A summer and winter’s tale: factors influencing avian community composition and species abundances in lowland subtropical floodplain forests in central eastern Australia
Mark Fitzgerald A , Simon Gorta B and Robert M. Kooyman C D
C D
A Mark Fitzgerald Ecological Consultant, Mullumbimby, NSW 2482, Australia.
B Centre for Ecosystem Science, School of Biological, Earth and Environmental Sciences, University of New South Wales, NSW 2052, Australia.
C Department of Biological Sciences, Macquarie University, NSW 2019, Australia.
D Corresponding author. Email: robert.kooyman@mq.edu.au
Pacific Conservation Biology 28(3) 247-260 https://doi.org/10.1071/PC21009
Submitted: 24 February 2021 Accepted: 21 June 2021 Published: 9 July 2021
Journal Compilation © CSIRO 2022 Open Access CC BY
Abstract
The ecology of avian community assembly in subtropical climate areas with seasonal and year-to-year variability is complex and poorly understood. To test for variation in year–year and seasonal (summer–winter) avian community composition and species abundances, we established 10 transects (200 m long) and sampled twice yearly for 7 years. To differentiate year–year and seasonal (summer–winter) patterns from the potential disturbance effects caused by human activities associated with music festivals (events), we monitored community composition and species abundances on sites close to disturbance areas (impact) and sites distant from disturbances (control). Impacts from large scale music events included loud noise, and thousands of vehicle and people movements on a daily basis. Raw count, abundance-weighted, and feeding guild data were analysed using multivariate and univariate methods. Seasonal (summer–winter) patterns of food resource availability in dominant forest trees (fruit and nectar resources) were identified. We found strong signals for the influence of seasonality and seasonal resource availability on community composition and feeding guild representation (nectivores and frugivores). We detected somewhat weaker effects for location relative to disturbance (control vs impact) and weak effects for sample timing associated with disturbance from the events. Avian community composition showed high similarity between control and impact sites, was dynamic in space and time (year to year) and showed strong local and regional trends in response to seasonality. Avian species abundances were greater in less disturbed (control) forest habitats, highlighting the fundamental value of conservation reserves in protecting higher quality avian habitats.
Keywords: avian community assembly, feeding guilds, human activity, lowland subtropical forest, multivariate generalised linear models, seasonal resources, time-series.
Introduction
Fauna communities inhabiting forest ecosystems represent diverse assemblages of species and foraging guilds (Garnett et al. 2015). Our current understanding of community assembly and the functional level shifts associated with different forest disturbances is limited (Wilson et al. 2020). While species and assemblage level responses to large-scale and severe disturbances such as deforestation and conversion of habitat to urban and agricultural use have been well-documented (Hansen et al. 2013; Bregman et al. 2014), the effects of less conspicuous human disturbances to these ecosystems, such as recreational use of remaining natural areas, has only recently begun to be investigated (Naidoo and Burton 2020).
Resource quality and abundance, and predation risk, can influence how animals use and are distributed across available habitat. The former favour choices that optimise energy and nutrient input, and the latter reflects survival (Frid and Dill 2002; Cooper and Frederick 2007). Those factors set the feeding strategies and choices made by species into a framework of risk and trade-offs (Wilson et al. 2020; Cunningham et al. 2021). The extent to which human activities are perceived by animals as a threat influences their reaction to humans as potential predators (Price 2008; LaManna and Martin 2016; Gaynor et al. 2019). However, the effects and ecological consequences of human activities that induce shifts in animal behaviour remain largely under-investigated (Wilson et al. 2020), and the ecology and dynamics of community assembly are variable, complex, and often poorly understood (Ricklefs 1987, 2011; Mac Nally 1996; Naidoo and Burton 2020).
Avian communities in forests occupy a range of habitats and foraging niches (Garnett et al. 2015) and provide an ideal study system to assess the possible disturbance effects of human activities at species, functional group, and community levels. In forest ecosystems, avian avoidance responses may reflect both foraging behaviour and the strata from they feed. In that context, birds feeding in lower strata and on the ground may be more affected by the perception of risk generated by different terrestrial human activities (Gaynor et al. 2019). In contrast, when food resource quality is high and the perceived risk of harm from human disturbance is low, birds are less likely to leave their foraging or breeding grounds (Frid and Dill 2002; Cooper and Frederick 2007); for example, when tree canopy nectar and fruit feeders are high above pedestrian-based terrestrial human activities. Logically, avian avoidance of human-induced disturbance might also be expected to influence habitat selection and distribution more when disturbances are long-lasting and/or intense (Price 2008; LaManna and Martin 2016; Gaynor et al. 2019). Avian community composition also shifts seasonally, with many species in temperate climates undertaking annual migrations to minimise exposure to resource poor winters and adverse thermal regimes (Somveille et al. 2015). Species in regions with less severe climatic variation often move seasonally and follow resource availability in the landscape (Howe 1984). For example, variation in the abundance of resources such as nectar, fruit and insects, and the patchy distribution of forest, has been shown to result in local and regional scale avian nomadism in subtropical eastern Australia (Howe et al. 1981). Avian communities can be dominated by non-equilibrium dynamics and show great spatial and temporal variation (Mac Nally 1996; sensu May 1977). In such cases, long time series rather than repeated intra-seasonal surveys are required to establish trends of appropriate explanatory power and predictive use for impact assessment incorporating birds (Thogmartin et al. 2007). In addition, tests of effects need to be able to differentiate the relative influence of explanatory variables (Wang et al. 2012).
The extent of clearing of lowland forests in subtropical eastern Australia has resulted in many of these ecosystems, including most of those sampled here, being listed as endangered ecological communities (NSW Biodiversity Conservation Act 2016). We investigated the temporal dynamics of avian communities in these subtropical forest habitats over 7 years, in summer and winter. In addition, using measures of avian community composition and species abundances, we investigated the impacts of two annual music festival events (one in summer, one in winter) at several locations in the study area.
The impacts of human recreational use of natural areas have often been evaluated relative to evolved anti-predator responses to threatening stimuli, such as loud noises and rapidly approaching objects. The disturbance-risk hypothesis suggests that from an evolutionary perspective, disturbance stimuli should be analogous to predation risk, and should induce similar responses (Frid and Dill 2002). Disturbance from music events includes loud noise, constant vehicle movements, and tens of thousands of people resident for multiple days. The weeks prior to and following events are characterised by the construction and removal of temporary infrastructure, which involves hundreds of people and vehicle movements per day. As a consequence, we expected the influence of disturbance from human activities associated with such events to be both detectable and significant in relation to species responses that affect community composition, species abundances, and feeding guild representation. In addition, we expected seasonal variation (summer–winter) (Hawkins 2014), and year-to-year and seasonal resource availability (Malizia 2001; Holt 2008) to be major factors influencing avian assemblage composition in these forest communities. In response to shifts in seasonal resources, we expected that high levels of nomadism might lead to high levels of convergence of avian assemblages in smaller (impact) and larger (control) forest patches (Howe et al. 1981; Holt 2008). We allocated species to feeding/foraging guilds to further test for seasonal avian assemblage variation reflecting feeding resources, and specific guild responses to seasonal resource availability.
Materials and methods
Study area
The study area is in the north-east of NSW and represents a mosaic of remnant lowland forest, including conservation reserve areas, and agricultural lands. Parts of the study area have a history of agriculture including cattle grazing, sugar cane and banana growing, and bee-keeping. The research area includes a 97 ha music festival (event) site located at Wooyung in the Byron Shire Local Government area. Climate is coastal subtropical with mean annual rainfall of 1650 mm, 70% of which falls in late summer. Rainfall records for 2012–2020 from the nearest Australian Bureau of Meteorology weather station (North Murwillumbah–Tweed River No. J8186; 25 km from study site) with complete data ranged from 747 to 2046 mm annually, with a mean of 1488 mm for the study period. The study area experiences extreme climatic events, including storms, cyclones and droughts, and has an elevational range from sea level to ~40 m, and lower-lying areas are flood prone. A low east-west ridge separates two coastal floodplains, one to the north and one to the south. The soils of the area include free-draining coastal sands, sand over clay pans (drainage impeded), heavy black clays (drainage impeded), and hillslope clay loams (derived from meta-sediments).
One of several NSW National Parks and Wildlife Service managed conservation reserves on the floodplains of the region, Billinudgel Nature Reserve (BNR; 713 ha) was the focus of the sampling presented here. We sampled a diverse range of vegetation types representative of the coastal vegetation of the region and present in the reserve. The vegetation sampling allowed us to characterise the range of habitat variables and the dominant forest types to inform the transect location selections. The results of floristic sampling of the forests of the area are provided as a dendrogram (see Fig. S1). The vegetation sampled by the transects included: Swamp Sclerophyll Forest dominated by Broad-leaved Paperbark (Melaleuca quinquenervia, Myrtaceae); Swamp Box (Lophostemon suaveolens, Myrtaceae), Casuarina glauca (Casuarinaceae) and Swamp Mahogany (Eucalyptus robusta, Myrtaceae). Both control and impact samples included a transect sampling the Blackbutt forest type (Eucalyptus pilularis with mesic understory), and a Brush Box (Lophostemon confertus) and Pink Bloodwood (Corymbia intermedia, Myrtaceae) association on low hillslopes largely surrounded by low-lying Paperbark (Melaleuca quinquenervia) dominated swamp forest.
The predominantly cleared (grassed) event area surrounded several smaller patches of swamp sclerophyll forest dominated by Melaleuca quinquenervia and adjoined areas of subtropical floodplain forest dominated by species in Eucalyptus (Myrtaceae). The event area was regularly mowed and had an extensive road network and drainage infrastructure, with structures including communications towers and visitor utility/amenity buildings that facilitate on-site camping. The number of people present each day during the 4 days of each event (two events per year) was ~25 000. The site had no permanent residents, but maintenance staff were present most days.
Bird surveys
To test for variation in year–year and seasonal (summer–winter) avian community composition and species abundances in the lowland floodplain forests of the study area we established 10 transects (200 m long) in 2013 and sampled twice yearly for 7 years. The sampling method was based on three samples (before, during and after each event) of each of 10 transects, totalling 90 samples for each event. Sampling duration was 6 years for the summer sample and 7 years for the winter sample. Due to weather and other factors over the 7-year period, the actual number of samples was 521 of a possible 540 for summer, and 627/630 for winter, making a total of 1148 from a scheduled total of 1170 (Table 1).

|
To differentiate year–year and seasonal (summer–winter) patterns from the potential disturbance effects caused by human activities, we monitored the same 10 transects in relation to community composition and species abundances on sites in disturbance areas (impact) and sites distant from disturbances (control). Impact transects were located close to event areas, with two positioned in isolated forest blocks in the actual event area, and two located directly adjacent in the adjoining nature reserve (BNR). The six control transects were located approximately 2 km to the east of the impact sites deeper into BNR. Where possible, the vegetation communities sampled in control and impact sites were replicated in terms of forest type, structure, and floristics. In all cases locations were matched in terms of forest type and dominant tree species. To further explore the response of the local and regional avian fauna to year–year and seasonal (summer–winter) resource availability and intermittent human disturbances, we allocated species to feeding guilds. Here, we test our expectations against a working null hypothesis of no difference between years, summer and winter, control vs impact sites, and samples representing before (B), during (D) and after (A) events (Underwood 1994).
Before (B) equates with the time prior to the bump-in period preceding the events. The bump-in is around 3 weeks in duration and is characterised by hundreds of truck and vehicle movements daily, extensive site preparation (e.g. tractor mowing), the placement of large temporary structures (performance stages and marquees), and hundreds of people movements per day. All (B) samples were completed prior to the commencement of the bump-in (i.e. the transect samples were completed ~1 week prior to bump-in activities commencing). During (D) equates with the actual time period of the events with hundreds to thousands of vehicle movements per day, 25 000 people resident or on site each day (event numbers were capped at 25 000 per day), and loud music from midday (1200 hours) to midnight (0000 hours) for 4 days. After (A) equates with a period of 2–3 weeks after the events, referred to as bump-out. Bump-out is characterised by tens of thousands of people leaving the site, initially thousands then hundreds of truck and vehicle movements, extensive rubbish removal, site cleaning and repair (e.g. tractor work), the removal of temporary structures, and hundreds of people movements related to work on-site per day. All (After) samples were completed after the bump-out was completed (i.e. the transect samples were completed ~1 week after the bump-out activities were completed). The time between (B) and (A) samples was 9 weeks (comprising 4 weeks before the event, 1 week of the event, and 4 weeks after the event).
At sampling at each transect (n = 10) before (B), during (D) and after (A) events in winter and summer, birds were counted in a 20-min early morning survey period if seen or heard within 50 m either side of a flagged (marked) central transect line. This represents a consistent time- and spatially-constrained census strategy (Bibby et al. 2000). At the 10 transect sites, three separate early morning samples were undertaken on consecutive days for each of the (B), (D) and (A) counts. Counts were usually undertaken simultaneously, initially with three, and later two observers. In all cases, the data acquired reflect the number of birds and species that an experienced observer could detect under the circumstances. To further equalise the survey effort, repeat measures (three separate samples for each of B, D and A counts) were used, and sampling was further randomised by rotating the different observers between transects to remove bias and to ensure consistency in and repeatability of the counts. We acknowledge that bird counts do not represent a precise transect-based census of the avifauna in a location. However, the method is widely used (Bibby et al. 2000) and accepted as capable of representing avian community composition and abundance. In some cases where large numbers of a single species were present (e.g. honeyeaters moving and vocalising in tall blossoming eucalypts), only estimates of the number of individual birds present could be made. In all cases, the application of transect sampling methods included protocols to avoid over-representing species abundances. The protocols included directions to avoid double counting individuals moving along the transect, avoid the over-estimation of numbers in large feeding aggregations, and ignore individuals detected outside the designated transect sample extent, or time allocated for sampling.
A yearly and ubiquitous irruptive emergence of cicadas in the Australian summer required a cicada noise score to be allocated based on the duration of noise during each count. Temperature variations between years influenced cicada noise levels, and both impact and control transects were affected. More generally, climatic variations between years affected noise levels from ocean swells in coastal transects (controls), and surveys were not carried out in extremely windy or wet conditions.
The optimal time to count birds (early mornings) did not overlap with the times of highest intensity of daily activities related to the events. Our working assumption was that if noise or other factors were having an effect over the duration of the event (including B-D-A and in control vs impact sites), this would be evidenced in the comparative methods used to analyse community composition and the species counts from day to day. That is, the data would reflect any changes in bird activity the morning following the most intense activities during events, and across the duration of the sampling including before, during, and after events.
Analysis of bird responses to resource availability was based on allocating species to foraging guilds reflecting their primary dietary preferences and included nectivore, frugivore, insectivore, carnivore, omnivore and herbivore. The single herbivorous species (Pacific Black Duck Anas superciliosa) was omitted from subsequent analyses due to the low number of records of this species in the sample.
In the presentation of results and discussion, we use the scientific and common names provided by Birdlife Australia (https://birdlife.org.au/).
Statistical analyses
Assemblage level data were first entered into a repeated measures site by species abundance-weighted matrix of bird counts. After square root transformation of the rectangular matrix, a triangular resemblance matrix was generated using pairwise binomial deviance as the resemblance measure (Gower 1966; Clarke et al. 2014). The relationships among sites and samples and species abundance were then analysed in a non-metric multidimensional scaling ordination (Fig. 1). Identified factors included seasons (summer–winter), and B-D-A samples split by impact and control sites. Controls represented similar habitats in the nearby nature reserve (BNR) and provided relatively undisturbed conditions. Additional analyses were undertaken using species counts grouped into feeding guilds and using the same analysis methods described above for count data.
Average taxonomic distinctness (AvTD, Clarke and Gorley 2015) was used to measure pairwise taxonomic distances between species in assemblage samples and test for phylogenetic clustering (Fig. 2). AvTD is known to be orthogonal to richness and, importantly, is unaffected by either sample size or sampling effort. The measure describes the relatedness of taxa within a sample at a given richness and uses a simple taxonomic (relatedness) tree with equalised branch lengths, based on the background list of species, genera and families (see Table S1). Expected values at a given richness represent a null (no taxonomic structure) derived from 1000 random draws from the available pool of species. Lower values outside the 95% confidence intervals (CIs) for AvTD in relation to random draws from the full pool occur when species assemblages have lower taxonomic breadth at a level of richness than expected under a null model. In that case, species are more related than expected by chance (clustered). Higher values reflect greater taxonomic breadth at a given richness in relation to the null (species are less related than by chance) and this equates with overdispersion or evenness.
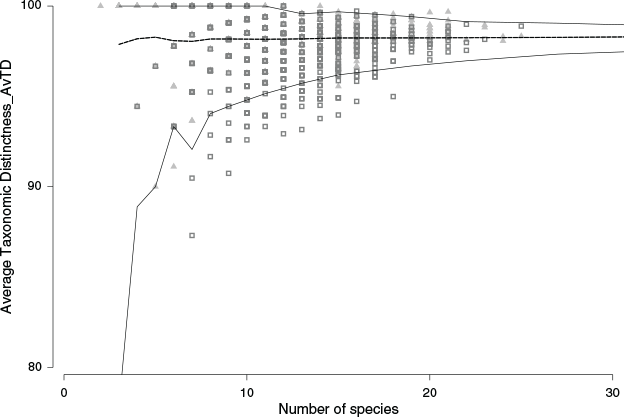
|
Multivariate analysis of bird species was completed using the ‘mvabund’ package (Wang et al. 2012) in the R statistical environment (R Core Team 2020). This method (using the manyglm function) fits a single Generalised Linear Model to each response variable with a common set of predictors, generating significance values by resampling (999 permutations), thus enabling greater statistical power and the ability to account for the mean-variance relationship of the data (Wang et al. 2012). Raw counts of the species recorded on surveys were included in the analysis (species level).
Additional analyses were undertaken using the species counts grouped into their respective feeding guilds. To test for effects of multiple response variables we used the following model formula to determine multivariate responses of the avian community across different years (Year), seasons (Season, i.e. summer vs winter) and disturbance (Disturbance, i.e. control vs impact). We used the interaction between disturbance and the three stages of disturbance (Disturbance × Stage, i.e. B-D-A) to determine if events affected community composition:
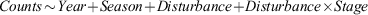
A negative binomial distribution was used for both models, as is appropriate for count data, and assessed using simulated Dunn-Smyth residuals (Wang et al. 2012; Warton et al. 2016; Brooks et al. 2017). Univariate responses (responses of individual species and functional groups) were identified using the p.uni = adjusted function, which incorporates the Holm method to correct significance values for multiple hypothesis testing (Wang et al. 2012). Statistical support for rejection of the null was determined at P < 0.1, and further separated into weak (0.05 ≥ P < 0.1) and strong (P < 0.05) statistical support. Tabulated results show residuals (d.f.), deviance and P-values for multivariate model outputs, and deviance and P-values for the univariate.
Results
Over 7 years of surveys (seven winter and six summer), we detected a total of 37 427 individuals representing 125 avian species, 97 genera and 44 families. Of the 125 species, 14 were recorded only once (singletons) in surveys. The avian community showed strong, and statistically significant seasonal (summer–winter) responses over the 7-year study period (Fig. 1; Tables 1–4). We detected weaker effects for disturbance reflecting the combination of sample locations (control vs impact transects) and times (B-D-A) (Tables 1–4; for B-D-A results, see Table S2). Most community assemblage samples showed expected taxonomic structure (AvTD) for species richness levels. However, a small number of samples were positioned outside 95% CIs for expected taxonomic diversity at specific species diversity levels. Both over-dispersion (AvTD higher than expected, mostly summer samples) and under-dispersion (AvTD less than expected representing mostly winter samples) were observed (Fig. 2). Over- and under-dispersion included samples from both control and impact sites across years, seasons, and the timing of disturbances (B-D-A) (for additional results, see Fig. S2.1–3).
At both species and feeding guild levels, there was strong statistical support for interannual and seasonal differences in community composition, as well as differences between control and impact sites, and the interaction between sites and the different stages of the disturbance related to events (Fig. 1; Tables 1, 2).
We found statistical support for interannual variation in abundance for 17 bird species (~14%, Table 4), including Australasian Figbird (Sphecotheres vieilloti), Black-faced Cuckoo-shrike (Coracina novaehollandiae), Brown Thornbill (Acanthiza pusilla), Channel-billed Cuckoo (Scythrops novaehollandiae), Great Egret (Ardea alba), Eastern Spinebill (Acanthorhynchus tenuirostris), Little Lorikeet (Glossopsitta pusilla), Noisy Friarbird (Philemon corniculatus), Rainbow Bee-eater (Merops ornatus), Rainbow Lorikeet (Trichoglossus haematodus), Torresian Crow (Corvus orru), White-bellied Cuckoo-shrike (Coracina papuensis), White-browed Scrubwren (Sericornis frontalis), White-throated Honeyeater (Melithreptus albogularis), and White-throated Treecreeper (Cormobates leucophaea). We found weak statistical support for interannual variation in the abundance of Oriental Dollarbird (Eurystomus orientalis) and White-throated Gerygone (Gerygone albogularis), and no statistical support for interannual variation in abundance for the remaining species (~84%) (Table 4).
There were three feeding guilds for which there was strong statistical support for interannual variation in abundance, specifically: frugivores, granivores, and nectivores (Table 3). There was no statistical support for interannual variation in abundance for carnivores, insectivores, and omnivores (Table 3).
We found statistical support for seasonal variation in abundance for 45 species (~36%) in the study region (Table 4). Of these, 24 species (19%) showed greater abundances during summer than winter, while 21 species (17%) showed greater abundances during winter than summer (Table 4; Fig. 2). We found strong statistical support for greater abundance in summer than winter for Australian King-parrot (Alisterus scapularis), Brown Goshawk (Accipiter fasciatus), Brush Cuckoo (Cacomantis variolosus), Channel-billed Cuckoo, Eastern Rosella (Platycercus eximius), Brown-capped Emerald-Dove (Chalcophaps longirostris), Great Egret, Grey Butcherbird (Cracticus torquatus), Grey Shrike-thrush (Colluricincla harmonica), Laughing Kookaburra (Dacelo novaeguineae), Leaden Flycatcher (Myiagra rubecula), Little Corella (Cacatua sanguinea), Little Wattlebird (Anthochaera chrysoptera), Oriental Dollarbird, Pacific Baza (Aviceda subcristata), Eastern Koel (Eudynamys orientalis), Rose-crowned Fruit-dove (Ptilinopus regina), Sacred Kingfisher (Todiramphus sanctus), Spangled Drongo (Dicrurus bracteatus), White-bellied Cuckoo-shrike, and White-throated Needletail (Hirundapus caudacutus). There was weak statistical support for greater abundance in summer than winter for Olive-backed Oriole (Oriolus sagittatus), Oriental Cuckoo (Cuculus optatus), and Pheasant Coucal (Centropus phasianinus).
Conversely, there was strong statistical support for greater abundance in winter than summer for Australasian Figbird, Brown Honeyeater (Lichmera indistincta), Brown Thornbill, Eastern Spinebill, Eastern Whipbird (Psophodes olivaceus), Fan-tailed Cuckoo (Cacomantis flabelliformis), Grey Fantail (Rhipidura albiscapa), Lewin’s Honeyeater (Meliphaga lewinii), Rainbow Bee-eater, Rainbow Lorikeet, Scarlet Honeyeater (Myzomela sanguinolenta), Shining Bronze-cuckoo (Chalcites lucidus), Silvereye (Zosterops lateralis), Spotted Pardalote (Pardalotus punctatus), Striated Pardalote (Pardalotus striatus), Topknot Pigeon (Lopholaimus antarcticus), White-eared Monarch (Carterornis leucotis), White-headed Pigeon (Columba leucomela), and Yellow-faced Honeyeater (Lichenostomus chrysops). There was weak statistical support for greater abundance in winter than summer for Rose Robin (Petroica rosea) and Scaly-breasted Lorikeet (Trichoglossus chlorolepidotus). There was no statistical support for seasonal variation in abundance for the remaining 78 species (~62%) (Table 4).
Strong statistical support for seasonal variation in abundance was found for four feeding guilds (Table 3). Carnivores and granivores showed greater abundance during summer than winter, while frugivores and nectivores showed greater abundance during winter than summer (Table 3). There was no statistical support for seasonal variation in abundance for insectivores and omnivores (Table 3).
There were 24 species (~19%) for which there was statistical support for variation in abundance in response to disturbance (Table 4). Five species (~4%) showed greater abundances at impact sites vs control sites, while 19 species (~15%) showed greater abundance at control sites. In relation to proximity to disturbance, there was strong statistical support for greater abundance in control sites than sites closer to disturbance for Bar-shouldered Dove (Geopelia humeralis), Brown Cuckoo-dove (Macropygia amboinensis), Brown Thornbill, Eastern Spinebill, Eastern Whipbird, Little Wattlebird (Anthochaera chrysoptera), Logrunner (Orthonyx temminckii), Noisy Friarbird, Peaceful Dove (Geopelia striata), Rainbow Bee-eater, Rufous Whistler (Pachycephala rufiventris), Scarlet Honeyeater, Sulfur-crested Cockatoo (Cacatua galerita), Variegated Fairywren (Malurus lamberti), White-bellied Sea-eagle (Haliaeetus leucogaster), White-browed Scrubwren, and White-cheeked Honeyeater (Phylidonyris niger). There was weak statistical support for greater abundance in control sites than sites closer to disturbance for Little Shrike-thrush (Colluricincla megarhyncha), and Regent Bowerbird (Sericulus chrysocephalus).
Conversely, there was strong statistical support for greater abundance in sites closer to disturbance than control sites for Scaly-breasted Lorikeet, White-throated Treecreeper (Cormobates leucophaeus), and Willie Wagtail (Rhipidura leucophrys), and weak statistical support for this pattern for Australasian Figbird and Australian Brush-turkey (Alectura lathami). There was no statistical support for variation in abundance in response to proximity to disturbance for the remaining 101 species (~81%) (Table 4).
There were three feeding guilds for which there was strong statistical support for variation in abundance in response to proximity to disturbance (Table 3). Granivores, insectivores, and nectivores all showed greater abundance at control sites than at sites closer to disturbance, while there was no statistical support for any response to proximity to disturbance in carnivores, frugivores, and omnivores (Table 3).
We found strong statistical support for the interaction between disturbance and stage of disturbance for only one species, reflecting increased abundance of the omnivorous Australian Brush-turkey (Table 4). As a consequence, only omnivores showed strong statistical support for the interaction between disturbance and stage of disturbance (Table 4).
Discussion
Spatial and temporal variation in avian community assemblages primarily reflected species level responses to seasonal feeding resources in the lowland floodplain forests. We found significant variation in subtropical avian community composition and abundance at both an interannual and seasonal scale, limited difference between control and impact sites, and little difference among stages of human disturbance associated with two annual music festival events attracting ~25 000 people. Grouping species into feeding guilds showed significant differences at interannual and seasonal scales, suggesting that fruit and seed availability at interannual scale, and nectar and fruit at seasonal scale, are key drivers of avifaunal composition in these communities (Davis et al. 2014; Hawkins 2014).
Differences between control and impact sites reflected consistently higher numbers (as avian abundance) in control than impact samples, likely reflecting the higher quality of habitat in control locations. In contrast, it is likely that high levels of nomadism resulted in the convergence of community composition in smaller (impact) and larger (control) forest patches in response to seasonal resources (Howe et al. 1981; Franklin and Noske 1999). Overall, there was little differentiation between species level and feeding guild patterns in control and impact locations relative to timing of samples and events, suggesting that avian responses to short-duration human activities were not a major factor shaping assemblage variation.
Specifically, there was no statistical support for impacts of events from the B-D-A sample comparisons except for an increase in the omnivorous Australian Brush Turkey at impact sites during events. This likely reflected an increase in the availability of food resources (food scraps) suitable for that species (Warnken et al. 2004). No other species (or feeding guilds) showed significant responses to actual disturbance events, again suggesting that the short-duration of the event-disturbances resulted in little detectable impact. However, there were differences between control and impact sites for 24 species (Table 4). In that case, the statistical support for variations in the abundance of species on control vs impact sites may reflect factors other than event-based disturbance. For example, the variation detected might reflect the influence of factors such as proximity to other habitat types, land-use history, proximity to the cleared (grassy) event area, and differences in the forest vegetation between control and impact transects.
The effects of less conspicuous human disturbances to forest ecosystems, such as recreational use of remaining natural areas, has only recently begun to be investigated (Naidoo and Burton 2020). To date, findings are mixed with often variable responses to different disturbance factors by diverse fauna. For example, managed hunting and recreation in protected forests in eastern USA produced measurable effects on the distribution of some species; however, these were relatively minor in comparison with the importance of habitat covariates associated with land use and habitat fragmentation (Kays et al. 2016).
In relation to differences between the vegetation on transects, vegetation condition assessments at establishment showed that some habitat elements were more abundant and better developed at the control transects. These ranked vegetation assessments showed deeper overall leaf litter accumulations, areas of the invasive scrambler Lantana camara, and native Saw-sedges such as Gahnia clarkei. In combination such vegetation features can provide dense cover and may have led to the detected increased abundances of ground and lower strata foraging species including Eastern Whipbird, Logrunner, White-browed Scrubwren and Brown Thornbill (Billerman et al. 2020) at control sites.
Transects also varied in forest habitat structure relative to land-use (conservation reserve vs privately owned agricultural land) and disturbance histories, including cattle grazing and clearing. Two of the impact transects (on private land) were grazed and trampled by cattle for long periods prior to commencement of the study (2013), and ground-layer vegetation at those sites was less well-developed than at control transects (in the conservation reserve) where cattle had been excluded. The ground-layer impacts of cattle grazing were most evident in the early years of monitoring. In addition, the more coastal location of control transects in the BNR may help explain the greater abundance there of White-cheeked Honeyeater and Peaceful Dove. White-bellied Sea-eagles nesting on a control transect likely explains the greater abundance of the species on control sites, while the presence of large tree hollows (old-growth eucalypt forest) on another control transect may explain the higher incidence of the hollow nesting Sulfur-crested Cockatoo in controls.
The three species that were more abundant at impact sites include Willie Wagtail, which prefer more open and edge habitats (Boles 2020), White-throated Treecreeper, and Scaly-breasted Lorikeet. White-throated Treecreeper abundance may be explained by preference for taller tree stands and more suitable bark substrate that were present at one impact transect in tall forest located away from any forest edges (Noske 2020). Scaly-breasted Lorikeets may have been easier to count in the more open impact transects and may have needed to undertake more frequent movements to access scattered blossom resources in the more disturbed landscape.
Habitat use and species behaviours relative to position in the forest strata may also have influenced the response of birds to the mostly terrestrial human and vehicle activity in the event landscape. Foraging height is known to influence bird assemblage structure (Remsen and Robinson 1990), with tolerance of human intrusion reported as lower for species active on the ground (Gutzweiler et al. 1998). However, it was clear from our analysis of abundance-weighted community composition that differences between control and impact sites were not reflected in the B-D-A analyses. Therefore, any differences in bird community composition or abundances detected likely reflected differences between the habitat characteristics of control and impact forest sites (reflecting landscape context and land-use history), rather than disturbance from intermittent human activities.
Pulsed resource dynamics are widespread phenomena (Yang et al. 2008) and can include responses to rainfall (Herrera 1998; Tischler et al. 2013) and to rainfall related insect abundance (Frith 1984). We found strong statistical support for interannual variation in abundance for 17 species. One explanation for this could be nomadism in response to local and regional resource availability (Howe et al. 1981). The underlying patterns in interannual variation likely reflect climatic variation (e.g. temperature, and total and seasonal rainfall) that affects plant growth and phenology and the related abundance of resources such as pollen, nectar, and insects. For example, the flowering phenology of local eucalypts can include large-scale variation, from regular annual flowering to intermittent or sometimes no flowering over longer time periods (Law et al. 2000). In addition, we acknowledge that regional scale patterns of decline and increase of species may contribute to these patterns (Ricklefs 1987, 2011), but such data are not available.
The 47 species that had statistical support for seasonal variation in abundance in the study region included mostly seasonal migrants and locally nomadic species, likely also responding to resource availability. Summer visitors to the region include Channel-billed Cuckoo, Oriental Dollarbird, Leaden Flycatcher, Eastern Koel, Spangled Drongo and White-throated Needletail (Billerman et al. 2020). Resident species that are more vocal and thus more conspicuous in summer include Brush Cuckoo, Pacific Baza, and Sacred Kingfisher, notwithstanding that low abundance and richness in summer were occasionally correlated with low detection rates because of high cicada noise. Seasonal variations in seed production and resource availability likely explain the increase in abundance of granivores such as King Parrot, Emerald Dove, and Little Corella. Carnivorous species such as Brown Goshawk and Laughing Kookaburra may be responding to increased abundance and activity in avian, reptile and mammal prey in summer (e.g. the seasonal increase in abundance of breeding birds, lizards including small skinks, small snakes, and irruptive breeding in mice and rats) (Singleton 2008; Yang et al. 2008).
Summer and winter resource pulses engender varying responses from resident, migratory, and regionally nomadic species (Munro et al. 1993; Franklin and Noske 1999; Yang et al. 2008). Seasonal patterns have been reported for regionally nomadic rainforest fruit-doves (Crome 1978; Frith 1980; Hawkins 2014), insectivores (Coughlan and Pearson 2004), and nectivores (Franklin and Noske 1998, 1999). In our study, frugivores dominated some samples in response to seasonal fruit abundance, including winter fruit crops of the exotic tree Camphor Laurel (Cinnamomum camphora, Lauraceae). Both Pied Currawongs (Strepera graculina) and Australasian Figbirds were observed taking this fruit during events, despite significant human activity around the impact transects within the event footprint. In winter, large foraging aggregations of species such as Olive-backed Oriole and Australasian Figbird occur in response to fruit availability (Peters et al. 2010) and coincide with the dispersal of juveniles of other species (e.g. Brown Thornbill). Interestingly, our data showed no statistical support for increased seasonal abundance of insectivores, suggesting that in the subtropical climate, winter flowering of many tree species may maintain insect numbers and insectivore activity throughout the year. In this case, the influence of nectar-producing trees on bird numbers and species composition was particularly evident in winter when blossom crops of Swamp Mahogany (Eucalyptus robusta) and Broad-leaved Paperbark (Melaleuca quinquenervia) overlapped. These patterns in fruit and nectar availability may help explain the detected taxonomic clustering evident in some winter assemblages (Fig. 2). Between the summer–winter seasons, Coast Banksia (Banksia integrifolia) provides important autumn nectar resources for Eastern Spinebills (Ford and Pursey 1982) and other honeyeaters.
Based on Mac Nally (1994, 1996; sensu May 1977) it has been suggested that there is a low likelihood of detecting or quantifying the effects of disturbance events on the non-equilibrium dynamics of bird community assembly processes through time. By using comprehensive multivariate model-based analyses (Wang et al. 2012), we were able to detect and quantify the influence of multiple factors on bird species, feeding guilds, and community assembly, and explain variations in community composition and species abundances through seasons and time. However, and consistent with May (1977), we also found the dynamics of bird community assembly were shaped by resource availability and habitat quality across a range of scales and seasonal and year–year variation.
Conclusions
Bird species are dynamic in space and time and respond differently to habitat and resource availability, land-use and disturbance histories that affect habitat quality, localised disturbances, and seasonal variations. Avian community assembly reflects non-equilibrium dynamics in populations of species and explains why seasonal resource variations make predictions about community assembly composition and species abundances probabilistic not deterministic. However, by measuring the relationship of species and species groups to response variables and a common set of predictors, we were able to differentiate the relative influence of selected variables, and to some extent identify the legacies of past land-use. It is clear from our 7-year study that the key factors influencing avian community assembly and species abundances in these subtropical floodplain forests of central eastern Australia are related to the habitat attributes that regulate the quality and quantity of food resources both seasonally and year-year. Avian community composition was similar between control and impact sites, but species abundances were higher in less disturbed forest habitats with larger tree sizes, greater structural and floristic diversity in lower strata, and greater abundance of resources (fruit and nectar). In combination, those factors highlight the fundamental value of the conservation reserves in protecting higher quality habitat and regional avian community dynamics in these lowland forests.
Data availability
Mvabund is an R-package. All R code for the mvabund analyses is open source and the working example of our code for the project can be provided. Site by species and feeding guild data used in the analyses can be provided on request. Standard data use agreements apply.
Conflict of interest
The authors declare no conflicts of interest.
Declaration of funding
Byron Venue Management funded the field work for this study. The associated research and writing did not receive any other specific funding.
Ethics statement
Bird sampling was based on observations (visual) and call recognition only. Capture and playback were not part of the research methods and the activities did not require additional approvals to the standard licensing arrangements. In that regard, surveys were carried out under Scientific Licence SL 100 719, and an Animal Research Authority issued to MF.
Acknowledgements
We thank two anonymous reviewers for constructive and very helpful comments that significantly improved the manuscript. Bird counts were carried out by MF with assistance from Steve Phillips, David Rohweder, Graeme Lloyd and John Callaghan. Allocation of computer time and assistance with modifications to R-code to run models was provided by Jason Bragg (Royal Botanic Gardens, Sydney). NSW Office of Heritage National Parks and Wildlife Service provided access for research in Billinudgel Nature Reserve. Surveys were carried out under Scientific Licence SL 100 719, and an Animal Research Authority issued to MF. MF and RK conceived the ideas and designed the methodology; MF collected the data; MF and RK managed the data; RK and SG analysed the data and SG did the mvabund coding and analyses; MF and RK led the writing of the manuscript, while all authors contributed critically and substantially to the drafts and gave final approval for publication.
References
Bibby, C. J., Burgess, N. D., Hill, D. A., and Mustoe, S. H. (2000). ‘Bird Census Techniques.’ (Academic Press, London, UK.)| Crossref |
Billerman, S. M., Keeney, B. K., Rodewald, P. G., and Schulenberg, T. S. (Eds). (2020). ‘Birds of the World.’ (Cornell Laboratory of Ornithology, Ithaca, NY, USA.) https://birdsoftheworld.org/bow/home
Boles, W. (2020). Willie-wagtail (Rhipidura leucophrys), version 1.0. In ‘Birds of the World.’ (Eds S. M. Billerman, B. K. Keeney, P. G. Rodewald and T. S. Schulenberg). (Cornell Laboratory of Ornithology, Ithaca, NY, USA.) https://birdsoftheworld.org/bow/support/citations-and-references
Bregman, T. P., Sekercioglu, C. H., and Tobias, J. A. (2014). Global patterns and predictors of bird species responses to forest fragmentation: implications for ecosystem function and conservation. Biological Conservation 169, 372–383.
| Global patterns and predictors of bird species responses to forest fragmentation: implications for ecosystem function and conservation.Crossref | GoogleScholarGoogle Scholar |
Brooks, M. E., Kristensen, K., van Benthem, K. J., Magnusson, A., Berg, C. W., Nielsen, A., Skaug, H. J., Machler, M., and Bolker, B. M. (2017). glmmTMB balances speed and flexibility among packages for zero-inflated generalized linear mixed modeling. The R Journal 9, 378–400.
| glmmTMB balances speed and flexibility among packages for zero-inflated generalized linear mixed modeling.Crossref | GoogleScholarGoogle Scholar |
Clarke, K. R., and Gorley, R. N. (2015). ‘Primer v7: User manual/tutorial.’ (PRIMER-E: Plymouth.)
Clarke, K. R., Gorley, R. N., Somerfield, P. J., and Warwick, R. M. (2014). ‘Changes in marine communities: an approach to statistical analysis and interpretation.’ 3rd edn. (PRIMER-E: Plymouth.)
Cooper, W. E., and Frederick, W. G. (2007). Optimal flight initiation distance. Journal of Theoretical Biology 244, 59–67.
| Optimal flight initiation distance.Crossref | GoogleScholarGoogle Scholar | 16949619PubMed |
Coughlan, J., and Pearson, R. G. (2004). The bird communities of dry rainforest and surrounding woodlands in North Queensland. In ‘Conservation of Australia’s Forest Fauna.’ (Ed Daniel Lunney) pp. 474–492 (Royal Zoological Society of New South Wales, Australia.)
| Crossref |
Crome, E. H. J. (1978). Foraging ecology of an assemblage of birds in lowland rainforest in North Queensland. Australian Journal of Ecology 3, 195–202.
| Foraging ecology of an assemblage of birds in lowland rainforest in North Queensland.Crossref | GoogleScholarGoogle Scholar |
Cunningham, S. J., Gardner, J. L., and Martin, R. O. (2021). Opportunity costs and the response of birds and mammals to climate warming. Frontiers in Ecology and the Environment 19, 300–307.
| Opportunity costs and the response of birds and mammals to climate warming.Crossref | GoogleScholarGoogle Scholar |
Davis, A., Major, R. E., and Taylor, C. E. (2014). The association between nectar availability and nectarivore density in urban and natural landscapes. Urban Ecosystems 18, 503–515.
| The association between nectar availability and nectarivore density in urban and natural landscapes.Crossref | GoogleScholarGoogle Scholar |
Ford, H. A., and Pursey, J. F. (1982). Status and feeding of the Eastern Spinebill Acanthorhynchus tenuirostris at New England National Park, north-eastern NSW. Emu 82, 203–211.
| Status and feeding of the Eastern Spinebill Acanthorhynchus tenuirostris at New England National Park, north-eastern NSW.Crossref | GoogleScholarGoogle Scholar |
Franklin, D. C., and Noske, R. A. (1998). Local movements of honeyeaters in a subcoastal vegetation mosaic in the Northern Territory. Corella 22, 97–103.
Franklin, D. C., and Noske, R. A. (1999). Birds and nectar in a monsoonal woodland: correlation at three spatio-temporal scales. Emu 99, 15–28.
| Birds and nectar in a monsoonal woodland: correlation at three spatio-temporal scales.Crossref | GoogleScholarGoogle Scholar |
Frid, A., and Dill, L. (2002). Human-caused disturbance stimuli as a form of predation risk. Conservation Ecology 6, 11–26.
| Human-caused disturbance stimuli as a form of predation risk.Crossref | GoogleScholarGoogle Scholar |
Frith, H. J. (1980). Pigeons and Doves of Australia. Rigby, Adelaide.
Frith, D. W. (1984). Foraging ecology of birds in an upland tropical forest in North Queensland. Australian Wildlife Research 11, 325–347.
| Foraging ecology of birds in an upland tropical forest in North Queensland.Crossref | GoogleScholarGoogle Scholar |
Garnett, S. T., Duursma, D. E., Ehmke, G., Guay, P. J., Stewart, A., Szabo, J. K., Weston, M. A., Bennett, S., Crowley, G. M., Drynan, D., and Dutson, G. (2015). Biological, ecological, conservation and legal information for all species and subspecies of Australian bird. Scientific Data 2, 1–6.
| Biological, ecological, conservation and legal information for all species and subspecies of Australian bird.Crossref | GoogleScholarGoogle Scholar |
Gaynor, K. M., Brown, J. S., Middleton, A. D., Power, M. E., and Brashares, J. S. (2019). Landscapes of fear: spatial patterns of risk perception and response. Trends in Ecology and Evolution 34, 355–368.
| Landscapes of fear: spatial patterns of risk perception and response.Crossref | GoogleScholarGoogle Scholar | 30745252PubMed |
Gower, J. C. (1966). Some distance properties of latent root and vector methods used in multivariate analysis. Biometrika 53, 325–338.
| Some distance properties of latent root and vector methods used in multivariate analysis.Crossref | GoogleScholarGoogle Scholar |
Gutzweiler, K. J., Marcum, H. A., Harvey, A. B., Roth, J. D., and Anderson, S. H. (1998). Bird tolerance to human intrusion in Wyoming montane forest. The Condor 100, 519–527.
| Bird tolerance to human intrusion in Wyoming montane forest.Crossref | GoogleScholarGoogle Scholar |
Hansen, M. C., Potapov, P. V., Moore, R., Hancher, M., Turubanova, S. A., Tyukavina, A., Thau, D., Stehman, S. V., Goetz, S. J., Loveland, T. R., and Kommareddy, A. (2013). High-resolution global maps of 21st-century forest cover change. Science 342, 850–853.
| High-resolution global maps of 21st-century forest cover change.Crossref | GoogleScholarGoogle Scholar | 24233722PubMed |
Hawkins, B. A. (2014). Birds, fruit and nectar: spatio-temporal patterns of regional abundance and food availability in sub-tropical eastern Australia. Ph. D. Thesis. Monash University Faculty of Science, School of Biological Sciences.
Herrera, C. M. (1998). Long term dynamics of Mediterranean frugivorous birds and fleshy fruits: a 12 year study. Ecological Monographs 68, 511–538.
| Long term dynamics of Mediterranean frugivorous birds and fleshy fruits: a 12 year study.Crossref | GoogleScholarGoogle Scholar |
Holt, R. (2008). Theoretical perspectives on resource pulses. Ecology 89, 671–681.
| Theoretical perspectives on resource pulses.Crossref | GoogleScholarGoogle Scholar | 18459331PubMed |
Howe, R. W. (1984). Local dynamics of bird assemblages in small forest habitat islands in Australia and North America. Ecology 65, 1585–1601.
| Local dynamics of bird assemblages in small forest habitat islands in Australia and North America.Crossref | GoogleScholarGoogle Scholar |
Howe, R. W., Howe, T. D., and Ford, H. A. (1981). Bird distributions on small rainforest remnants in New South Wales. Australian Wildlife Research 8, 637–651.
| Bird distributions on small rainforest remnants in New South Wales.Crossref | GoogleScholarGoogle Scholar |
Kays, R., Parsons, A. W., Kalies, E. L., Forrester, T., Costello, R., Rota, C. T., Millspaugh, J. J., and McShea, W. J. (2016). Does hunting or hiking affect wildlife communities in protected areas? Journal of Applied Ecology 54, 242–252.
| Does hunting or hiking affect wildlife communities in protected areas?Crossref | GoogleScholarGoogle Scholar |
LaManna, J. A., and Martin, T. E. (2016). Costs of fear: Behavioural and life‐history responses to risk and their demographic consequences vary across species. Ecology Letters 19, 403–413.
| Costs of fear: Behavioural and life‐history responses to risk and their demographic consequences vary across species.Crossref | GoogleScholarGoogle Scholar | 26900087PubMed |
Law, B., Mackowski, C., Schoer, L., and Tweedie, T. (2000). The flowering phenology of myrtaceous trees and their relation to environmental and disturbance variables in northern New South Wales. Austral Ecology 25, 162–178.
| The flowering phenology of myrtaceous trees and their relation to environmental and disturbance variables in northern New South Wales.Crossref | GoogleScholarGoogle Scholar |
Mac Nally, R. (1994). Habitat-specific guild structure of forest birds in south-eastern Australia: a regional scale perspective. Journal of Animal Ecology 63, 988–1001.
| Habitat-specific guild structure of forest birds in south-eastern Australia: a regional scale perspective.Crossref | GoogleScholarGoogle Scholar |
Mac Nally, R. (1996). A winter’s tale: among year variation in bird community structure in a southeastern Australian forest. Australian Journal of Ecology 21, 280–291.
| A winter’s tale: among year variation in bird community structure in a southeastern Australian forest.Crossref | GoogleScholarGoogle Scholar |
Malizia, L. R. (2001). Seasonal fluctuations of birds, fruits, and flowers in a subtropical forest of Argentina. The Condor 103, 45–61.
| Seasonal fluctuations of birds, fruits, and flowers in a subtropical forest of Argentina.Crossref | GoogleScholarGoogle Scholar |
May, R. M. (1977). Thresholds and breakpoints in ecosystems with a multiplicity of stable states. Nature 269, 471–477.
| Thresholds and breakpoints in ecosystems with a multiplicity of stable states.Crossref | GoogleScholarGoogle Scholar |
Munro, U., Wiltschko, W., and Ford, H. (1993). Changes in the migratory direction of Yellow-faced Honeyeaters Lichenostomus chrysops (Meliphagidae) during autumn migration. Emu 93, 59–62.
| Changes in the migratory direction of Yellow-faced Honeyeaters Lichenostomus chrysops (Meliphagidae) during autumn migration.Crossref | GoogleScholarGoogle Scholar |
Naidoo, R., and Burton, A. C. (2020). Relative effects of recreational activities on a temperate terrestrial wildlife assemblage. Conservation Science and Practice 2, e271.
| Relative effects of recreational activities on a temperate terrestrial wildlife assemblage.Crossref | GoogleScholarGoogle Scholar |
Noske, R. (2020). White-throated Treecreeper(Cormobates leucophaea), version 1.0. In Birds of the World. Available at https://birdsoftheworld.org/bow/support/citations-and-references
Peters, V. R., Mordecai, R., Carroll, C. R., Robert, J., Cooper, R. J., and Greenberg, R. (2010). Bird community response to fruit energy. Journal of Animal Ecology 79, 824–835.
| Bird community response to fruit energy.Crossref | GoogleScholarGoogle Scholar |
Price, M. (2008). The impact of human disturbance on birds: a selective review. In ‘Too close for comfort: contentious issues in human-wildlife encounters.’ (Eds Daniel Lunney Adam Munn and Will Meikle.) pp. 163–196. (Royal Zoological Society of New South Wales, Mosman, NSW, Australia.)
R Core Team. (2020). R: A language and environment for statistical computing.
Remsen, J. V., and Robinson, S. K. (1990). A classification scheme for foraging behavior of birds in terrestrial habitats. Studies in Avian Biology 13, 144–160.
Ricklefs, R. E. (1987). Community diversity: relative roles of local and regional processes. Science 235, 167–171.
| Community diversity: relative roles of local and regional processes.Crossref | GoogleScholarGoogle Scholar | 17778629PubMed |
Ricklefs, R. E. (2011). Applying a regional community concept to forest birds of eastern North America. Proceedings of the National Academy of Sciences USA 108, 2300–2305.
| Applying a regional community concept to forest birds of eastern North America.Crossref | GoogleScholarGoogle Scholar |
Singleton, G. R. (2008). House Mouse Mus musculus in ‘The Mammals of Australia’ 3rd edn. Queensland Government, Queensland Museum.
Somveille, M., Rodrigues, A. S., and Manica, A. (2015). Why do birds migrate? A macroecological perspective. Global Ecology and Biogeography 24, 664–674.
| Why do birds migrate? A macroecological perspective.Crossref | GoogleScholarGoogle Scholar |
Thogmartin, W. E., Gray, B. R., Gallagher, M., Young, N., Rohweder, J. J., and Knutson, M. G. (2007). Power to detect trend in short-term time series of bird abundance. The Condor 109, 943–948.
| Power to detect trend in short-term time series of bird abundance.Crossref | GoogleScholarGoogle Scholar |
Tischler, M., Dickman, C. R., and Wardle, G. M. (2013). Avian functional group responses to rainfall across four vegetation types in the Simpson Desert, central Australia. Austral Ecology 38, 809–819.
| Avian functional group responses to rainfall across four vegetation types in the Simpson Desert, central Australia.Crossref | GoogleScholarGoogle Scholar |
Underwood, A. J. (1994). On beyond BACI: sampling designs that might reliably detect environmental disturbances. Ecological Applications 4, 3–15.
| On beyond BACI: sampling designs that might reliably detect environmental disturbances.Crossref | GoogleScholarGoogle Scholar |
Wang, Y. I., Naumann, U., Wright, S. T., and Warton, D. I. (2012). mvabund–an R package for model-based analysis of multivariate abundance data. Methods in Ecology and Evolution 3, 471–474.
| mvabund–an R package for model-based analysis of multivariate abundance data.Crossref | GoogleScholarGoogle Scholar |
Warnken, J., Hodgkison, S., Wilda, C., and Jones, D. (2004). The localized environmental degradation of protected areas adjacent to bird feeding stations: a case study of the Australian brush-turkey Alectura lathami. Journal of Environmental Management 70, 109–118.
| The localized environmental degradation of protected areas adjacent to bird feeding stations: a case study of the Australian brush-turkey Alectura lathami.Crossref | GoogleScholarGoogle Scholar | 15160737PubMed |
Warton, D. I., Lyons, M., Stoklosa, J., and Ives, A. R. (2016). Three points to consider when choosing a LM or GLM test for count data. Methods in Ecology and Evolution 7, 882–890.
| Three points to consider when choosing a LM or GLM test for count data.Crossref | GoogleScholarGoogle Scholar |
Wilson, M. W., Ridlon, A. D., Gaynor, K. M., Gaines, S. D., Stier, A. C., and Halpern, B. S. (2020). Ecological impacts of human-induced animal behaviour change. Ecology Letters , .
| Ecological impacts of human-induced animal behaviour change.Crossref | GoogleScholarGoogle Scholar | 32705769PubMed |
Yang, L. H., Bastow, J. L., Spence, K. O., and Wright, A. N. (2008). What can we learn from resource pulses? Ecology 89, 621–634.
| What can we learn from resource pulses?Crossref | GoogleScholarGoogle Scholar | 18459327PubMed |






