Post-fire clumping of seedlings of Cape Proteaceae species: ecological, evolutionary and conservation implications
J. J. Midgley A * , M. D. Cramer A , A. L. Schutte-Vlok B D and A. Veldtman CA
B
C
D Present address:
Abstract
Spatial clumping of plants results in intense competition.
We analysed the extent of clumping of Proteaceae seedlings across the Cape Floristic Region after fire.
To demonstrate the extent of clumping in 23 species, we analysed the seedling and adult densities in 290 post-fire surveys each of 100 × 1 m2 plots using standard indices of clumping.
We detected clumping of Cape Proteaceae seedlings, whereas parent plants were less clumped. The clumping is not due to limited safe sites for seedlings because the number of plots at a site with at least one seedling was positively related to the number of seedlings at the site. Sites with seedlings were enriched in nutrients relative to those without.
The possible benefits of clumping are saturation of granivores and co-location of buried seeds with nutrient-rich patches of fire-derived ash and debris. A cause of clumping is seeds sticking together. Clumping, which is strongly intraspecific, has implications for trait evolution and the dominance of reseeders over resprouters. Strong clumping reduces the usefulness of the widely used seedling:parent index to determine whether fires were favourable or not.
Clumping has implications for understanding seed dispersal distances, seedling versus adult traits, fire responses and demography.
Keywords: Leucadendron, Protea, seedling clumping, seed dispersal, seedlings, self-thinning, serotiny, South Africa.
Introduction
After fires in the mediterranean-climate Cape Floristic Region ‘fynbos’ shrublands, the hairy single-seeded diaspores (hereafter referred to as seeds) of serotinous Protea seeds accumulate in large clumps (Figs 1, 2) and this leads to dense clumps of seedlings (>200 m−2, Fig. 1b). Here, ‘clumping’ refers to whether individuals are aggregated together rather than being evenly or randomly distributed across a landscape. This high extent of clumping of seeds and seedlings has not been documented previously but is relevant to several ecological and evolutionary processes in these shrublands, as well as having conservation implications. This clumping may be related to the type and distance of seed dispersal, the seed characteristics (e.g. appendages) and characteristics of the post-fire environment. Winged seeds of the dominant serotinous southwest Australian shrubland Proteaceae genera of Hakea and Banksia tend to occur in specific post-fire microsites (e.g. among unburned leaf litter or microweirs) rather than in open sandy sites (Enright and Lamont 1989; Lamont et al. 1993). At these sites, seeds are more deeply buried than in open sites, and thus Lamont et al. (1993) concluded that debris sites are ‘safe for seeds (greater burial and thus lower loss of viability of seeds over the period before winter germination) but unsafe for seedlings’ (greater mortality due to intense competition in multi-seedling clumps). Our first question was to determine what was the extent of clumping in serotinous Cape Proteaceae, and to determine whether it varies with dispersal type. In the Cape Proteaceae, black winged seeds are found in Leucadendron (e.g. L. laureolum), but unlike in southwestern Australia, hairy seeds are also very common. Protea, Aulax and some Leucadendron species (e.g. L. linifolium) have hairy seeds. Some species have seeds with parachutes (e.g. L. rubrum). All of these have the potential to disperse once on the ground (Bond 1988). Thus, our expectation was that there would be a lower extent of clumping among hairy seeds (compared with winged seeds) because the extent of clumping should decline with increasing dispersal distances.
Clumping of seeds of Protea repens (a) soon after fire, and clumping of seedlings after winter rainfall (b) (lat. −34.3335, long. 19.1117). The identification numbers on white background are superimposed on sheets of A4 paper in (b) and copied onto image in (a). The number of seedlings in each of these patches was counted, the area of the patch measured and the result expressed as number per square metre (no. m−2, yellow numbers, average ± s.e. = 106 ± 26 m−2).

Dense clumps of orange/brown hairy Protea repens seeds after fire but before germination. Insert shows individual Protea repens seeds.

The actual distances moved by serotinous Proteaceae seeds in the post-fire fynbos environment have not been measured, but they have been modelled. The models of Schurr et al. (2005) produced maximum dispersal distances of 59 km for the hairy seeds of Protea repens versus only 3 m for winged seeds of Leucadendron salignum. This indicated that long-distance dispersal of hairy seeds is important in understanding the distribution and spatial niche size of contemporary Cape plants (Pagel et al. 2020). However, whether long-distance dispersal occurs is controversial because the modelling was based on data from obstacle-free flat smooth beaches that do not match the broken post-fire environment (rocks of various sizes, burned plant remains, depressions) and that is rarely flat. Also, the most common seed dispersal mechanism in species of the Cape Proteaceae is short-distance ant dispersal (i.e. myrmecochory, Bond and Slingsby 1983). As the post-fire environment is the best recruitment arena and fires are frequent (return every 7–21 years; Kraaij and van Wilgen 2014), there is no need for seeds to disperse to post-fire recruitment sites. Thus, the expectation is that wind dispersal of serotinous species should be similarly short-distance dispersal, to avoid dispersing away from the fire that stimulated the opening of cones. Furthermore, it is common to observe accumulations of seeds (which include fertile and non-fertile seeds) in patches in Proteaceous shrublands after fire (Figs 1a, 2), indicating that seed dispersal distances are short, partly owing to being limited by the formation of clumps of seeds.
The next factor to be considered is whether the extent of clumping in Cape Proteaceae differs between dioecious (Leucadendron) and hermaphroditic (Protea) taxa. The prediction is that dioecious plants should have greater clumping of seedlings because, compared with equally abundant hermaphrodites, the females should typically release double the number of seeds but from only half the individuals (Heilbuth et al. 2001). Thus, there should be a higher extent of clumping around dioecious females than around hermaphrodites due to the greater concentration of seeds.
If clumping exists, then it is relevant to ask whether clumps frequently contain more than one species. A predominance of intraspecific competition will favour traits such as rapid seedling growth to survive intense seedling competition, whereas a predominance of inter-specific competition may favour trait differentiation. At present, traits that allow functional niche differentiation among co-occurring adult Cape Proteaceae is emphasised, such as wood density, leaf longevity, plant height and ramification (Treurnicht et al. 2020). Many of these traits would be inversed with rapid seedling growth given the trade-offs of the live-fast and die-young versus slow growth and greater longevity spectrum (Reich 2014). Intense clumping of seedlings may select for rapid seedling growth. Although the costs of clumping are clear (heightened competition for resources), the advantages are less obvious. Here, we tested whether seeds that were clumped following fires were co-located with nutrient-enriched areas due to the accumulation of fire-derived ash and debris that would serve to reduce competition for nutrients (Fig. 1).
Clumping of seedlings also has implications for conservation and management. Bond et al. (1984) and others subsequently (Midgley 1989; Kraaij et al. 2013) have shown that more prolific seedling recruitment, as measured by seedling:parent ratios, occurs after fires in mature, rather than young, vegetation and after summer/autumn burns rather than winter/spring burns. Management burning practices are designed to maximise seedling:parent ratios. Thus, fires in winter or spring are avoided because of low seedling:parent ratios. However, if the more prolific initial regeneration is merely associated with a higher extent of clumping, then this reduces these seasonal differences, because relatively more seedlings after prolific recruitment will be lost to self-thinning in dense clumps.
We asked whether the extent of clumping depends on the density of seedlings and how it interacts with seedling:parent ratios. We predicted that if clumping occurs, it would be correlated with dispersal attributes (winged versus hairy seeds), the breeding system (hermaphrodite versus dioecious) and the extent of seedling recruitment (more seedlings resulting in greater clumping). We also compared the soil nutrient status of seed clumps with areas without clumps to determine whether clumping has a potential survival benefit.
Methods
The data
CapeNature manages most conservation land of the Cape Province and routinely collects parent and seedling information for overstorey non-sprouting Protea and Leucadendron (Proteaceae) species in 60–100 plots each 1 m2 and 5 m apart within 1 year to 18 months after fires on their custodial land, with approximately a 100-plot transect for every 500 ha of fire up to a maximum of four transects per fire across 258 sites and 274 fires (Fig. 3). The data are collected to determine the effect of the pre-fire veld age (i.e. fire return interval) and the season of burn on the recruitment success of these Proteaceae indicator species. Mature parents can be identified after fires by their remaining cones and counted at this stage as they take many years to decay and fall over. The overwhelming majority of records are on serotinous species of Protea (15 species) and Leucadendron (eight species). We ignored non-serotinous taxa. A limitation of our data is that seedlings within a clump (i.e. within a few centimetres of another seedling) but outside of the 1 m2 plot would not be recorded, and thus this analysis is a conservative estimate of clumping (see below).
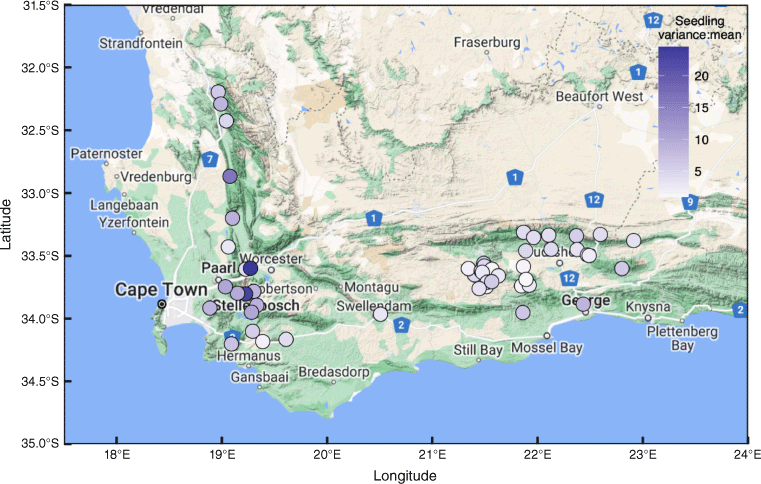
The geographic distribution of study sites within southwestern South Africa. The colour of the points shows the average variation of the seedling Fisher’s index (variance:mean) for each survey site (>1 indicates clumping, i.e. all sites showed clumping). Major towns are named, and roads are shown for reference with route identification (pentagon symbols).
As Leucadendron is dioecious, only females can be counted as a source of seeds. In this case, to get realistic parent:seedling ratios, the number of females is usually doubled (e.g. Midgley 1989; Kraaij et al. 2013). However, the protocol used across sites and times was not clear and given this potential for error of parent numbers and because the present paper is focussed on seedlings, we ignored parent numbers for Leucadendron. For most analyses, we only used sites where at least 10 parents or seedlings were recorded.
Measuring clumping
As we wished to compare clumping of seedlings and parents in relation to seed dispersal types and breeding systems, we needed an appropriate clumping index. There are many clumping indices and de Freitas Alves and de Santana (2021) showed that, for their study taxon, most of these clumping indices produced similar results. We analysed clumping using most of these indices as well as a focussing on one of these (variance:mean ratio, i.e. Fisher’s Index). Morisita’s index was calculated using the dispindmorisita function in vegan (Oksanen et al. 2022) in R (R Core Team 2022). Hurlbert (1990) cautioned against indiscriminate use of indices of clumping, especially when underlying distributions differ widely. However, because our comparisons were among very similar Proteaceae species, with fairly similar abiotic dispersal attributes (e.g. no animal-dispersed seeds) and only varying in post-fire seedling densities, this does not apply to our study. Hurlbert (1990) argued that researchers should devise locally appropriate biological and statistical tests. We thus used various clumping indices as well as our own methods of showing clumping.
A further problem is that clumping is related to the scale it is measured at; if plots are large, clumping is more likely. Our 1 m2 plots were considered an appropriate size as they could support only a few adults (given plants are typically 2–3 m maximum height), but several seedlings. de Freitas Alves and de Santana (2021) noted that clumping indices do not show the scale of clumping, nor do they produce insights into the cause of clumping. We investigated the likely cause of the clumping in several ways; we noted whether the extent of clumping varied with seedling number, dispersal type and dispersibility.
Clumping of seedlings can result from several processes: limited safe sites (i.e. seed and seedling survival is restricted to a few sites), limited seed dispersal, high seed output, low adult density and seeds aggregating to form clumps or being trapped behind obstacles or in depressions. If seeds do not move far and if adult plants produce many seeds, seedlings will be clumped around adult plants. If safe sites were limited and/or seeds stuck together, we would expect greater clumping with a greater density of seedlings, whereas if safe sites were not limiting, we would expect clumping to be invariant with seedling number; more seed would increase the probability of finding a safe site at which clumping could occur.
Soil sampling and site analysis
In April 2022, prior to germination, a single site (34.333°S, 19.112°E, Fig. 2) that burnt in January 2022 was photographed. In December 2022 (after germination), photographing of the site was repeated and a 5 × 100 m long transect surveyed with 100 × 1 m2 plots located every 5 m. At each plot, the number of adults and seedlings were counted and whether there was fire-derived ash and debris within the plot noted.
At the photographed site (Fig. 1), five soil samples were collected in January 2023 from patches with ash/debris and seed clumps and from five adjacent sites with seedlings, but no accumulation of ash/debris or seed clumps. The surface organic material was scraped away to expose the mineral soil and samples collected by excavating a small hole with a trowel and then sampling the exposed surface with a trowel to a depth of 0.2 m. The soil was air-dried for 1 week and then sieved through a 2-mm sieve and soils extracted as described in The Non-Affiliated Soil Analyses Work Committee (1990). Resistance and electrical conductivity were measured on saturated paste. pH was measured in 1.0 M KCl. Extractable acidty was determined through titration with 0.05 M NaOH on 1 M KCl extracts. Bray II P was extracted with 95% NH4F and 32% HCl and total extractable cations (i.e. K, Ca, Mg and Na) with 0.2 M ammonium acetate. Organic C was extracted using the Walkley–Black method. Zn, Mn, Cu and Fe were extracted with 0.02 M (NH4)2EDTA using a 1:2 hot water ratio. Sulfur (S) was extracted with concentrated H3PO4 (pH 4) according to the method described by Pansu and Gautheyrou (2006). The extracted solutions were analysed with a Varian (Palo Alto, CA, USA) inductively coupled plasma optical emission spectrometer (ICP-OES). Both total C and N content of soil were determined through total combustion using a Leco Truspec (St Joseph, MI, USA) CHN analyser.
Do seeds stick together?
To determine whether seeds were mobile in wind, we placed 10 individual seeds on a coarse sand surface at three wind speeds (14.8, 16.0 and 16.3 km h−1) in a wind tunnel (design following Fister et al. 2012), either oriented into the wind or facing downwind. To determine whether seeds stick together, we placed three groups of 10 Protea repens seeds together in a wind tunnel on a levelled coarse sand surface, exposed them to varying winds speeds at ground level and noted how many left the group (by moving at least 10 cm). The groups of seeds were either oriented into the wind, downwind or randomly.
Results
Clumping analysis
Seedlings and parents are both clumped, with seedlings more heavily clumped by all indices than adults (Fig. 4). A Fisher’s index of <1 indicates regular spread and >1 indicates clumping, and Fisher’s index can be calculated per species and per site (that is, where all seedlings and parents at a site are lumped together). There is a power law ratio between log mean and log variance for both seedlings and parents, and the exponent is higher for the seedlings than parents (Fig. 5). Also the log-log relationship exponent being >1.1 indicates that the variance is at least an order of magnitude greater than the mean (i.e. indicative of strong clumping). The mean of Fisher’s index for seedlings and parents at the plot level is 7.86 and 0.84, and at the species level is 7.22 and 0.85, respectively, again indicating greater clumping in seedlings compared with parents. If absences are ignored (i.e. plots where no parents or seedlings are recorded), Fisher’s index remains >1 and higher for seedlings than parents (14.45 versus 4.11). There are small but significant differences among seed types (hairy versus wings versus parachutes) and breeding systems (hermaphrodites versus dioecious) in the extent of clumping (Fig. 6). For Protea species (Leucadendron species were not included owing to uncertainty of parent counts: see Methods), seedling to parent ratios were >1 for five of nine species.
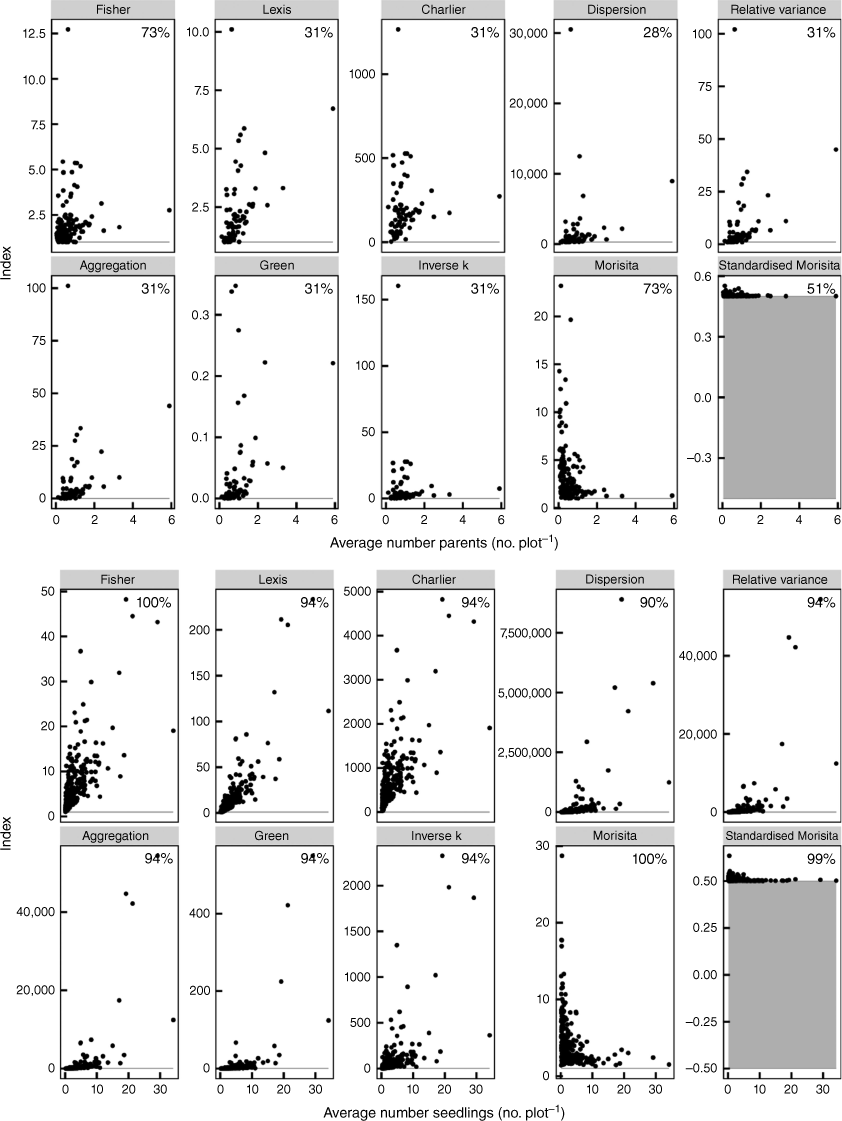
Indices of aggregation specified by de Freitas Alves and de Santana (2021). Each point represents the aggregation index value for a site (generally comprising 100 plots) plotted against the average number of parents or seedlings per plot in each site. The indices are shown for both parental plants (top) and seedlings (bottom) without differentiating species. The percentage values in each panel represent the proportion of sites in which the aggregation was significantly (P < 0.05) different from random. The horizontal grey line or band represents the critical value for non-significant results.
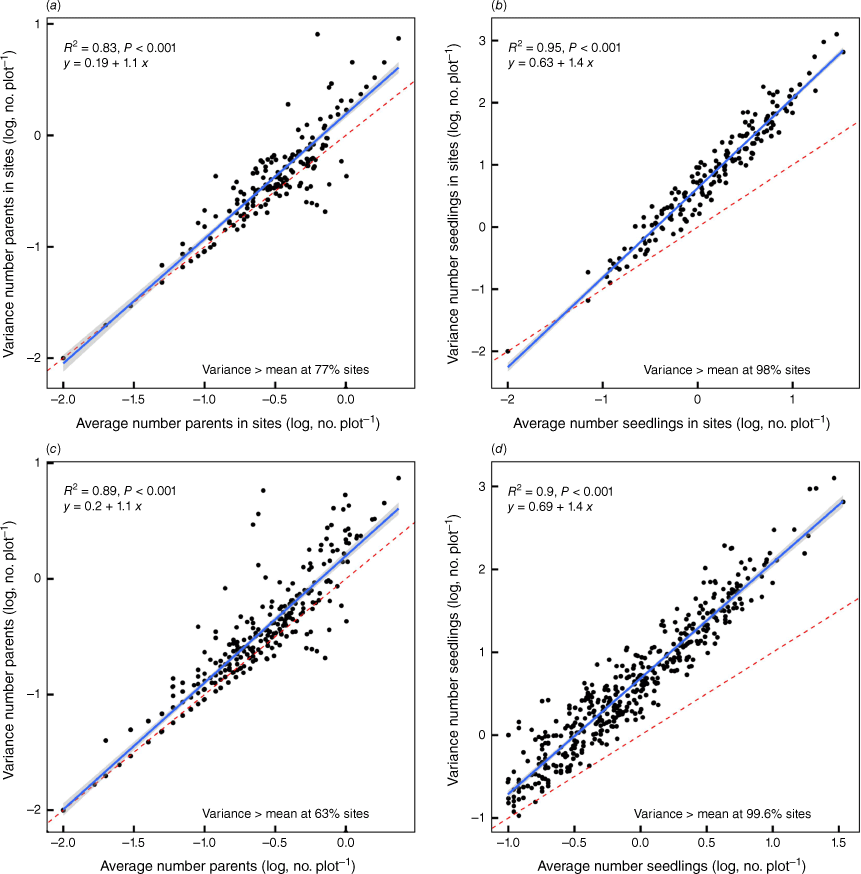
Variation in the variance in the number of parent plants and seedlings at each site with the average number of parent plants and seedlings, respectively. Each point represents the variance/mean of a site consisting of ~100 plots. The blue line represents the linear fit to the data for which the equation and the coefficient of determination and the significance of the relationship is shown. The broken red line indicates the 1:1 line and the percentage of sites at which the variance exceeded the mean is shown for each. The grey band shows the 95% confidence interval. The upper panels (a, b) show all species treated separately within sites and the lower panels (c, d) show the data for sites, but ignoring species.
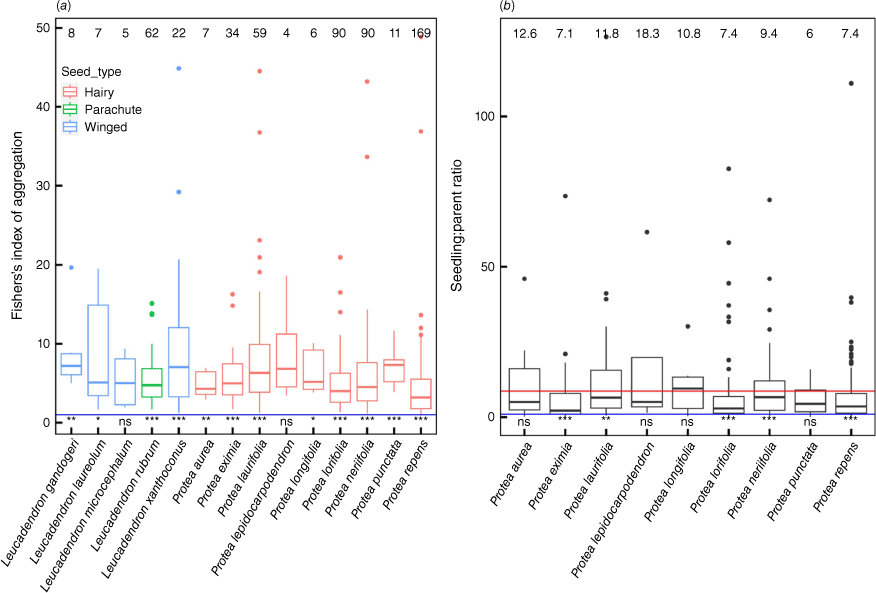
(a) Fisher’s index of aggregation for Leucadendron spp. seedlings (mean = 7.62) and for Protea spp. seedlings (mean = 5.81; F = 5.53, d.f. = 1/433, P = 0.019). These differences are both between dioecious Leucadendron species and monoecious Protea species and between, predominantly, winged seeds versus hairy seeds. The numbers along the top axis indicate the average Fisher’s index of aggregation. (b) Seedling to parent ratio for Protea species. The numbers along the top axis indicate the average seedling:parent ratio. The average seedling to parental ratio across all species is shown by the red line. The results of one-sided students t-test (mu = 1, blue line) are shown along the bottom axis (ns, not significant; **P < 0.05; ***P < 0.001).
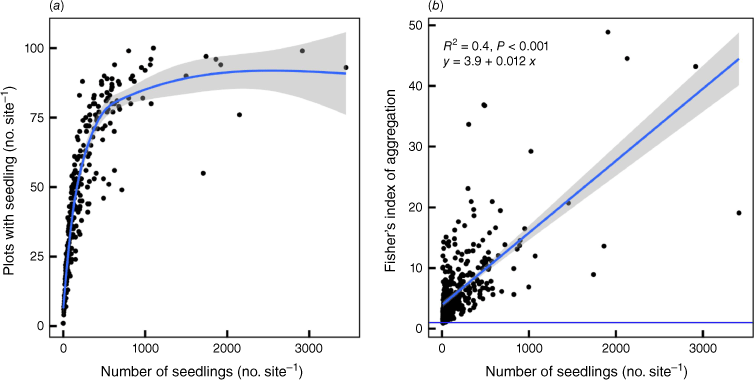
The extreme clumping of seedlings is reflected in the way the number of plots with at least one seedling in a site (total plot number = 100 per site) rises asymptotically with the total number of seedlings such that after ~1000 seedlings per 100 × 1 m2 plots, only 80% of plots have at least one seedling (Fig. 7). This suggests that for approximately every 12 (or >100 seeds; see later discussion) seedlings, a new 1 m−2 plot is colonised, presumably because there are many obstacles and because seeds thereafter clump together. Overall, this indicates that suitable 1 m−2 plots are essentially unlimited, although it takes many seeds to ‘find’ them. Thus, the greater the number of seedlings in a site, the greater the sizes of the clumps but the extent of aggregation across sites is fairly constant (Fig. 6). The mean parent density at the plot level was 40.4 per 100 × 1 m2 plots, whereas it was 236.2 for seedlings. At the species level, these values were 23.4 and 136.9 respectively. Owing to high variability among sites and species, mean seedling:parent ratios per plot were 9.49 and per species 8.63 rather than 5.9 per plot (i.e. 236.2/40.4) and 5.9 per species (i.e. 136.9/23.4). Near-neighbour interactions were overwhelmingly intraspecific rather than inter-specific. Of the 59,096 1 m2 plots that were examined, 32.2% had at least one seedling. Of these, 83.3, 15.5 and 1.2% had one, two and three species per plot respectively.
(a) The variation in the number of plots within the sites (n = 258) that had seedlings versus the number of seedlings (all species) in each site. The blue line with grey band represents a smoothing function (generalised additive model) and the confidence interval of the fit. This shows that for <1000 seedlings per site, the more seedlings there are in a site, the more uniform their distribution. (b) The variation in Fisher’s index (variance:mean) of aggregation with the average numbers of seedlings per plot (all species). The blue line with grey band represents the linear best fit for which the equation is specified along with the coefficient of determination and the P value. The horizontal blue line represents the threshold for clumping (Fisher’s index = 1).
Soil sampling and site analysis
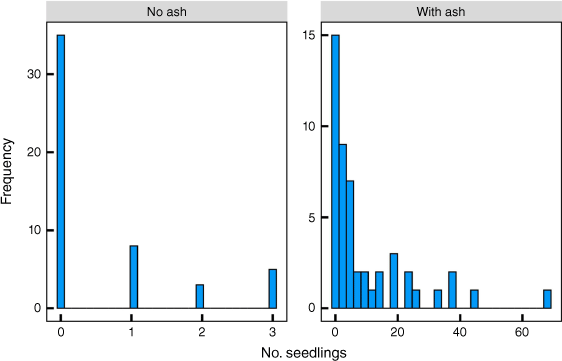
Seedlings were strongly associated with ash and debris. The total number of seedlings in plots without ash/debris was less than a maximum of three and with ash/debris less than a maximum of 70 (Fig. 8). Across the landscape, 49% of all plots had an accumulation of ash and debris. Only 16% of the plots had seedlings without ash/debris and 39% had neither ash/debris nor seedlings. The average density of seedlings was 5.15 m−2 and of adult skeletons 0.25 m−2. Average Fisher’s index values across the 100 plots for adults and seedlings were 0.84 and 23.0, respectively. There were 0.57 ± 0.13 (mean ± s.e.) seedlings m−2 without ash/debris and 9.9 ± 2.0 seedlings m−2 with ash/debris (Student’s t = 4.64, P < 0.001). Of plots with ash/debris clumps from the fire, 92% had seedlings as well. There was significantly (P < 0.05) greater C, Bray II P, K and extractable Mg, K, Na and S in the mineral soil below patches with ash/debris and seed than in areas without debris (Table 1). Notably, pH and N did not differ. The lower resistance of soil with debris indicates greater electrical conductivity.
Frequency distribution of Protea repens seedling numbers in December 2022 (lat −34.3335, lon 19.1117; Fig. 1) along a 500 m transect with 100 × 1 m2 plots every 5 m.
| Measured | Ash/debris | No ash/debris | P value | |
|---|---|---|---|---|
| pH (1 M KCl) | 5.02 ± 0.13 | 4.68 ± 0.09 | 0.067 | |
| Resistance (Ω) | 2160 ± 94 | 3876 ± 550 | 0.015 | |
| H+ (cmol+ kg−1) | 0.77 ± 0.05 | 0.88 ± 0.06 | 0.163 | |
| Total N (%) | 0.068 ± 0.01 | 0.058 ± 0.01 | 0.419 | |
| C (%) | 1.58 ± 0.09 | 1.10 ± 0.16 | 0.029 | |
| Bray II P (mg kg−1) | 17.6 ± 3.8 | 8.0 ± 0.7 | 0.039 | |
| K (mg kg−1) | 71 ± 14 | 30 ± 4 | 0.019 | |
| Ca (cmol+ kg−1) | 2.50 ± 0.33 | 1.70 ± 0.15 | 0.057 | |
| Mg (cmol+ kg−1) | 1.10 ± 0.15 | 0.67 ± 0.04 | 0.022 | |
| K (cmol+ kg−1) | 0.18 ± 0.03 | 0.07 ± 0.01 | 0.016 | |
| Na (cmol+ kg−1) | 0.20 ± 0.02 | 0.12 ± 0.01 | 0.013 | |
| Cu (mg kg−1) | 1.52 ± 0.83 | 0.17 ± 0.01 | 0.143 | |
| Zn (mg kg−1) | 7.41 ± 5.21 | 0.23 ± 0.05 | 0.205 | |
| Mn (mg kg−1) | 43.4 ± 25.4 | 2.4 ± 0.5 | 0.145 | |
| B (mg kg−1) | 0.44 ± 0.04 | 0.19 ± 0.01 | <0.001 | |
| Fe (mg kg−1) | 61 ± 13 | 38 ± 3 | 0.114 | |
| S (mg kg−1) | 9.92 ± 1.53 | 3.64 ± 0.82 | 0.007 |
The results are the mean ± s.e. (n = 5) and P values were calculated using Student’s t-tests (values in bold are significant, P < 0.05). The larger value for significantly different variables is indicated in Bold.
Wind effect on seeds
Protea repens seeds have hair radially arranged to form a cone with the seed at its apex (Fig. 2). When dropped into a wind stream, all seeds orientated so that the seed faced into the wind with the hair trailing downwind, regardless of whether the seed awn was present or not. Seeds placed on the sand surface with the seed facing downwind all re-orientated so that the seed faced into the wind. Over three trials at varying wind speeds of the 90 seeds in groups exposed to winds, only three left the groups, which strongly indicates that seeds trap each other and then as a group resist being moved.
Discussion
All serotinous Proteaceae seedlings in the survey, regardless of dispersal type or sexual system, were strongly clumped rather than uniformly or randomly dispersed and adults were less clumped than seedlings. This pattern did not change when plots without seedlings and/or parents were excluded from the analysis (log(variance:mean) seedling = 2.3 and parents = 4.2). Unfortunately, there are no winged species that are hermaphrodites and most of the hairy species are hermaphrodites. This means it is not possible to disentangle sexual systems from dispersal as determinants of clumping. Assuming stable co-existence, the mean fitness of Leucadendron and Protea must be the same and therefore a female Leucadendron must produce double the number of seedlings of a Protea (Midgley et al. 2019). This implies more seeds are released from dioecious Leucadendron females than from hermaphroditic Protea individuals. For example, mean seed stores can be nearly 2500 seeds for two Protea species and 15,000 seeds for females of two co-occurring Leucadendron species (Mustart et al. 1994). Given that only half the adult Leucadendron population, the females, produce and release seeds, our results of similar levels of clumping in Protea and Leucadendron indicate that dispersal must be more limited in Protea.
We observed that most individuals have conspecifics as nearest neighbours. Given intense clumping, this high level of intraspecific competition among seedlings is an important evolutionary process. For example, it has implications for understanding the balance between the importance of adult traits and seedling traits. We predict rapid seedling growth to overcome intense clumping is an important trait and this is at the expense of adult traits, e.g. those used by Treurnicht et al. (2020). For example, wood density is negatively correlated with growth rates (Wright et al. 2010) but low wood density may compromise longevity. Also, given that most seedling interactions are among conspecifics, this reduces the importance of niche differentiation in ecophysiological traits for co-existence. Self-thinning strongly reduces the impact of variability in recruitment levels after fires, as seedlings will be lost to competition as a function of initial number. In Australia, Enright and Lamont (1989) found that thinning effectively equalised seedling to parent ratios after fires in different seasons. Thus, high seedling numbers do not translate into similarly high parent numbers, and conversely high parent numbers do not translate into high seedling numbers. As this self-thinning seems to represent a huge fitness cost, the question arises as to what causes clumping and what, if any, advantages there might be to clumping.
Regarding the proximate causes of seedling clumps, Leucadendron and Protea seeds are single-seeded fruits and therefore clumping does not result from dispersal of multi-seed units. Long-distance dispersal is unlikely to produce this clumped distribution at the 1 m2 plot scale, because it will reduce dispersal overlap from different parent plants and will thus cause a few sites (of 1 m2) to have one or a few seedlings and most others to have none. Genotyping could resolve whether the low dispersal distances suggested by us are appropriate or whether long distances suggested by Schurr et al. (2005) more accurately predict dispersal distances. The post-fire recruitment method (counting seedlings and parents in small (1 m2) plots) devised by Bond (1984) depends on low seed dispersal. If seeds dispersed far, it would not make sense to measure seedling to parent ratios as many seeds would disperse far away from parents. Thus, in a forest with bird seed dispersal, it would not be efficient to measure seedling:parent ratios in small plots. Clumping partially arises out of limited dispersal distances, although potential dispersal distances become less important if the seeds either form clumps or are trapped by landscape heterogeneities (Figs 1, 2). Hairy Protea seeds do aggregate, and this reduces seed dispersal distances along the ground. Our analysis suggests that safe sites are not limiting (i.e. that obstacles to seed movement on the ground are ubiquitous) and seed clumps indicate that seed dispersal distances of hairy seeds are low. This is in strong conflict with the modelling of Schurr et al. (2005).
What then is the ultimate reason for clumping given that the resulting clumping appears to ‘waste’ seedlings? One possibility is that seed clumping reduces seed predation, especially by birds. Hairy seeds and winged seeds match local soil and debris colour, thus potentially protecting them against granivores that search using colour vision (White and Midgley 2022). In addition, clumps are co-located with significant deposits of fire-derived ash and debris (leaves, stems, ash and charcoal) that result in a nutrient-enriched mineral soil below the patch. Preliminary information indicates that hairy seeds are not buried deeply (mean seedling emergence depth of P. repens 2.05 cm, n = 30) but this requires further analysis to determine whether this is active (e.g. due to movement of hairs) or passive burial. The extractable P values measured here using Bray II extraction with and without ash/debris were 70 and 30%, respectively, of global average values (Olsen P = 26 mg kg−1; McDowell et al. 2023). In this particularly P-scarce environment (Stock and Verboom 2012), it could be important to seedlings that soil P is enriched >2-fold by the accumulation of ash and debris. However, the competition resulting from intense clumping we found may subsequently, at least partially, over-ride the benefits to seedlings (e.g. Lamont et al. 1993).
Our data allow for an overall demographic perspective of serotinous Cape plants that gives insight into the evolution of various life histories, such as an explanation for the predominance of reseeding Proteaceae versus resprouters (Le Maitre 1992). Serotinous species are functionally monocarpic; they flower, set and store seeds over many years but these seeds typically only germinate in the first winter (winter-rainfall region) after fire kills parents. This provides the opportunity for calculating the chance that a seedling becomes a parent. Given approximately stable population sizes, initial seedling to parent ratios of 6–18.3 (Fig. 5) indicate an extremely high chance (~10–20%) of seedlings surviving the first year or two and becoming an adult. For comparison, Moles and Westoby (2004) suggest that 0.5% survival would be high. Thus, despite a high extent of clumping, the survival of seedlings is greater than 1 in 10. This seems high compared with the small percentage of seedlings of forest tree species that survive to maturity. Regarding the transition from seed to seedling, Maze and Bond (1996) argued for conversion rates of ~2:1, compared with 5–10:1 for Australian proteoids and 200–2000:1 for chapparal species. Given the extensive mats of seeds and the average seedling number (106 ± 26 m−2; Fig. 1), the ratio, at least for P. repens, is likely to be at the higher end of this range. Their 2:1 ratio combined with our ratio suggests a 5% chance that a seed becomes an adult plant, which appears to be a very high success rate. However, the ratio obtained by Maze and Bond (1996) may have overestimated this rate because mean seed stores of some Cape proteoid individuals can be nearly 2500 seeds for two Protea species and 15,000 seeds for females of two Leucadendron species (Mustart et al. 1994). This suggests a seed:adult ratio of between 1:250 and 1:750 (given Leucadendron is dioecious). This indicates that seed to seedling is a more unlikely transition than seedling to parent. Clumping may thus be a response to high seed deaths outside of clumps,intr for example, by causing local predator satiation (e.g. Bond 1984) or overcoming nutrient limitations. Nevertheless, a transition from seed to adult of say 1:500 is extremely high and may favour the re-seeding life history that predominates in Cape Proteaceae. Also, because clumping of seedlings is intense, the fewer seedlings produced by sprouters compared with seeders (Le Maitre 1992) may select against the sprouting life history.
Lamont et al. (1993) showed that seedling density in litter patches in western Australia was ~40-fold that in sand but that litter patches only contributed 12.6% of the area (sand the remainder). The nett outcome of this was those competitive interactions over the first year had already led to a 57% mortality rate in the litter patches (from an initial mean of 50.7 seedlings m−2) versus 20% mortality from initial mean density of 8.8 m−2 in sand. This means that the relative ratio of seedlings in patches:sand is 275:615 (1:2.24) and one can expect self-thinning to continue in the seedlings clumped in litter patches, thus increasing this ratio. At our site, we found 17.4-fold more seedlings in ash/debris patches than without ash/debris, but those patches occupied 49% of the landscape. This may indicate that competitive interactions are lower in our study area relative to that of Lamont et al. (1993).
However, this clumping does not mean that seedlings resulting from dispersal to litter patches are unsafe or that dispersal is maladaptive. Firstly, it is a widespread phenomenon (winged seeds occur in Australian Proteaceae, and both winged and hairy seeds of Cape Proteaceae) and thus cannot be unsafe dispersal or maladaptive. Secondly, seedling numbers regularly exceed adult numbers, indicating that adult plants are likely replacing themselves owing to successful seedling establishment. Thirdly, adults too are clumped, indicating that thinning does not reduce densities to less than 1 m−2 if there is more than one seedling. Fourthly, the transition from seed to seedling is quite successful. Finally, there is no seed dispersal mechanism that can lead to an even spread of seeds, such as in both sites of environmental heterogeneities (e.g. obstacles, depressions and litter) and homogeneities (e.g. smooth sand). This is especially the case when there is no selection for long-distance dispersal. Thus, clumpedness reflects a kind of directed dispersal to litter sites that appears to benefit some seeds (e.g. from burial, protection from predation) and seedlings (e.g. from nutrients after seed nutrients have been depleted). When seedling numbers are high, loss through intraspecific competition in clumps is an unavoidable consequence that reduces variation in nett seedling:adult replacement rates.
Finally, from a conservation management perspective, our analysis of clumping suggests that parent:seedling ratios that have been widely used as an index of fire suitability (e.g. Bond et al. 1984) can be a poor measure because they do not consider clumping and subsequent self-thinning. For recruitment success estimates, we suggest that the percentage of plots with at least one seedling is also recorded.
Acknowledgements
We thank CapeNature for access to these data, Dr Andrew Turner (CapeNature) for assistance with sample collection and Professor Adam West for the photograph used in Fig. 2.
References
Bond WJ (1984) Fire survival of Cape Proteaceae – influence of fire season and seed predators. Vegetatio 56, 65-74.
| Crossref | Google Scholar |
Bond WJ (1988) Proteas as ‘tumbleseeds’: wind dispersal through the air and over soil. South African Journal of Botany 54, 455-460.
| Crossref | Google Scholar |
Bond WJ, Slingsby P (1983) Seed dispersal by ants in shrublands of the Cape Province and its evolutionary implications. . South African Journal of Science 79, 231-233 https://hdl.handle.net/10520/AJA00382353_2316.
| Google Scholar |
Bond WJ, Vlok J, Viviers M (1984) Variation in seedling recruitment of Cape Proteaceae after fire. Journal of Ecology 72, 209-221.
| Crossref | Google Scholar |
de Freitas Alves G, de Santana DG (2021) Why do traditional dispersion indices used for analysis of spatial distribution of plants tend to become obsolete? Population Ecology 64, 80-92.
| Crossref | Google Scholar |
Enright NJ, Lamont BB (1989) Seed banks, fire season, safe sites and seedling recruitment in five co-occurring Banksia species. Journal of Ecology 77, 1111-1122.
| Crossref | Google Scholar |
Fister W, Iserloh T, Ries JB, Schnidt R-G (2012) A portable wind and rainfall simulator for in situ soil erosion measurements. Catena 91, 72-84.
| Crossref | Google Scholar |
Heilbuth JC, Ilves KL, Otto SP (2001) The consequences of dioecy for seed dispersal: modelling the seed-shadow handicap. Evolution 55, 880-888.
| Crossref | Google Scholar | PubMed |
Hurlbert SH (1990) Spatial distribution of the Montane Unicorn. Oikos 58, 257-271.
| Crossref | Google Scholar |
Kraaij T, Cowling RM, van Wilgen BW, Schutte‐Vlok AL (2013) Proteaceae juvenile periods and post‐fire recruitment as indicators of minimum fire return interval in eastern coastal fynbos. Applied Vegetation Science 16, 84-94.
| Crossref | Google Scholar |
Lamont BB, Witkowski ETF, Enright NJ (1993) Post-fire litter microsites: safe for seeds, unsafe for seedlings. Ecology 74, 501-512.
| Crossref | Google Scholar |
Le Maitre DC (1992) The relative advantages of seedling and sprouting in fire-prone environments: a comparison of life histories of Protea neriifolia and Protea nitida. In ‘Fire in South African Mountain Fynbos’. (Eds BW van Wilgen, DM Richardson, FJ Kruger, HJ van Hensbergen) pp. 123–144. (Springer-Verlag: Berlin, Germany)
Maze KE, Bond WJ (1996) Are Protea populations seed limited? Implications for wildflower harvesting in Cape fynbos. Australian Journal of Ecology 21, 96-105.
| Crossref | Google Scholar |
McDowell RW, Noble A, Pletnyakov P, Haygarth PM (2023) A global database of soil plant available phosphorus. Scientific Data 10, 125.
| Crossref | Google Scholar | PubMed |
Midgley JJ (1989) Season of burn of serotinous fynbos Proteaceae: a critical review and further data. South African Journal of Botany 55, 165-170.
| Crossref | Google Scholar |
Midgley JJ, West AG, Cramer MD (2019) Female and male costs of reproduction must be equal in dioecious Cape plant genus Leucadendron (Proteaceae). Australian Journal of Botany 67, 517-520.
| Crossref | Google Scholar |
Moles AT, Westoby M (2004) Seedling survival and seed size: a synthesis of the literature. Journal of Ecology 92, 372-383.
| Crossref | Google Scholar |
Mustart PJ, Cowling RM, Dunne TT (1994) Reproductive traits of two closely related species-pairs on adjacent, different soil types in South African Fynbos. Vegetatio 111, 161-171.
| Crossref | Google Scholar |
Oksanen J, Simpson G, Blanchet F, Kindt R, Legendre P, Minchin P, O’Hara R, Solymos P, Stevens M, Szoecs E, Wagner H, Barbour M, Bedward M, Bolker B, Borcard D, Carvalho G, Chirico M, De Caceres M, Durand S, Evangelista H, FitzJohn R, Friendly M, Furneaux B, Hannigan G, Hill M, Lahti L, McGlinn D, Ouellette M, Ribeiro Cunha E, Smith T, Stier A, Ter Braak C, Weedon J (2022) _vegan: Community Ecology Package_. R package version 2.6-4, Available at https://CRAN.R-project.org/package=vegan
Pagel J, Treurnicht M, Bond WJ, Kraaij T, Nottebrock H, Schutte-Vlok A, Tonnabel J, Esler KJ, Schurr FM (2020) Mismatches between demographic niches and geographic distributions are strongest in poorly dispersed and highly persistent plant species. Proceedings of the National Academy of Sciences of the United States of America 117, 3663-3669.
| Crossref | Google Scholar | PubMed |
R Core Team (2022) ‘R: A language and environment for statistical computing.’ (R Foundation for Statistical Computing: Vienna, Austria) Available at https://www.R-project.org/
Reich PB (2014) The world-wide ‘fast–slow’ plant economics spectrum: a traits manifesto. Journal of Ecology 102, 275-301.
| Crossref | Google Scholar |
Schurr FM, Bond WJ, Midgley GF, Higgins SI (2005) A mechanistic model for secondary seed dispersal by wind and its experimental validation. Journal of Ecology 93, 1017-1028.
| Crossref | Google Scholar |
Stock WD, Verboom GA (2012) Phylogenetic ecology of foliar N and P concentrations and N:P ratios across mediterranean-type ecosystems. Global Ecology and Biogeography 21, 1147-56.
| Crossref | Google Scholar |
Treurnicht M, Pagel J, Tonnabel J, Esler KJ, Slingsby JA, Schurr FM (2020) Functional traits explain the Hutchinsonian niches of plant species. Global Ecology and Biogeography 29, 534-545.
| Crossref | Google Scholar |
White JDM, Midgley JJ (2022) Cryptic polymorphic Proteaceae seeds reduce detection by visually cued predators on post-fire soils. South African Journal of Botany 146, 538-545.
| Crossref | Google Scholar |
Wright SJ, Kitajima K, Kraft NJB, Reich PB, Wright IJ, Bunker DE, Condit R, Dalling JW, Davies SJ, Diaz S, Engelbrecht BMJ, Harms KE, Hubbell SP, Marks C, Ruiz-Jaen MC, Salvador CM, Zeanne AE (2010) Functional traits and the growth–mortality trade-off in tropical trees. Ecology 91, 3664-3674.
| Crossref | Google Scholar |


