battaglia M, Smith FW, Sheppard WD (2009) Predicting mortality of ponderosa pine regeneration after prescribed fire in the Black Hills, South Dakota, USA. International Journal of Wildland Fire 18, 176-190.
| Crossref | Google Scholar |
Battipaglia G, Savi T, Ascoli D, Castagneri D, Esposito A, Mayr S, Nardini A (2016) Effects of prescribed burning on ecophysiological, anatomical and stem hydraulic properties in Pinus pinea L. Tree Physiology 36, 1019-1031.
| Crossref | Google Scholar | PubMed |
Beadle NCW (1940) Soil temperatures during forest fires and their effect on the survival of vegetation. Journal of Ecology 28, 180-192.
| Crossref | Google Scholar |
Belehradek JAR (1935) Temperature and living matter. Protoplasma Monographs 8, 1-277.
| Google Scholar |
Bison NN, Partelli-Feltrin R, Michaletz ST (2022) Trait phenology and fire seasonality co‐drive seasonal variation in fire effects on tree crowns. New Phytologist 234(5), 1654-1663.
| Crossref | Google Scholar | PubMed |
Bita CE, Gerats T (2013) Plant tolerance to high temperature in a changing environment: scientific fundamentals and production of heat stress-tolerant crops. Frontiers in Plant Science 4, 273.
| Crossref | Google Scholar | PubMed |
Brando PM, Nepstad DC, Balch JK, Bolker B, Christman MC, Coe M, Putz FE (2012) Fire-induced tree mortlaity in a neotropical forest: the roles of bark traits, tree size, wood density, and fire behavior. Global Change Biology 18, 630-641.
| Crossref | Google Scholar |
Brock TD (1967) Life at high temperatures. Science 158, 1012-1019.
| Crossref | Google Scholar | PubMed |
Brodribb TJ, Cochard H (2009) Hydraulic failure defines the recovery and point of death in water-stressed conifers. Plant Physiology 149(1), 575-584.
| Crossref | Google Scholar | PubMed |
Brown JK, DeByle NV (1987) Fire damage, mortality, and suckering in aspen. Candian Journal of Forest Research 17(9), 1100-1109.
| Crossref | Google Scholar |
Butler BW, Dickinson MB (2010) Tree injury and mortality in fires: developing process-based models. Fire Ecology 6(1), 55-79.
| Crossref | Google Scholar |
Cansler CA, Hood SM, Varner JM, van Mantgem PJ, Agne MC, Andrus RA, Ayres MP, Ayres BD, Bakker JD, Battaglia MA, Bentz BJ, Breece CR, Brown JK, Cluck DR, Coleman TW, Corace RG, Covington WW, Cram DS, Cronan JB, Crouse JE, Das AJ, Davis RS, Dickinson DM, Fitzgerald SA, Fulé PZ, Ganio LM, Grayson LM, Halpern CB, Hanula JL, Harvey BJ, Kevin Hiers J, Huffman DW, Keifer M, Keyser TL, Kobziar LN, Kolb TE, Kolden CA, Kopper KE, Kreitler JR, Kreye JK, Latimer AM, Lerch AP, Lombardero MJ, McDaniel VL, McHugh CW, McMillin JD, Moghaddas JJ, O'brien JJ, Perrakis DDB, Peterson DW, Prichard SJ, Progar RA, Raffa KF, Reinhardt ED, Restaino JC, Roccaforte JP, Rogers BM, Ryan KC, Safford HD, Santoro AE, Shearman TM, Shumate AM, Sieg CH, Smith SL, Smith RJ, Stephenson NL, Stuever M, Stevens JT, Stoddard MT, Thies WG, Vaillant NM, Weiss SA, Westlind DJ, Wooley TJ, Wright MC (2020) The Fire and Tree Mortality Database, for empirical modeling of individual tree mortality after fire. Scientific Data 7, 194.
| Crossref | Google Scholar | PubMed |
Cayla T, Le Hir R, Dinant S (2019) Live-cell imaging of fluorescently tagged phloem proteins with confocal microscopy. In ‘Phloem. Methods in Molecular Biology. Vol. 2014’. (Ed. J Liesche) (Humana: New York, NY, USA) 10.1007/978-1-4939-9562-2_8
Chatziefstratiou EK, Bohrer G, Bova AS, Subramanian R, Frasson RP, Scherzer A, Butler BW, Dickinson MB (2013) FireStem2D – A two-dimensional heat transfer model for simulating tree stem injury in fires. Plos One 8(7), 70110.
| Crossref | Google Scholar | PubMed |
Chirico D (2019) Effects of shortleaf pine seedling stock (bareroot vs containerized) on growth and survival in the absence and presence of fire. MS Thesis, North Carolina State University, USA. Available at http://www.lib.ncsu.edu/resolver/1840.20/36802
Choat B, Brodribb TJ, Brodersen CR, Duursma RA, López R, Medlyn BE (2018) Triggers of tree mortality under drought. Nature 558, 531-539 7711.
| Crossref | Google Scholar | PubMed |
Curtis OF (1936) Comparative effects of altering leaf temperatures and air humities on vapor pressure gradients. Plant Physiology 11, 595-603.
| Crossref | Google Scholar | PubMed |
Dickinson MB, Johnson WA (2004) Temperature-dependent rate models of vascular cambium cell mortality. Canadian Journal of Forest Research 34(3), 546-559.
| Crossref | Google Scholar |
Dickinson MB, Jolliff J, Bova AS (2004) Vascular cambium necrosis in forest fires: using hyperbolic temperature regimes to estimate parameters of a tissue-response model. Australian Journal of Botany 52, 757-763.
| Crossref | Google Scholar |
Dickman LT, Jonko AK, Linn RR, Altintas I, Atchley AL, Bär A, Collins AD, Dupuy J-L, Gallagher MR, Hiers JK, Hoffman CM, Hood SM, Hurteau MD, Jolly WM, Josephson A, Loudermilk EL, Ma W, Michaletz ST, Nolan RH, O’Brien JJ, Parsons RA, Partelli-Feltrin R, Pimont F, Resco de Dios V, Restaino J, Robbins ZJ, Sartor KA, Schultz-Fellenz E, Serbin SP, Sevanto S, Shuman JK, Sieg CH, Skowronski NS, Weise DR, Wright M, Xu C, Yebra M, Younes N (2023) Integrating plant physiology into simulation of fire behavior and effects. New Phytologist 238(3), 952-970.
| Crossref | Google Scholar | PubMed |
Dieterich JH, Swetnam TW (1984) Dendrochronology of a fire-scarred ponderosa pine. Forest Science 30(1), 238-247.
| Crossref | Google Scholar |
dos Santos TB, Ribas AF, de Souza SGH, Budzinski IGF, Domingues DS (2022) Physiological responses to drought, salinity, and heat stress in plants: a review. Stresses 2(1), 113-135.
| Crossref | Google Scholar |
Ducrey M, Duhoux F, Huc R, Rigolot E (1996) The ecophysiological and growth response of Aleppo pine (Pinus halepensis) to controlled heating applied to the base of the trunk. Canadian Journal of Forest Research 26, 1366-1374.
| Crossref | Google Scholar |
Espinosa J, Martin-Benito D, Rodríguez de Rivera Ó, Hernando C, Guijarro M, Madrigal J (2021) Tree growth response to low-intensity prescribed burning in Pinus nigra stands: effects of burn season and fire severity. Applied Sciences 11, 7462.
| Crossref | Google Scholar |
Falkowski MJ, Shuman J, Boland J, Kauffman T, Lefer B, Martin MM (2024) The NASA FireSense Project: responding to stakeholder needs across the fire life cycle. In ‘IGARSS 2024 - 2024 IEEE International Geoscience and Remote Sensing Symposium’, Athens, Greece. pp. 2356–2359. 10.1109/IGARSS53475.2024.10642820
Farooq M, Wahid A, Kobayashi N, Fujita D, Basra SMA (2009) Plant drought stress: effects, mechanisms and management. Agronomy for Sustainable Development 29, 153-188.
| Crossref | Google Scholar |
Finney MA, Cohen JD, Forthofer JM, McAllister SS, Gollner MJ, Gorham DJ, Saito K, Akafuah NK, Adam BA, English JD (2015) Role of buoyant flame dynamics in wildfire spread. Proceedings of the National Academy of Sciences 112(32), 9833-9838.
| Crossref | Google Scholar | PubMed |
Fowler JF, Sieg CH, McMillin J, Allen KK, Negron JF, Wadleigh LL, Anhold JA, Gibson KE (2010) Development of post-fire crown damage mortality thresholds in ponderosa pine. International Journal of Wildland Fire 19(5), 583-588.
| Crossref | Google Scholar |
French NHF, Graham J, Whitman E, Bourgeau-Chavez LL (2020) Quantifying surface severity of the 2014 and 2015 fires in the Great Slave Lake area of Canada. International Journal of Wildland Fire 29(10), 892-906.
| Crossref | Google Scholar |
Gonzalez-Benecke CA, Teskey RO, Martin TA, Jokela EJ, Fox TR, Kane MB, Noormets A (2016) Regional validation and improved parameterization of the 3-PG model for Pinus taeda stands. Forest Ecology and Management 361, 237-256.
| Crossref | Google Scholar |
Grayson LM, Progar RA, Hood SM (2017) Predicting post-fire tree mortality for 14 conifers in the Pacific Northwest, USA: model evaluation, development, and thresholds. Forest Ecology and Management 399, 213-226.
| Crossref | Google Scholar |
Gutsell SL, Johnson EA (1996) How fire scars are formed: coupling a disturbance process to its ecological effect. Candian Journal of Forest Research 26, 166-174.
| Crossref | Google Scholar |
Hanan EJ, Kennedy MC, Ren J, Johnson MC, Smith AMS (2022) Missing climate feedbacks in fire models: limitations and uncertainties in fuel loadings and the role of decomposition in fine fuel succession. Journal of Advances in Modeling Earth Systems 14(3), e2021MS002818.
| Crossref | Google Scholar |
Hare RC (1965a) Bark surfaces and cambial temperatures in simulated forest fires. Journal of Forestry 63, 437-440.
| Crossref | Google Scholar |
Hare RC (1965b) Contribution of bark to fire resistance of southern trees. Journal of Forestry 63(4), 248-251.
| Crossref | Google Scholar |
Hassan MU, Chattha MU, Khan I, Chattha MB, Barbanti L, Aamer M, Iqbal MM, Mawaz M, Mahmood A, Ali A, Aslam MT (2021) Heat stress in cultivated plants: nature, impact, mechanisms, and mitigation strategies—a review. Plant Biosystems 155(2), 211-234 11263504.2020.1727987.
| Crossref | Google Scholar |
Hengst GE, Dawson JO (1993) Bark properties and fire resistance of selected tree species from the central hardwood region of North America. Canadian Journal of Forest Research 24, 688-696.
| Crossref | Google Scholar |
Hoffmann WA, Rodrigues AC, Uncles N, Rossi L (2021) Hydraulic segmentation does not protect stems from acute water loss during fire. Tree Physiology 41(10), 1785-1793.
| Crossref | Google Scholar | PubMed |
Hoffmann WA, Sherry CDK, Donnelly TM (2024) Stem heating results in hydraulic dysfunction in Symplocos tinctoria: implications for post-fire tree death. Tree Physiology 44(3),.
| Crossref | Google Scholar | PubMed |
Hood SM, Lutes D (2017) Predicting post-fire tree mortality for 12 western US conifers using the First Order Fire Effects Model (FOFEM). Fire Ecology 13, 66-84.
| Crossref | Google Scholar |
Hood SM, McHugh CW, Ryan KC, Reinhardt E, Smith SL (2007) Evaluation of a post-fire tree mortality model for western USA conifers. International Journal of Wildland Fire 16(6), 679-689.
| Crossref | Google Scholar |
Hood SM, Varner JM, van Mantgem P, Cansler CA (2018) Fire and tree death: understanding and improving modeling of fire induced tree mortality. Environmental Research Letters 13, 113004.
| Crossref | Google Scholar |
Hudiburg T, Mathias J, Bartowitz K, Berardi DM, Bryant K, Graham E, Kolden CA, Betts RA, Lynch L (2023) Terrestrial carbon dynamics in an era of increasing wildfire. Nature Climate Change 13, 1306-1316.
| Crossref | Google Scholar |
Jimenez E, Vega JA, Fernandez X, Perez-Gorostiaga P, Cuinas P, Fonturbel T, Alonso M, Rozados MJ, Bara S (2012) Change in Eucalyptus globulus Labill. saplings growth and physiological paramaters following fire-induced stem and crown damage in a plantation in north-western Spain. European Journal of Forest Research 131, 1967-1978.
| Crossref | Google Scholar |
Johnson DM, Katul G, Domec J-C (2022) Catastrophic hydraulic failure and tipping points in plants. Plant, Cell and Environment 45(8), 2231-2266.
| Crossref | Google Scholar | PubMed |
Jolly WM, Johnson DM (2018) Pyro-ecophysiology: shifting the paradigm of live wildland fuel research. Fire 1(1), 8.
| Crossref | Google Scholar |
Jones JL, Webb BW, Jimenez , Reardon J, Butler B (2004) Development of an advanced one-dimensional stem heating model for application in surface fires. Canadian Journal of Forest Research 34, 20-30.
| Crossref | Google Scholar |
Jones JL, Webb BW, Butler BW, Dickinson MD, Jimenez D, Reardon J, Bova AS (2006) Prediction and measurement of thermally-induced cambial tissue necrosis in tree stems. International Journal of Wildland Fire 15, 3-17.
| Crossref | Google Scholar |
Jump AS, Ruiz-Benito P, Greenwood S, Allen CD, Kitzberger T, Fensham R, Martínez-Vilalta J, Lloret F (2017) Structural overshoot of tree growth with climate variability and the global spectrum of drought-induced forest dieback. Global Change Biology 23(9), 3742-3757.
| Crossref | Google Scholar | PubMed |
Kavanagh KL, Dickinson MB, Bova AS (2010) A way forward for fire-caused tree mortality prediction: modeling a physiological consequence of fire. Fire Ecology 6(1), 80-94.
| Crossref | Google Scholar |
Kelsey RG, Westlind DJ (2017) Physiological stress and ethanol accumulation in tree stems and woody tissues at sublethal temperatures from fire. BioScience 67(5), 443-451.
| Crossref | Google Scholar |
Kremens R, Smith AMS, Dickinson M (2010) Fire metrology: current and future directions in physics-based measurements. Fire Ecology 6(1), 13-35.
| Crossref | Google Scholar |
Kreye JK, Brewer NW, Morgan P, Varner JM, Smith AMS, Hoffman CH, Ottmar RD (2014) Fire behavior in masticated fuels: a review. Forest Ecology and Management 314, 193-207.
| Crossref | Google Scholar |
Kuljian H, Varner JM (2013) Foliar consumption across a sudden oak death chronosequence in laboratory fires. Fire Ecology 9, 33-44.
| Crossref | Google Scholar |
Kurtz EB (1958) Chemical basis for adaptation in plants. Understanding of heat tolerance in plants may permit improved yields in arid and semiarid regions. Science 128, 1115-1117.
| Crossref | Google Scholar | PubMed |
Legendre P (1993) Spatial autocorrelation: trouble or new paradigm? Ecology 74(6), 1659-1673.
| Crossref | Google Scholar |
Li N, Euring D, Cha JY, Lin Z, Lu M, Huang LJ, Kim WY (2021) Plant hormone-mediated regulation of heat tolerance in response to global climate change. Frontiers in Plant Science 11, 2318.
| Crossref | Google Scholar | PubMed |
Linn RR, Goodrick SL, Brambilla S, Brown MJ, Middleton RS, O’Brein JJ, Hiers JK (2020) QUIC-fire: a fast-running simulation tool for prescribed fire planning. Environmental Modelling and Software 125, 104616.
| Crossref | Google Scholar |
Lodge AG, Dickinson MB, Kavanagh KL (2018) Xylem heating increases vulnerability to cavitation in longleaf pine. Environmental Research Letters 13, 055007.
| Crossref | Google Scholar |
Lombardo KJ, Sewtnam TW, Baisan CH, Borchert MI (2009) Using bigcone Douglas-fir fire scars and tree rings to reconstruct interior chaparral fire history. Fire Ecology 5, 35-56.
| Crossref | Google Scholar |
Maestri E, Klueva N, Perrotta C, Gulli M, Nguyen HT, Marmiroli N (2002) Molecular genetics of heat tolerance and heat shock proteins in cereals. Plant Molecular Biology 48, 667-681.
| Crossref | Google Scholar | PubMed |
Malini MK, Lekshmy VS, Pal M, Chinnusamy V, Kumar MN (2020) Unfolded protein response (UPR) mediated under heat stress in plants. Plant Physiology Reports 25, 569-582.
| Crossref | Google Scholar |
Massmann WJ, Frank JM, Mooney SJ (2010) Advancing investigation and physical modeling of first-order fire effects on soils. Fire Ecology 6(1), 36-54.
| Crossref | Google Scholar |
Mazorra LM, Nunez M, Echerarria E, Coll F, Sanchez-Blanco MJ (2002) Influence of brassinosteroids and antioxidant enzymes activity in tomato under different temperatures. Plant Biology 45, 593-596.
| Crossref | Google Scholar |
McCulloh KA, Domec J-C, Johnson DM, Smith DD, Meinzer FC (2019) A dynamic yet vulnerable pipeline: integration and coordination of hydraulic traits across whole plants. Plant, Cell and Environment 42(10), 2789-2807.
| Crossref | Google Scholar | PubMed |
McHugh CW, Kolb TE (2003) Ponderosa pine mortality following fire in northern Arizona. International Journal of Wildland Fire 12(1), 7-22.
| Crossref | Google Scholar |
Michaletz ST (2018) Xylem dysfunction in fires: towards a hydraulic theory of plant responses to multiple disturbance stressors. New Phytologist 217(4), 1391-1393.
| Crossref | Google Scholar | PubMed |
Michaletz ST, Johnson EA (2007) How forest fires kill trees: a review of the fundamental biophysical processes. Scandinavian Journal of Forest Research 22, 500-515.
| Crossref | Google Scholar |
Michaletz ST, Johnson EA, Tyree MT (2012) Moving beyond the cambium necrosis hypothesis of post-fire tree mortality: cavitation and deformation of xylem in forest fires. New Phytologist 194, 254-263.
| Crossref | Google Scholar | PubMed |
Moncrieff GR, Kruger LM, Midgley JJ (2008) Stem mortality of Acacia nigrescens induced by the synergistic effects of elephants and fire in Kruger National Park, South Africa. Journal of Tropical Ecology 24, 655-665.
| Crossref | Google Scholar |
Moore GM, Rowan KS, Blake TJ (1977) Effects of heat on the physiology of seedlings of Eucalyptus obliqua. Functional Plant Biology 4(2), 283-288.
| Crossref | Google Scholar |
Niccoli F, Pacheco-Solana A, Delzon S, Kabala JP, Asgharinia S, Castaldi S, Valentini R, Battipaglia G (2023) Effects of wildfire on growth, transpiration and hydraulic properties of Pinus pinaster Aiton forest. Dendrochonologia 482, 118813.
| Crossref | Google Scholar |
Niklasson M, Drakenberg B (2001) A 600-year tree-ring fire history from Norra Kvills National Park, southern Sweden: implications for conservation strategies in the hemiboreal zone. Biological Conservation 101(1), 63-71.
| Crossref | Google Scholar |
Nolan RH, Reed CC, Hood SM (2024) Mechanisms of fire-caused tree death are far from resolved. Tree Physiology 44, tpae073.
| Crossref | Google Scholar | PubMed |
O’Brien JJ, Hiers JK, Mitchell RJ, Varner JM, Mordecai K (2010) Acute physiological stress and mortality following fire in a long-unburned longleaf pine ecosystem. Fire Ecology 6(2), 1-10.
| Crossref | Google Scholar |
O’Brien JJ, Hiers JK, Varner JM, Hoffman CM, Dickinson MB, Michaletz ST, Loudermilk EL, Butler BW (2018) Advances in mechanistic approaches to quantifying biophysical fire effects. Current Forestry Reports 4, 161-177.
| Crossref | Google Scholar |
Ottmar RD, Hiers JK, Butler BW, Clements CB, Dickinson MB, Hidak AT, O’Brien JJ, potter BE, Rowell EM, Strand TM, Zajkowski TJ (2015) Measurements, datasets and preliminary results from the RxCADRE project – 2008, 2011 and 2012. International Journal of Wildland Fire 25(1), 1-9.
| Crossref | Google Scholar |
Partelli-Feltrin R, Johnson DM, Sparks AM, Adams HD, Kolden CA, Nelson AS, Smith AMS (2020) Drought increases vulnerability of Pinus ponderosa saplings to fire-induced mortality. Fire 3, 56.
| Crossref | Google Scholar |
Partelli-Feltrin R, Smith AMS, Adams HD, Kolden CA, Johnson DM (2021) Short- and long-term effects of fire on stem hydraulics in Pinus ponderosa saplings. Plant, Cell, and Environment 44(3), 696-705.
| Crossref | Google Scholar | PubMed |
Partelli-Feltrin R, Smith AMS, Adams HD, Thompson RA, Kolden CA, Yedinak KM, Johnson DM (2023) Death from hunger or thirst? Phloem dysfunction, rather than xylem hydraulic failure, as a driver of fire-induced conifer mortality. New Phytologist 237(4), 1154-1163.
| Crossref | Google Scholar | PubMed |
Peacocke AR, Walker IO (1962) The thermal denaturation of sodium deoxyribonucleate. II. Kinetics. Journal Molecular Biology 5, 560-563.
| Crossref | Google Scholar | PubMed |
Peterson DL (1985) Crown scorch and scorch height: estimates of post-fire condition. Canadian Journal of Forest Research 15, 596-598.
| Crossref | Google Scholar |
Pinard MA, Huffman J (1997) Fire resistance and bark properties of trees in a seasonally dry forest in eastern Bolivia. Journal of Tropical Ecology 13, 727-740.
| Crossref | Google Scholar |
Piper FI, Paula S (2020) The role of non-structural carbohydrates storage in forest resilience under climate change. Current Forestry Reports 6, 1-13.
| Crossref | Google Scholar |
Pita P, Lopez R, Gil L (2023) The effect of hot wind on needle and stem water status: response strategies in resprouting and non-resprouting pine species. Forests 14, 2174.
| Crossref | Google Scholar |
Potter VE, Andersen JA (2002) A finite-difference model of temperatures and heat flow within a tree stem. Canadian Journal of Forest Research 32, 548-555.
| Crossref | Google Scholar |
Prichard S, Larkin NS, Ottmar R, French NHF, Baker K, Brown T, Clements C, Dickinson M, Hudak A, Kochanski A, Linn R, Liu Y, Potter B, Mell W, Tanzer D, Urbanski S, Watts A (2019) The Fire and Smoke Model Evaluation Experiment—a plan for integrated, large fire–atmosphere field campaigns. Atmosphere 10, 66.
| Crossref | Google Scholar | PubMed |
Prior LD, French BJ, Bowman DMJS (2018) Effect of experimental fire on seedlings of Australian and Gondwanan trees species from a Tasmanian montane vegetation mosaic. Australian Journal of Botany 66, 511-517.
| Crossref | Google Scholar |
Reed CC, Hood SM (2024) Non-structural carbohydrates explain post-fire tree mortality and recovery patterns. Tree Physiology 44, tpad155.
| Crossref | Google Scholar | PubMed |
Rigolot E (2004) Predicting post-fure mortlaity of Pinus halepensis Mil. and Pinus pinea L. Plant Ecology 171, 139-154.
| Crossref | Google Scholar |
Robberecht R, Defosse GE (1995) The relative sensitivity of two bunchgrass species to fire. International Journal of Wildland Fire 5, 127-134.
| Crossref | Google Scholar |
Ryan KC, Reinhardt ED (1988) Predicting postfire mortality of seven western conifers. Canadian Journal of Forest Research 18, 1291-1297.
| Crossref | Google Scholar |
Ryan KC, Peterson DL, Reinhardt ED (1988) Modeling long-term fire-caused mortality of Douglas-fir. Forest Science 34(1), 190-199.
| Crossref | Google Scholar |
Ryan KC, Rigolot E, Botelho H (1994) Comparative analysis of fire resistance and survival of Mediterranean and Western North American conifers. In ‘Proceedings of the 12th International Conference on Fire and Forest Meteorology’. SAF Pub. 94-02. pp. 701–708. (Society of American Foresters: Bethesda, MD, USA)
Sakamoto A, Murata N (2002) The role of glycine betaine in the protection of plants from stress: clues from transgenic plants. Plant Cell and Environment 25, 163-171.
| Crossref | Google Scholar | PubMed |
Salladay RA, Pittermann J (2023) Using heat plumes to simulate post-fire effects on cambial viability and hydrualic performance in Sequoia sempervirens stems. Tree Physiology 43(5), 769-780.
| Crossref | Google Scholar | PubMed |
Shearman TM, Varner JM, Hood SM, van Mantgem PJ, Cansler CA, Wright M (2022) Predictive accuracy of post-fire conifer death declines over time in models based on crown and bole injury. Ecological Applications 33(2), e2760.
| Crossref | Google Scholar | PubMed |
Shirley HL (1936) Lethal high temperatures for conifers, and the cooling effect of transpiration. Journal of Agricultural Research 53(4), 239-258.
| Google Scholar |
Shuman JK, Balch JK, Barnes RT, Higuera PE, Roos CI, Schwilk DW, Stavros EN, Banerjee T, Bela MM, Bendix J, Bertolino S, Bililign S, Bladon KD, Brando P, Breidenthal RE, Buma B, Calhoun D, Carvalho LMV, Cattau ME, Cawley KM, Chandra S, Chipman ML, Cobian-Iñiguez J, Conlisk E, Coop JD, Cullen A, Davis KT, Dayalu A, De Sales Sales F, Dolman M, Ellsworth LM, Franklin S, Guiterman CH, Hamilton M, Hanan EJ, Hansen WD, Hantson S, Harvey BJ, Holz A, Huang T, Hurteau MD, Ilangakoon NT, Jennings M, Jones C, Klimaszewski-Patterson A, Kobziar LN, Kominoski J, Kosovic B, Krawchuk MA, Laris P, Leonard J, Loria-Salazar SM, Lucash M, Mahmoud H, Margolis E, Maxwell T, McCarty JL, McWethy DB, Meyer RS, Miesel JR, Moser WK, Nagy RC, Niyogi D, Palmer HM, Pellegrini A, Poulter B, Robertson K, Rocha AV, Sadegh M, Santos F, Scordo F, Sexton JO, Sharma AS, Smith AMS, Soja AJ, Still C, Swetnam T, Syphard AD, Tingley MW, Tohidi A, Trugman AT, Turetsky M, Varner JM, Wang Y, Whitman T, Yelenik S, Zhang X (2022) Reimagine fire science for the Anthropocene. PNAS Nexus 1(3), pgac115.
| Crossref | Google Scholar | PubMed |
Sidoroff K, Kuuluvianen T, Tanskanen H, Vanha-Majamaa I (2007) Tree mortality after low-intensity prescribed fires in managed Pinus sylvestris stands in southern Finland. Scandinavian Journal of Forest Research 22(1), 2-12.
| Crossref | Google Scholar |
Smith AMS, Tinkham WT, Roy DP, Boschetti L, Kumar S, Sparks AM, Kremens RL, Falkowski MJ (2013) Quantification of fuel moisture effects on biomass consumed derived from fire radiative energy retrievals. Geophysical Research Letters 40, 6298-6302.
| Crossref | Google Scholar |
Smith AMS, Kolden CA, Tinkham WT, Talhelm AF, Marshall JD, Hudak AT, Boschetti L, Falkowski MJ, Greenberg JA, Anderson JW, Kliskey A, Alessa L, Keefe RF, Gosz J (2014) Remote Sensing the vulnerability of vegetation in natural terrestrial ecosystems. Remote Sensing of Environment 154, 322-337.
| Crossref | Google Scholar |
Smith AMS, Sparks AM, Kolden CA, Abatzoglou JT, Talhelm AF, Johnson DM, Boschetti L, Lutz JA, Apostol KG, Yedinak KM, Tinkham WT, Kremens RJ (2016) Towards a new paradigm in fire severity research using dose–response experiments. International Journal of Wildland Fire 25, 158-166.
| Crossref | Google Scholar |
Smith AMS, Talhelm AF, Johnson DM, Sparks AM, Yedinak KM, Apostol KG, Tinkham WT, Kolden CA, Abatzoglou JT, Lutz JA, Davis AS, Pregitzer KS, Adams HD, Kremens RL (2017) Effects of fire radiative energy density doses on Pinus contorta and Larix occidentalis seedling physiology and mortality. International Journal of Wildland Fire 26(1), 82-94.
| Crossref | Google Scholar |
Smith AMS, Kolden CA, Bowman DMJS (2018) Biomimicry can help humans to sustainably coexist with fire. Nature Ecology and Evolution 2, 1827-1829.
| Crossref | Google Scholar | PubMed |
Sparks AM, Kolden CA, Talhelm AF, Smith AMS, Apostol KG, Johnson DM, Boschetti L (2016) Spectral indices accurately quantify changes in tree physiology following fire: toward mechanistic assessments of landscape post-fire carbon cycling. Remote Sensing 8(7), 572.
| Crossref | Google Scholar |
Sparks AM, Smith AMS, Talhelm AF, Kolden CA, Yedinak KM, Johnson DM (2017) Impacts of fire radiative flux on mature Pinus ponderosa growth and vulnerability to secondary mortality agents. International Journal of Wildland Fire 26(1), 95-106.
| Crossref | Google Scholar |
Sparks AM, Talhelm AF, Partelli-Feltrin R, Smith AMS, Johnson DM, Kolden CA, Boschetti L (2018a) An experimental assessment of the impact of drought and fire on western larch mortality and recovery. International Journal of Wildland Fire 27(7), 490-497.
| Crossref | Google Scholar |
Sparks AM, Kolden CA, Smith AMS, Boschetti L, Johnson DM, Cochrane MA (2018b) Fire intensity impacts on post-fire response of temperate coniferous forest net primary productivity. Biogeosciences 15(4), 1173-1183.
| Crossref | Google Scholar |
Sparks AM, Blanco AS, Wilson DR, Schwilk DW, Johnson DM, Adams HD, Bowman DMJS, Hardman DD, Smith AMS (2023a) Fire intensity impacts on physiological performance and mortality in Pinus monticola and Pseudotsuga menziesii: a dose–response analysis. Tree Physiology 43(8), 1365-1382.
| Crossref | Google Scholar | PubMed |
Sparks AM, Smith AMS, Hudak AT, Carrao MV, Kremens RL, Keefe RF (2023b) Integrating active fire behavior observations and multitemporal airborne laser scanning data to quantify fire impacts on tree growth: a pilot study in mature Pinus ponderosa stands. Forest Ecology and Management 545, 121246.
| Crossref | Google Scholar |
Sparks AM, Blanco AS, Lad LE, Smith AMS, Adams HD, Tinkham WT (2024) Pre-fire drought intensity drives post-fire recovery and mortality in Pinus monticola and Pseudotsuga menziesii saplings. Forest Science 70, 2, 189-201.
| Crossref | Google Scholar |
Steady WD, Partelli-Feltrin R, Johnson DM, Sparks AM, Kolden CA, Talhelm AF, Lutz JA, Boschetti L, Hudak AT, Nelson AS, Smith AMS (2019) The survival of Pinus Ponderosa saplings subjected to increasing levels of fire intensity and impacts on post-fire growth. Fire 2(2), 23.
| Crossref | Google Scholar |
Stenzel JE, Bartowitz KJ, Hartman MD, Lutz JA, Kolden CA, Smith A, Law BE, Swanson ME, Larson AJ, Parton WJ, Hudiburg TW (2019) Fixing a snag in estimating carbon emissions from wildfires. Global Change Biology 25(11), 3985-3994.
| Crossref | Google Scholar | PubMed |
Stephens SL, Finney MA (2002) Prescribed fire mortality of Sierra Nevada mixed conifer tree species: effects of crown damage and forest floor combustion. Forest Ecology and Management 162(2–3), 261-271.
| Crossref | Google Scholar |
Stephan K, Miller M, Dickinson MB (2010) First-Order Fire Effects on Herbs and Shrubs: Present Knowledge and Process Modeling Needs. Fire Ecology 6, 95-114.
| Crossref | Google Scholar |
Thiffault N, Jobidon R, Munson AD (2014) Comparing large containerized and bareroot conifer stock on sites of contrasting vegetation composition in a non-herbicide scenario. New Forests 45, 875-891.
| Crossref | Google Scholar |
Tohidi A, Gollner MJ, Xiao H (2018) Fire whirls. Annual Review of Fluid Mechanics 50, 187-213.
| Crossref | Google Scholar |
Tonet V, Carins-Murphy M, Deans R, Brodribb TJ (2023) Deadly acceleration in dehydration of Eucalyptus viminalis leaves coincides with high-order vein cavitation. Plant Physiology 191, 1648-1661.
| Crossref | Google Scholar | PubMed |
Trouvé R, Oborne L, Baker PJ (2021) The effect of species, size, and fire intenisty on tree mortality within a catastophic bushfire complex. Ecological Applicatins 31(6), e02383.
| Crossref | Google Scholar | PubMed |
Uhl C, Kauffman JB (1990) Deforestation, fire susceptibility, and potential tree responses to fire in the eastern Amazon. Ecology 49, 71-437.
| Google Scholar |
Van Mantgem P, Schwartz M (2003) Bark heat resistance of small trees in Californian mixed conifer forests: testing some model assumptions. Forest Ecology and Management 178(3), 341-352.
| Crossref | Google Scholar |
Van Wagner CE (1971) Two solitudes in forest fire research. Information Report PS-X-29. (Canadian Forest Service, Petawawa Forest Experiment Stations: Chalk River, ON) Available at https://ostrnrcan-dostrncan.canada.ca/entities/publication/94193076-d362-4b9f-9c4e-ded220a19f3f
Varner JM, Putz FE, O’Brien JJ, Hiers JK, Mitchell RJ, Gordon DR (2009) Post-fire tree stress and growth following smouldering duff fires. Forest Ecology and Management 258(11), 2467-2474.
| Crossref | Google Scholar |
Varner JM, Hood SM, Aubrey DP, Yedinak K, Hiers JK, Jolly WM, Shearman TM, McDaniel JK, O’Brien JJ, Rowell EM (2021) Tree crown injury from wildland fires: causes, measurement and ecological and physiological consequences. New Phytologist 231(5), 1676-1685.
| Crossref | Google Scholar | PubMed |
Vines RG (1968) Heat transfer through bark, and the resistance of trees to fire. Australian Journal of Botany 16(3), 499-514.
| Crossref | Google Scholar |
Wahid A, Gelani S, Ashraf M, Foolad MR (2007) Heat tolerance in plants: an overview. Environmental and Experimental Botany 61, 199-223.
| Crossref | Google Scholar |
Watanabe M (1953) Effect of heat application upon the pollen viability of Japanese black pine and Japanese red pine. Journal of the Japanese Forest Society 35, 248-251.
| Google Scholar |
Wener PA, Franklin DC (2010) Resprouting and mortality of juvenile eucalypts in an Australian savanna: impacts of fire season and annual sorghum. Australian Journal of Botany 58, 619-628.
| Crossref | Google Scholar |
Wesolowski A, Adams MA, Pfautsch S (2014) Insulation capacity of three bark types of temperate Eucalyptus species. Forest Ecology and Management 313, 224-232.
| Crossref | Google Scholar |
West AG, Nel JA, Bond WJ, Midgley JJ (2016) Experimental evidence for heat plume-induced cavitation and xylem deformation as a mechanism of rapid post-fire tree mortality. New Phytologist 211, 828-838.
| Crossref | Google Scholar | PubMed |
West AG, Bloy ST, Skelton RP, Midgley JJ (2023) Hydraulic segmentation explains differences in loss of branch conductance caused by fire. Tree Physiology 43, tpad108.
| Crossref | Google Scholar | PubMed |
Wilson LA, Spencer RN, Aubrey DP, O’Brien JJ, Smith AMS, Thomas RW, Johnson DM (2022) Longleaf pine seedlings are extremely resilient to the combined effects of experimental fire and drought. Fire 5(5), 128.
| Crossref | Google Scholar |
Woolley T, Shaw DC, Ganio LM, Fitzgerald S (2012) A review of logistic regression models used to predict post-fire tree mortality of western North American conifers. International Journal of Wildland Fire 21, 1-35.
| Crossref | Google Scholar |
Wooster MJ, Roberts GJ, Giglio L, Roy DP, Freeborn P, Boschetti L, Justice CO, Ichoku CM, Schroeder W, Davies DK, Smith AMS, Setzer A, Csiszar I, Strydom T, Frost P, Zhang T, Xu W, De Jong M, Johnson JM, Ellison L, Vardrevu KP, Sparks AM, Nguyen H, McCarty JL, Tanpipat V, Schmidt C, San-Miguel-Ayanz J (2021) Satellite remote sensing of active fires: history and current status, applications and future requirements. Remote Sensing of Environment 267, 112694.
| Crossref | Google Scholar |
Wyant JG, Omi PN, Laven RD (1986) Fire induced mortality in a Colorado ponderosa pine/Douglas fir stand. Forest Science 32, 49-59.
| Crossref | Google Scholar |
Yang X, Bassham DC (2015) Chapter one - New insight into the mechanism and function of autophagy in plant cells. In ‘International Review of Cell and Molecular Biology. Vol. 320’. (Ed. KW Jeo) pp. 1–40. (Academic Press) 10.1016/bs.ircmb.2015.07.005


