Phylogenetics of the skyhoppers (Kosciuscola) of the Australian Alps: evolutionary and conservation implications
Kate D. L. Umbers A B J , Rachel A. Slatyer C , Nikolai J. Tatarnic D E , Giselle R. Muschett F G , Shichen Wang H and Hojun Song I
A B J , Rachel A. Slatyer C , Nikolai J. Tatarnic D E , Giselle R. Muschett F G , Shichen Wang H and Hojun Song I
A School of Science, Western Sydney University, Penrith, NSW 2751, Australia.
B Hawkesbury Institute for the Environment, Western Sydney University, Penrith, NSW 2751, Australia.
C Division of Ecology & Evolution, Research School of Biology, The Australian National University, Canberra, ACT, Australia.
D Collections and Research, Western Australian Museum, 49 Kew Street, Welshpool, WA 6106, Australia.
E Centre for Evolutionary Biology, University of Western Australia, Crawley, WA 6009, Australia.
F Department of Biological Sciences, Macquarie University, North Ryde, NSW 2109, Australia.
G Vicerrectoría de Investigación, Pontificia Universidad Católica de Valparaíso, Valparaíso, Chile.
H Texas A&M AgriLife Research: Genomics and Bioinformatics Service, College Station, TX, USA.
I Department of Entomology, Texas A&M University, College Station, TX, USA.
J Corresponding author. Email: k.umbers@westernsydney.edu.au
Pacific Conservation Biology 28(3) 261-276 https://doi.org/10.1071/PC21015
Submitted: 12 March 2021 Accepted: 22 June 2021 Published: 15 July 2021
Journal Compilation © CSIRO 2022 Open Access CC BY-NC-ND
Abstract
The true biodiversity of Australia’s alpine and subalpine endemics is unknown. Genetic studies to date have focused on sub-regions and restricted taxa, but even so, indicate deep divergences across small geographic scales and therefore that the bulk of biodiversity remains to be discovered. We aimed to study the phylogeography of the Australian Alps by focusing on the skyhoppers (Kosciuscola), a genus of five species of flightless grasshoppers whose combined distributions both span the region and are almost exclusively contained within it. Our sampling covered 650 km on the mainland and several sites in Tasmania with total of 260 specimens used to reconstruct a robust phylogeny of Koscisucola. Phylogenies were based on single nucleotide polymorphism data generated from double-digested restriction-associated DNA sequencing. Skyhoppers diverged around 2 million years ago and have since undergone complex diversification seemingly driven by climatic oscillations throughout the Pleistocene. We recovered not 5 but 14 clades indicating the presence of many unknown species. Our results support conspicuous geographic features as genetic breaks; e.g. the Murray Valley, and inconspicuous ones; e.g. between the Bogong High Plains and Mt Hotham. Climate change is progressing quickly in the region and its impact, particularly on snow, could have severe consequences for the skyhoppers’ overwinter survival. The true diversity of skyhoppers highlights that biodiversity loss in the Alps as a result of climate change is likely to be far greater than what can be estimated based on current species numbers and that management including small geographical scales is key.
Keywords: alpine, climate change, conservation, flightless grasshoppers, insect decline, Kosciuscola, mountain, skyhoppers.
Introduction
Australia has weathered over millennia. The Great Dividing Range, which spans the entire eastern fringe of the continent from north to south, is 40–90 million years old and because of its extreme age is now decayed and disjointed (Holdgate et al. 2008). The Australian ‘high-country’ upland areas are typically reported to have remained geologically stable since the early Cretaceous (145–65 million years ago (mya)) (Holdgate et al. 2008), but in that time have experienced substantial climatic variability. The relatively warm and wet climates of the Miocene (23–5 mya) gave way to relatively cooler and drier conditions by the Quaternary (12 000 years before present (ybp)); conditions for cold adapted biotic assemblages to form are thought to have begun in the alps around 5 mya (Green and Osborne 1994). The following millennia were characterised by punctuated cold periods, including the last glacial maximum which peaked around 18 000 ybp and concluded around 12 000 ybp (Holdgate et al. 2008). In Australia, the extent of glaciation in the Pleistocene (2.5 mya–12 000 ybp) seems to have been quite different and had different consequences to that in the northern hemisphere. While there was extensive glaciation in Tasmania (~2000 km2), and a little (~25 km2) in the Snowy Mountains in New South Wales (NSW), there is no clear evidence of glaciation in the Victorian Alps (Green and Osborne 1994). Thus, compared with the northern hemisphere, glaciation in the Australian Alps was minimal, potentially allowing species adapted to high-elevation conditions to persist in place relatively undisturbed and/or expand downhill rather than contracting into refugia (Holdgate et al. 2008). In the present day, the Australian Alps harbour deep snow for only 4 months of the year, and their rolling plateaus are worn and rounded. The timber line sits at around 1800 m elevation (metres above sea level) in NSW, 1750 m in Victoria, and as low as approximately 750 m in Tasmania with just 200–300 vertical metres of alpine habitat across the alps (Green and Osborne 1994).
Given its position on an otherwise hot, dry and flat continent, it is no surprise that much of the biodiversity in the Australian Alps is unique. The sub-alpine ecosystem, the area between the timber line and the winter snow line, is characterised by the iconic snow gums (Eucalyptus pauciflora and E. coccifera) and great expanses of closed heath (Green and Osborne 1994). With increasing elevation, this gives way to open heath, alpine grasslands and meadows, and the upper windblown reaches still maintain small patches of fjeldmark, fens and endangered snowpatch communities (Green and Osborne 1994; Williams et al. 2015). Large glacial lakes and granite outcrops are visually prominent landscape features and provide critical habitat. The fauna is predominantly composed of insects, both in abundance and diversity, with numerous endemic grasshoppers, moths, beetles, flies and ants, many of which are threatened with extinction (Green and Osborne 1994; Mynott 2015; Rutter et al. 2021). Sawfly larvae can be found in abundance devouring sapling Eucalyptus, a fly-dominated pollinator assemblage buzzes over the alpine flower meadows, and millions to billions of bogong moths (Agrotis infusa) migrate to the alps every spring to overwinter in granite caves and crevices (Green et al. 2020; Milla and Encinas-Viso 2020; Goodwin et al. 2021). Crayfish (Euastacus), stoneflies (Thaumatoperla), mayflies (Coloburiscoides), amphipods (Neoniphargus), isopods (Coluboltelson), ancient mountain shrimp (Anaspides) and small galaxid fish occupy the streams (Furse and Coughran 2011; Mynott 2015; Rutter et al. 2021). Large spider holes and silvery orb webs are common sights among the vegetation. Oversized endemic earthworms (Green and Osborne 1994) and endemic springtails (Endo et al. 2015; Green and Slatyer 2019) represent a host of under-appreciated soil fauna (Green and Osborne 1994). This density of life supports iconic vertebrate species, many of which are also threatened, including the mountain pygmy possum, a hibernating marsupial (Geiser and Broome 1991; Mitrovski et al. 2007), northern and southern corroboree frogs (Hunter et al. 2010; Brannelly et al. 2015; Umbers et al. 2020), Guthega, she-oak, mountain rock, and log skinks (Haines et al. 2017; Pepper et al. 2018; Atkins et al. 2020), large flocks of ravens and other avian visitors (Green and Osborne 1994).
How did so many different lineages of organisms independently adapt to life in the Australian Alps? Are there common underlying patterns? And what are the implications for the effects of climate change on the region’s biodiversity? Elucidating biogeographical and diversification patterns can help us to address these questions by hinting at the processes that have given rise to present-day distributions. Phylogenetic studies of the organisms inhabiting the Australian Alps are few in number, but the taxa that have been examined show deep divergence among species and populations over fine geographic scales (Griffin and Hoffmann 2014; Slatyer et al. 2014; Endo et al. 2015; Hatley and Murphy 2016; Haines et al. 2017; Bell et al. 2018). For example, Australian alpine amphipods show remarkably deep divergence over extremely small spatial scales with clades in streams less than 2 km apart, last sharing a common ancestor between 7 and 40 mya (Hatley and Murphy 2016), and two clades of sympatric isopods have stayed reproductively isolated for 10 million years (Hatley and Murphy 2016). In terrestrial arthropods, divergence times are typically shallower than those reported for amphipods and isopods, but still deep compared with the divergence times found in alpine species in the northern hemisphere. Endo et al. (2015) reported on five arthropod species (two beetles, a grasshopper, a springtail and a millipede), all of which showed evidence of divergence times of around 1 mya, still far earlier than the last glacial maximum 18 000 ybp. Likewise, Bell et al.(2018) show deep genetic splits and high genetic diversity across seven alpine plant species – shrubs and herbs with variable pollination requirements and mating systems – across their Victorian range. However, Griffin and Hoffmann (2014) found minimal genetic structure among grasses such as Poa species. This is perhaps not surprising given their great capacity for dispersal not only among Australian high-elevation regions, but also across to Aotearoa/New Zealand.
Among Australian alpine insects, the Koscisucola grasshoppers or ‘skyhoppers’ (Sjöstedt 1934) (Orthoptera: Acrididae: Oxyinae) are widespread mountain endemics and are brachypterous (reduced-winged) and flightless, with limited dispersal capacity. The skyhoppers are thus an ideal group for understanding how the insect lineages have diversified across the entire Australian Alps (Fig. 1). This genus has a fascinating natural history. Kosciuscola species display a striking and unique trait among grasshoppers: temperature-mediated, reversible colour change (Key and Day 1954a, 1954b; Filshie et al. 1975; Umbers 2011; Umbers et al. 2013). The change is most conspicuous in male K. tristis, which changes from black to turquoise when body temperature exceeds 25°C (Umbers 2011; Umbers et al. 2013). This colour change has given the species its common names, the chameleon skyhopper and thermocolour skyhopper. Furthermore, Kosciuscola are the only grasshoppers known to engage in fierce male–male combat and the level and types of aggressive behaviour vary among the species (Umbers et al. 2012; Muschett 2016; Muschett et al. 2017).
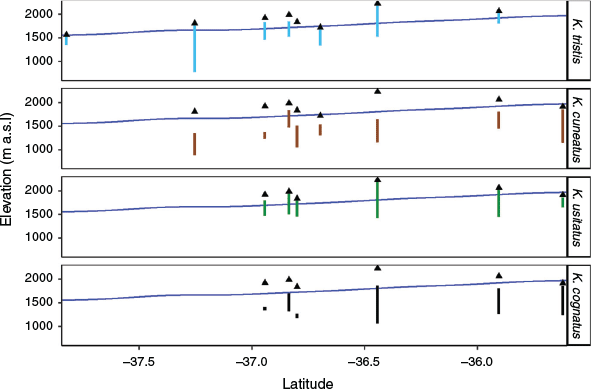
Kosciuscola belongs to the tribe Praxibulini in the paraphyletic subfamily Oxyinae, the water-loving grasshoppers (Rehn 1957). The Praxibulini currently includes four brachypterous genera endemic to Australia, of which two monotypic genera (Methiola and Methiolopsis) are confined to Queensland. The type genus, Praxibulus, is found throughout the lowlands of eastern Australia (Key 1989), and Kosciuscola appears to be the dominant group that has adapted to life in the mountainous south-east of Australia. Kosciuscola currently includes five described species with two subspecies (K. tristis, K. tristis restrictus, K. cuneatus, K. usitatus, K. cognatus, and K. tasmanicus), whose collective distributions extend from the Brindabella Ranges in the north (west of Canberra, ACT), through Australia’s highest peak, Mt. Kosciuszko in NSW and the Victorian Alps into Tasmania’s alpine region (Rehn 1957; Tatarnic et al. 2013). Kosciuscola are most commonly found at high elevations above 500 m in Tasmania all the way to the peak of Mt. Kosciuszko (Rehn 1957). The skyhoppers are mountain specialists, with each species appearing to have a specific habitat preference loosely corresponding with elevation, such that some species tend to occur at higher elevations than others (Slatyer et al. 2016) (Fig. 2).
|
Kosciuscola’s unique features and potential for revealing the biogeography of the Australian Alps have recently driven a number of studies to attempt to elucidate their phylogeny and population genetic structures (Tatarnic et al. 2013; Slatyer et al. 2014; Endo et al. 2015; Yadav et al. 2020). However, most of these studies so far have been based on relatively small datasets, or have focused on only one or two species, leading to incomplete understanding of the diversification patterns within the genus. In this study, we aimed to illuminate phylogeographic patterns across the whole of the Australian Alps by reconstructing a robust phylogeny of Koscisucola based on single nucleotide polymorphism (SNP) data generated from double-digested restriction-associated DNA sequencing (ddRAD-seq) and extensive taxon sampling across the entire range of the genus. We also aimed to examine diversification patterns of each species considering their distributions, phylogenetic relationships, and divergence time estimates. Given that the Australian Alps has many geographically isolated peaks separated by some large low-elevation distances and the improbably broad distributions of the currently recognised species, we predicted we would find strong structure within and between the skyhoppers. We predicted that we would recover genetically well-differentiated clades that are geographically clustered, thereby identifying previously unrecognised cryptic species and increasing known species diversity within the genus.
Materials and methods
Taxon sampling
Sampling for Kosciuscola was completed between 2012 and 2016 from 35 locations across the Australian Alps region (see Table S1). Grasshoppers were collected between February (late summer) and May (late autumn) when adults are active, as juveniles cannot be easily identified in the field. The four mainland species (K. tristis, K. cuneatus, K. cognatus, K. usitatus) were also sampled by hand along a 650 km transect of the Australian Alps, roughly following the Great Dividing Range (see Fig. S1) at intervals of approximately 20 km in autumn (March–April) 2016. These samples were collected and identified by R. Slatyer in the field using the shape of the prosternal process (Muschett 2016). For samples collected along the transect, a single hind femur was removed and stored in 100% ethanol until DNA extraction, and the grasshopper was then released. The rest of the samples were killed by freezing, and then stored in 100% ethanol in a freezer until extraction. Kosciuscola tasmanicus was collected from three locations in Tasmania, killed by freezing, and then stored in 100% ethanol in a freezer until DNA extraction. For this study, we extracted DNA from 260 samples and generated sequence data from these specimens.
DNA extraction
From ethanol-preserved hind femora, muscle tissues were dissected, and Gentra Puregene Tissue Kit (Qiagen) was used to extract high molecular weight DNA following the manufacturer’s guidelines. The quality and concentration of DNA extracts were initially measured using a DeNovix DS-11 Spectrophotometer, and more thoroughly analysed using Fragment Analyzer (Advanced Analytical Technologies, Inc.) prior to library preparation.
RAD-seq data generation
ddRAD-seq was carried out at the Texas A&M AgriLife Genomics and Bioinformatics Service. Total genomic DNA was digested with the restriction enzymes HindIII and MseI, and the resulting fragments were size selected for inserts ranging from 250 to 500 base pairs (bp). Sample libraries were prepared for the Illumina HiSeq. 4000 platform and sequenced for 2×150 cycles. A total of six lanes were used for sequencing to ensure sufficient coverage, given generally large genome sizes of grasshoppers (http://www.genomesise.com). The raw RAD-seq data used in this study were deposited to the NCBI SRA database (SUB9180426).
Raw sequence processing and quality control
Raw RAD sequences were processed using pyRAD ver. 3.0.66 (Eaton 2014). First, low quality base calls with a score less than 20 were trimmed from the 3′ end of reads. The quality-filtered reads were then clustered into ‘stacks’ using a similarity threshold of 88% for within and among sample clustering. Clusters with a minimum depth of coverage less than five reads per locus per individual were excluded. Loci containing more than two alleles after error correction were excluded, as they are mostly formed by potential paralogs. To keep as many loci as possible, any loci for which at least three samples contained data were retained for subsequent analyses. The parameters otherwise described here were kept as default.
Phylogenetic analyses of RAD data
We created six datasets containing different amounts of missing data, which we analysed in a maximum likelihood (ML) framework using RAxML ver. 8.2.11 (Stamatakis 2014) implemented in the Texas A&M University High Performance Research Computing (HPRC) Ada Cluster. Following the recommendation of Leaché et al. (2015), we analysed the datasets using the reconstituted DNA model implemented in RAxML by correcting for ascertainment bias. Because our SNP data were essentially composite data pulled from a large number of loci, it was not justifiable to apply a single model of rate heterogeneity according to typical model tests (Stamatakis 2014). Thus, we used a plain GTR model without any correction for rate heterogeneity by applying the following commands: -m ASC_GTRCAT -V --asc-corr Stamatakis. For each analysis, we performed a rapid bootstrap analysis with 100 replications and searched for the best scoring ML tree.
Species tree estimation
We also analysed the same six datasets in a coalescence framework using SVDQuartets (Chifman and Kubatko 2014), which has been shown to perform well for SNP datasets. Based on the well-defined clades recovered from the RAxML analyses (Fig. 3), we first grouped the individuals into 12 putative species and randomly sampled 100 000 quartets with 100 bootstrap replications. The analyses were performed using PAUP (v4.a165) on XSEDE (Extreme Science and Engineering Discovery Environment, https://www.xsede.org) through the CIPRES Science Gateway (Miller et al. 2012) as well as the TAMU HPRC Ada Cluster.
Mitochondrial gene data generation, alignment, and phylogenetic analysis
Because the RAD data only included Kosciuscola species, we needed an independent analysis to test the monophyly of the genus as well as the ingroup relationships relative to the outgroups. To achieve this, we also generated mitochondrial data for 63 specimens, which represented a subset of the taxon sampling used for RAD data. Using the aliquot from the same DNA extractions used for the RAD data generation, we used the Nextera XT DNA Library Prep Kit for library preparation and performed shotgun sequencing of genomic DNA via 150 bp paired-end sequencing using HiSeq. 4000. Library preparation and next generation sequencing were conducted at the Texas A&M Genomics and Bioinformatics Service. The resulting raw reads were quality-trimmed in Geneious Prime (Biomatters). We extracted mitochondrial genes from the shotgun sequence data by using the K. tristis mitochondrial genome (MG993402, MG993408, MG993414) as a reference, using the ‘Map to Reference’ tool in Geneious. We used the Geneious mapper with low sensitivity to search for short reads that mapped to the reference sequences. This approach was effective in extracting mitochondrial genes to assemble entire mitochondrial genomes in many cases, but there were also a number of instances where complete mitochondrial genome assembly was not possible. Grasshoppers are known to have the largest genome sizes among insects (Bensasson et al. 2001), and shotgun sequencing of genomic DNA often yields a very small proportion of mitochondrial sequences relative to nuclear genes (H. Song, unpubl. data). DNA sequence data generated for this study have been submitted to GenBank (see Table S1).
Initially, we created a total of 15 alignments representing 13 protein-coding genes and 2 ribosomal RNA genes. We also obtained mitochondrial genome sequences of one Hemiacridinae and three Oxyinae species as outgroups: Hieroglyphus tonkinensis (NC_030587), Oxya chinensis (NC_010219), Pseudoxya diminuta (NC_025765) and Caryanda sp. (NC_030165). For mitochondrial protein-coding genes, we aligned based on the conservation of reading frames by first translating into amino acids and aligning individually in MUSCLE (Edgar 2004) using default parameters in Geneious. The two rRNA genes (16S, 12S) were aligned in MAFFT using the E-INS-i setting, also in Geneious. Upon inspection of each alignment, we found that only three mitochondrial genes (COI, COII, COIII) had nearly complete alignment without missing data, while other genes had varying degrees of missing data. Thus, we decided to create a concatenated dataset consisting of these three mitochondrial genes using SequenceMatrix (Vaidya et al. 2011), which resulted in 3042 aligned nucleotides and 67 terminals. We divided the data into a total of nine data blocks (three mitochondrial genes divided into individual codon positions. We then used PartitionFinder 2 (Lanfear et al. 2017) using the ‘greedy’ algorithm (heuristic search) with branch lengths estimated as ‘unlinked’ to search for the best-fit scheme as well as to estimate the model of nucleotide evolution for each partition on the CIPRES Science Gateway. We inferred the phylogeny of Kosciuscola in a ML framework, and used a best-fit partitioning scheme (two partitions) recommended by PartitionFinder with the GTRCAT model applied to each partition and analysed using RAxML 8.2.12 (Stamatakis et al. 2008) on the CIPRES Science Gateway. Nodal support was evaluated using 1000 replications of rapid bootstrapping implemented in RAxML.
We also estimated divergence time based on the same dataset consisting of the three mitochondrial genes. Currently, there is no known fossil record available for Australian grasshoppers to use for establishing calibration points for our study. Recently, Song et al. (2018) included Kosciuscola in a large-scale phylogenetic analysis of Acrididae, and estimated that this genus diverged from the genus Hieroglyphus about 25.24 mya, which we used as a calibration point for our analysis. Koscisucola tasmanicus is only found in Tasmania, which was presumably colonised by flightless grasshoppers when the land bridge to the mainland was open around 43 000 years ago (Lambeck and Chappell 2001). Tasmania was again separated from the mainland around 14 000 years ago when the ocean level rose (Lambeck and Chappell 2001). Thus, we used the midpoint (28 500 ybp) as another internal calibration point for the common ancestor of K. tasmanicus, likely a conservative estimate. We created an XML file in BEAUti from the BEAST2 (ver. 2.6.3) package (Bouckaert et al. 2019), specifying the priors and monophyly constraints. We used the relaxed clock lognormal model for the clock model, the calibrated Yule model with a uniform distribution as a tree prior, and a lognormal distribution as a distribution prior for the older calibration point, and a normal distribution for the Tasmania calibration point. To assess convergence across independent runs, we conducted two separate analyses each for 100 million generations, sampling every 1000 generations on the CIPRES Science Gateway. We inspected the results using Tracer (Rambaut and Drummond 2003), discarded 25% of each run as burn-in, and combined and resampled the trees at every 5000 generations using LogCombiner (Drummond and Rambaut 2007). A maximum clade credibility tree was summarised in TreeAnnotator (Drummond and Rambaut 2007) and visualised in FigTree. All the phylogenetic datasets used in this study including both SNP and mitochondrial data were deposited to DRYAD (https://doi.org/10.5061/dryad.b5mkkwhcs).
Results
After filtering, a total of 101 892 loci were determined to be paralogs, duplicates, or sequences with too many indels or too many SNPs than the parameters specified and were excluded from further analyses. The pyRAD pipeline resulted in a filtered dataset consisting of 636 038 loci with a total of 128 373 607 aligned nucleotides. From this dataset, we exported six datasets that contained different amounts of missing data by adjusting the min. ind. parameter from the pyRAD pipeline. The total taxon sampling included 260 terminals, and we varied the number of terminals with data at a locus ranging from 175, 150, 125, 100, 75, and to 50. The proportion of missing data accordingly ranged from 28.3%, 36.9%, 48.0%, 56.7%, 66.7%, and to 75.7%, respectively (see Table S2; Fig. S2). As we increased the amount of missing data, the number of loci and variable sites increased.
In general, phylogenetic resolution among the lineages increased as the number of SNPs and the amount of missing data increased (Fig. S2). Even with the smallest number of SNPs (dataset s175 with 298 SNPs), K. tristis was recovered as a monophyletic group, and this pattern continued with data size increase. On the other hand, the monophyly of three species (K. cuneatus, K. usitatus and K. tasmaniscus) was only recovered when the dataset included at least 2554 SNPs (s125). Koscisucola cognatus was recovered as paraphyletic in all datasets because of the position of K. tasmanicus. While the basal position of K. tristis within the genus was consistent through this sensitivity analysis, the phylogenetic relationships among the other species shifted with increasing data size, and stabilised when the dataset included at least 6167 SNPs (s100). However, internal relationships continued to shift to achieve more resolution with increasing dataset size. Further discussion about the relationships is based on the s50 dataset (Fig. 3), which included the largest number of SNPs among the six datasets we analysed.
Based on the RAD phylogeny (Fig. 3), we recovered the following relationships within Kosciuscola: (K. tristis (K. cuneatus, (K. usitatus, (K. cognatus, K. tasmanicus)))). However, within K. tristis, K. cuneatus and K. cognatus, we identified a number of geographically restricted clades with strong nodal support, which we recognised here as putative cryptic species. Specifically, within K. tristis, we identified four subclades restricted to the following geographic ranges: (1) ‘Hotham & Sth Vic Clade’ in the Northern and Southern Alps in Victoria, including Mt. Baw Baw; (2) ‘Buffalo Clade’ at Mt. Buffalo (K. t. restrictus); (3) ‘Bogong Clade’ in the Bogong High Plains; and (4) ‘NSW Clade’ in Northern and Southern Alps of NSW. For K. cuneatus, we also identified four subclades restricted to the following regions: (1) ‘ACT & Nth NSW Clade’ in the ACT and NSW; (2) ‘Sth NSW Clade’ in the Southern Alps in NSW; (3) ‘Bogong Clade’ in Northern Victoria’s Bogong High Plains; and (4) ‘Sth Vic & Buffalo Clade’ in the Southern Alps in Victoria, including Mt. Buffalo. In K. usitatus, there is a broad distribution throughout ACT and NSW, the ‘ACT & NSW Clade’, and there was relatively strongly resolved structure in Victoria in the Bogong High Plains and Mt Hotham, the ‘Bogong & Hotham Clade’, despite the lack of reciprocal monophyly. For K. cognatus, which was not monophyletic, we identified two geographically overlapping subclades ‘ACT & NSW’ and ‘Vic Clade’ which spans a wide area into relatively low elevations; and the ‘NSW & Vic Clade’ which includes individuals from the higher elevations in NSW and the Northern Victorian Alps. Finally, K. tasmanicus, which was nested within K. cognatus, is endemic to Tasmania and showed little geographical structure.
The multicoalescence species tree analyses based on the 12 putative species (see Fig. S3), identified from the RAD phylogeny of the s50 dataset, yielded largely congruent relationships with some minor differences. Similar to the pattern that we observed in the RAD-based analyses, the nodal support values increased as the number of SNPs increased. s75 and s50 datasets resulted in identical relationships (see Fig. S3), which we further elaborate. Within K. tristis, we found that the lineage occurring in the Victorian Alps to be the earliest diverging lineage. The lineages found in the Bogong High Plains and Mt. Buffalo (K. t. restrictus) were more closely related to each other than the one found in the NSW Alps. For K. cuneatus, the lineage distributed in the ACT and NSW Alps was the earliest to diverge, and the lineages found in the Bogong High Plains and the Victorian Alps were more closely related to each other than to the one found in the NSW Alps. Unlike the RAD-based analyses (Fig. 3; see Fig. S2), K. cognatus was recovered as a monophyletic group, and K. tasmanicus was recovered as sister to K. cognatus.
The ML phylogeny based on three cytochrome c oxidase genes (COI, COII, COIII) (Fig. 4a) recovered Kosciuscola as a monophyletic group, and K. tristis as the earliest diverging lineage within the genus. However, it failed to recover K. cuneatus and K. usitatus as monophyletic taxa. Similar to the RAD phylogeny, K. cognatus was recovered as paraphyletic, with K. tasmanicus nested within. The Bayesian phylogeny inferred from the BEAST analysis (Fig. 4b) recovered a similar topology to the ML tree, with only minor differences. The analysis estimated that Kosciuscola and Hieroglyphus shared a common ancestor around 17 mya (95% credibility interval, 23.35–11.11 my), and that the common ancestor of all Kosciuscola species diverged around 1.95 mya (CI, 3.13–0.95 my). The analysis also estimated the age of the common ancestor of all K. tristis lineages to be 0.99 mya (CI, 1.62–0.48 my), and the age of the common ancestor of the remaining species to be 1.51 mya (CI, 2.37–0.78 my). Because the internal relationships among species other than K. tristis were not fully resolved based on this dataset, we do not comment further about their divergence times.
Discussion
Based on our comprehensive phylogeny (Fig. 3) of Australia’s skyhoppers, Kosciuscola, and with divergence time estimates (Fig. 4b), we suggest that the genus was likely present towards at least the beginning of the Pleistocene and diversified throughout this period. Our study reveals that Kosciuscola consists of more than the five currently described species and supports up to nine additional geographically isolated and genetically distinct species (Fig. 3), confirming the taxonomic assessment by Muschett (2016) to be formalised in future works. In comparison with previous studies on other mountain specialists in Australia, our data concur with deep divergence times, and support some of the previously reported centres of endemism and geographic breaks. However, our study also highlights major differences in genetic structuring even among closely related species, reinforcing the notion that nuanced conservation planning is required to maintain biodiversity in the Australian Alps as climate change progresses quickly in the region.
Phylogeographic patterns in Kosciuscola
Our study suggests that the ancestral Kosciuscola likely diverged sometime in the early Pleistocene, and we hypothesise that this divergence was not due to cold adaptation alone, because alpine conditions at the highest elevations of the Australian Alps are thought to have appeared much earlier, between 5 and 2.5 mya (Gallagher et al. 2003). Previous studies have shown that different Kosciuscola species exhibit different morphological, ecological, physiological, and behavioural traits (Slatyer et al. 2014; Muschett 2016). For example, a preliminary examination of internal male genitalia shows clear species-specific morphological differences (Muschett 2016), different species occupy somewhat different elevational niches (Fig. 2) (Campbell and Dearn 1980), thermal tolerance differs among species (Slatyer et al. 2016), the extreme fighting behaviour characteristic of this genus varies among species (G. M. Muschett, C. J. Painting, M. E. Herberstein, K. D. L. Umbers, unpubl. data), as does their temperature-dependent physiological colour change (K. D. L. Umbers, J. C. O’Hanlon, M. E. Herberstein, unpubl. data). Collectively, these differences suggest that a number of evolutionary processes, such as ecological divergence, sexual selection, as well as allopatric divergence, have been important drivers of diversification in Kosciuscola.
Our data suggest that the ancestral skyhoppers gave rise to two ecologically divergent lineages, one adapted to the high-elevation alpine habitat that eventually led to the speciation of K. tristis, and the other adapted to the subalpine habitat that led to the remaining Kosciuscola species. Taking each currently described species, below we discuss the phylogenetic structure supported by our analyses. As our data reveal several more cryptic species than previously described, many of which seem to be short range endemics, we also discuss the conservation implications of this new phylogenetic hypothesis.
Kosciuscola tristis
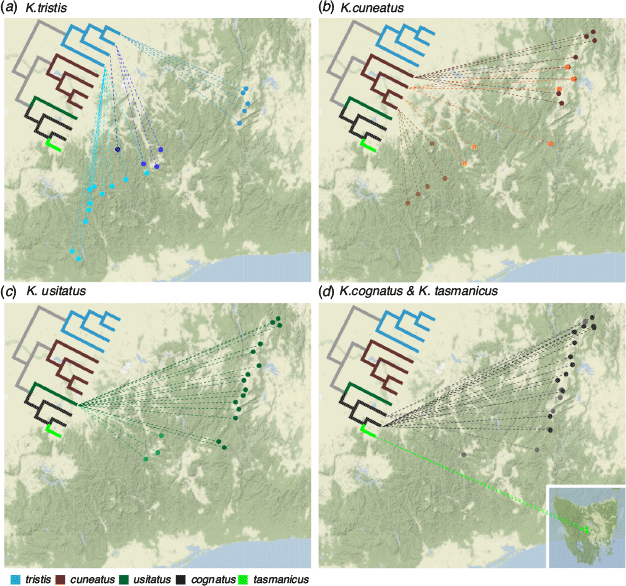
Kosciuscola tristis was originally described by Sjöstedt (1934) from a female specimen collected on Mt. Kosciuszko, with Rehn (1957) later dividing this into two subspecies based on minor morphological differences: the more widespread K. tristis, and K. tristis restrictus confined to Mt. Buffalo. Over the past 65 years since Rehn’s (1957) revision of the genus, hundreds of specimens that could be identified as K. tristis have been collected throughout the mountain regions of NSW and Victoria. However, exactly how many genetically distinct lineages of K. tristis exist, and how genetically distinct K. t. restrictus is from the nominal species had not been rigorously tested. Tatarnic et al. (2013) were the first to propose a phylogeny of Kosciuscola based on partial mitochondrial COI and COII genes, which tested the monophyly of the genus and of each known species. Including multiple specimens of K. tristis collected from different mountains in NSW and Victoria, they found geographical structuring within the species, albeit with weak support at some nodes (Tatarnic et al. 2013). Slatyer et al. (2014) focused on K. tristis populations from the Kosciuszko region, the Bogong High Plains, and southern Victoria to test for geographical structuring, and found that mitochondrial COI genes did not always resolve phylogeographic patterns with strong confidence, but that microsatellite data suggested significant population differentiation across small spatial scales, coupled with a strong signature of isolation-by-distance. In a comparative phylogeographic analysis of five Australian alpine arthropod species, Endo et al. (2015) showed that K. tristis exhibited strong phylogeographic structuring, showing a division between northern and southern Victorian lineages. Here, our results show that K. tristis is the earliest diverging clade within the genus, and that there is strong phylogeographic structure within K. tristis, corroborating previous findings (Tatarnic et al. 2013; Slatyer et al. 2014; Endo et al. 2015). We show that K. tristis consists of four clades across the Australian Alps that are geographically isolated from one another with little or no contemporary gene flow, and thus suggest that they merit recognition as four distinct species. These four clades are: K. t. tristis from the NSW Alps, hereafter called K. t. tristis (NSW clade); K. t. restrictus found on Mt. Buffalo; K. t. tristis (Bogong Clade) an undescribed species found on the Bogong High Plains; and K. t. tristis (Hotham & Sth Vic Clade) another undescribed species found on Mt. Hotham and the Southern Victorian Alps.
During cooler periods in the Pleistocene, the preferred habitat of the ancestral K. tristis was likely more contiguous across the Victorian and NSW Alps than today, potentially allowing these flightless grasshoppers to inhabit the region widely. In NSW, glaciation at elevations around 2000 m and above meant that these highest elevations were unable to support these grasshoppers during the glacial periods, with suitable habitat only existing at lower, somewhat more connected elevations. Similarly, despite Victoria’s peaks remaining ice-free, temperatures during times of glacial maxima probably also made them inhospitably cold. During periods of warming, the cool climes at the peaks would have receded, and the ancestral K. tristis could have inhabited higher-elevation habitat once more. This elevational displacement corresponding to warming and cooling periods is different to what is hypothesised to have led to the patterns of speciation in Melanoplus grasshoppers from the Rocky Mountains (USA) (Knowles 2000, 2001). In the Australian Alps, periods of glaciation may have given rise to range expansion and gene flow whereas in the Rockies, glaciation seems to have driven retreat into refugia and restricted gene flow. More recently, as a warmer climate prevailed, K. tristis populations would have become restricted to higher elevations, eventually becoming isolated, and leading to the pattern we see today.
Among the four clades of K. tristis sensu lato (s.l.) (Figs. 3, 5a), the earliest diverging clade is K. t. tristis (Hotham and St Vic Clade) found across the southern stretches of the Victorian high-country including Mt. Hotham and Mt. Baw Baw. We found that the individuals from Mt. Hotham clustered with strong support with those on the peaks across the rest of the southern Victorian Alps, but a remarkably clear split with individuals from locations only ~10 kms to the north on Mt. Feathertop and the Bogong High Plains. Barriers to gene flow between Mt. Hotham and its neighbouring northern peaks are enigmatic but appear to limit gene flow in other groups as well. For example, in the alpine bog skink Pseudomoia cryodroma, individuals from Mt. Hotham form a distinct clade from those of the Bogong High Plains (Haines et al. 2017).
|
Potential evidence of elevational displacement and isolation is apparent in the next clade to diverge, K. t. restrictus, found only on Mt. Buffalo. Mt. Buffalo is an isolated peak about 40 km from the locations at which K. t. tristis (Hotham & Sth Vic Clade) is found. It is separated by a small stretch of lowland at 300–500 m elevation to Mt. Buffalo’s east and south. It is possible that, during a cooling period, some of the ancestral populations from the southern Victorian Alps pushed down to lower elevation and expanded northward to Mt. Buffalo, moved up to the upper elevations (1333–1553 m) during the warming period and becoming permanently associated within this habitat. The relative geographic isolation of Mt. Buffalo must have been sufficient for limiting gene flow such that K. t. restrictus is now morphologically distinct enough from K. t. tristis (NSW Clade) for Rehn (1957) to describe it as a subspecies. Our observations confirm this, with the males of K. t. restrictus being very small and not undergoing pronounced colour change characteristic of K. t. tristis (NSW Clade) (K. D. L. Umbers, J. C. O’Hanlon, M. E. Herberstein, unpubl. data) and as such we recommend it is elevated to its own species. Support for a distinct Mt. Buffalo clade has been shown in previous genetic studies, each showing unique K. tristis haplotypes on Mt. Buffalo or genetic structuring between K. tristis on Mt. Buffalo and K. tristis from the main Victorian range (Tatarnic et al. 2013; Slatyer et al. 2014; Endo et al. 2015). Mt. Buffalo appears to be a biogeographically important region, with various other endemic organisms. For example, there are five haplotypes of the springtail Australotomurus baratus found only on Mt. Buffalo (Endo et al. 2015). Also, species of Poa snowgrass show genetic structure in the form of distinctiveness in eigenvector score between Mt. Buffalo and the Victorian main range at microsatellite markers (Griffin and Hoffmann 2014). Mt. Buffalo also harbours the Mt. Buffalo Glowworm Arachnocampa buffaloensis (Baker et al. 2008) and a subspecies of the Small Alpine Xenica butterfly, Oreixenica latialis theddora (Dunn 2019). In contrast, not all groups show this pattern, for example, K. cuneatus from Mt. Buffalo do not form an isolated clade, and instead cluster with individuals from other nearby southern Victoria locations.
The most recently diverged lineage, K. t. tristis (NSW Clade), is found furthest north, restricted to the tallest mountains in NSW, including the type locality on Mt. Kosciuszko, up to its most northerly populations on Mt. Jagungal, NSW. Its sister-clade, K. t. tristis (Bogong Clade) is distributed in the northern Victorian Alps from Mt. Feathertop and the Bogong High Plains. Our study supports a clear distinction between the northernmost clade, K. t. tristis (NSW Clade) and its sister clades found to the south across the wide Murray Valley in the Victorian Alps, but where the clade boundary lies is unknown. Sampling in the wilderness areas such as the Cobberras Range on the NSW–Victoria border, where K. tristis is known to occur (R. Slatyer, pers. obs.), is needed to precisely locate the geographic breaks. Interestingly, there is no K. tristis s.l. recorded from the more northern Brindabella Ranges of the ACT. Kosciuscola tristis s.l. has never been recorded from the seemingly suitable habitat in the ACT despite a long history of entomological work and collections in the area. This absence is supported by a lack of specimens in the Australian National Insect Collection (ANIC) which is rich in Kosciuscola and the personal observations of the authors of this paper who have conducted collections of Kosciuscola in the area many times over the past 13 or so years.
Kosciuscola cuneatus
Kosciuscola cuneatus s.l. was originally described from a male specimen from Lee’s Spring, ACT (Rehn 1957), it is morphologically and ecologically distinct and appears to be adapted to the wooded subalpine and montane areas. The phylogeography of this species has not previously been examined. Tatarnic et al. (2013) reported K. cuneatus but our study suggests, and revisiting the data confirms, that in Tatarnic et al. (2013) K. cuneatus is actually K. cognatus (ACT & NSW Clade). Although our taxon sampling for K. cuneatus s.l. was less extensive than that for K. tristis s.l., we nonetheless recovered four near-geographically separated clades (Figs. 3, 5b): K. cuneatus (ACT & NSW Clade) primarily from the ACT Alps, an undescribed species from the NSW Alps K. cuneatus (Sth NSW Clade), an undescribed species from the Bogong High Plains K. cuneatus (Bogong Clade), and an undescribed species from the Victorian Alps, including Mt. Buffalo, K. cuneatus (Buffalo & Sth Vic Clade). Unlike the four lineages of K. tristis where diversification appears to have occurred from south to northeast, the pattern of lineage splitting K. cuneatus begins in the northern limit of the Australian Alps in the ACT, with the most recent diversification/splitting occurring in the southwest. The geographic distinction between K. cuneatus (ACT & NSW Clade) and K. cuneatus (Sth NSW Clade) is seemingly less pronounced than between other clades and further work may show that the clades have elevational limits, but more detailed work on the species in the region of overlap is needed.
Because K. cuneatus s.l. appears restricted to lower-elevation wooded areas, we postulate that its distribution was probably less affected by the warming and cooling periods of the Pleistocene than K. tristis, for example, which seem to be more restricted to high elevations. Instead, we suggest that the current distribution of K. cuneatus s.l. most likely reflects the broad distribution of the Kosciuscola ancestor shared by K. tristis and the rest of Kosciuscola and that cooling and warming periods and related conditions gave rise to speciation across the alps that is currently maintained. Because of their limited dispersal capacity owing to brachyptery it seems plausible that genetic isolation was likely if we can invoke local extinction at the intermediate ranges. Among the three southern clades, we find a strong split between those from Sth NSW (K. cuneatus (Sth NSW Clade)) and the two from Victoria (K. cuneatus (Bogong Clade) and K. cuneatus (Sth Vic and Buffalo Clade)) suggesting the Murray Valley is an important break, but as for K. tristis, the precise location of that break requires sampling through wilderness areas on the NSW–Victoria state border.
In Victoria, individuals from the Bogong High Plains K. cuneatus (Bogong Clade) form a sister clade to the southern Victorian Alps clade K. cuneatus (Sth Vic and Buffalo Clade). This is different from the pattern found in K. tristis s.l., in which the Bogong clade is more closely related to the NSW clade, and perhaps suggest that K. cuneatus populations transverse low elevation corridors that K. tristis does not. Intriguingly in K. cuneatus s.l., there seems to be gene flow between the Mt. Buffalo population and the southern reaches of the main Victorian range. Our Mt. Buffalo samples cluster with those from the southern part of the Victorian range ~50 km to the south, but not with specimens from the northern part of the Victorian range, only around 40 km to the east. These flightless grasshoppers may be able to cross low-elevation divides to reach Mt. Buffalo from southern regions and vice versa regularly enough to maintain genetic connectivity due to its propensity to occur at lower elevations, or populations may be continuous across the region. If we can rule out migration, we know, for example, that K. tristis (NSW Clade) is semelparous (K. D. L. Umbers, unpubl. data) and thus that it is unlikely they are moving between mountains, then perhaps we expect to find continuous populations connecting Mt. Buffalo to the southern part of the Victorian range around places such as Abbeyard, Buckland, and Wobonga in Victoria. Kosciuscola cuneatus s.l. is not alone in showing connectivity between Mt. Buffalo and the main ranges, there is no clear evidence for separate haplotypes of the millipede Orocladosoma kosciuskovagum on Mt. Buffalo, or the springtail Australotomurus c.f. barbatus (Endo et al. 2015). More sampling in remote areas and information about species life history are required to understand how gene flow might be maintained. It is also of interest for future work to include samples of K. cuneatus s.l. from Mt. Hotham to see whether the enigmatic geographic break supported in other taxa (Haines et al. 2017), including K. tristis s.l., is supported in K. cuneatus s.l. too.
Kosciuscola usitatus
Kosciuscola usitatus was originally described from a male specimen from Mt. Gingera in the ACT (Rehn 1957) and is widely distributed throughout the mountain ranges in the ACT and NSW, but only reaching as far south as the Bogong High Plains and Mt. Hotham in northern Victoria. Because we found strong geographical structuring in K. tristis and K. cuneatus, we also expected K. usitatus to show a similar pattern. Surprisingly however, we found little geographic structuring within the species (Figs. 3, 5c). The only clearly defined and well-supported clade consisted of those individuals from the Bogong High Plains and Mt. Hotham within which eastern Bogong High Plains individuals form a clade and the western Bogong High Plains samples cluster with those from Mt. Hotham. Based on the same logic applied to other species, we feel justified to recognise this clade as a cryptic species that is genetically isolated from other populations, with a caveat that it is nested within the ACT & NSW Clade without reciprocal monophyly. Previous comparisons of museum specimens collected across the range where K. usitatus is found showed that there is little or no morphological variation within this species (Muschett 2016), which may indicate the genetic isolation of the Bogong & Hotham Clade could have been relatively recent. This species has a broad elevational distribution, concentrated at middle elevations but extending into the highest elevations (Fig. 2). Over the past decade collecting this genus, however, we have not found K. usitatus found at elevations lower than 1100 m. K. usitatus and K. cuneatus shared a common ancestor but the distribution of K. usitatus is reduced, they are missing from the majority of the Victorian Alps save for the far north (Bogong High Plains and Hotham). This same truncated distribution is shared by K. cognatus s.l., sister to K. usitatus.
Kosciuscola cognatus
Kosciuscola cognatus was originally described from a male specimen collected from Mt. Gingera, ACT (Rehn 1957) and has a different geographic spread to the other skyhoppers. K. cognatus extends into the surrounding montane forest at around 1000 m, from the Brindabella region in ACT and NSW Northern Alps, east to the coastal plateau (Brown Mountain, Mt. Antitangle). Similar to K. usitatus s.l., K. cognatus s.l.’s distribution extends only as far south as the Bogong High Plains and Mt Hotham in northern Victoria. It is sympatric with K. usitatus at middle elevations in the mountains and is also found at lower elevations outside the Australian Alps proper (Fig. 2). In this study the phylogeny only includes samples from the high-elevation regions of its distribution and recovers K. cognatus s.l. as paraphyletic due to K. tasmanicus being more closely related to the southern K. cognatus (NSW & Vic Clade) than to the northern K. cognatus (ACT & NSW Clade) (Figs. 3, 5d). That the two K. cognatus clades we identify here have broadly overlapping ranges may indicates that some factors other than geographic isolation, such as sexual selection or ecological divergence, could be maintaining reproductive isolation.
The K. cognatus (ACT & NSW Clade) samples in our study are exclusively found in NSW including the K. cognatus type locality. Tatarnic et al. (2013) included representatives of K. cognatus (ACT & NSW Clade) but therein misidentified it as K. cuneatus. In revisiting the results in Tatarnic et al. (2013) and combining them with ours, we suggest that K. cognatus (ACT & NSW Clade)’s range extends to the eastern reaches of the Great Dividing Range to Brown Mountain and Mt. Tantawanglo across the Monaro plateau as well as up into the Brindabella Ranges. Much more sampling at lower montane elevations between 1500 and 1000 m is required to discover the full geographic extent of this clade’s distribution.
The K. cognatus (NSW & Vic Clade), which is sister to K. tasmanicus, is likely an undescribed species. Tatarnic et al. (2013) also recovered different individuals from these locations in a clade as sister to K. tasmanicus, and thus our findings corroborate their study. Within K. cognatus (NSW & Vic Clade), we found a somewhat well supported split between populations from NSW and Victoria that corresponds to the Murray Valley, a dominant geographic feature that seems to be a barrier to gene flow in K. tristis and K. cuneatus as well. We hypothesise that the ancestral K. cognatus (NSW & Vic Clade), which is the ancestor of K. tasmanicus, was once widely distributed across the southeast to low enough elevations to cross the Bassian Plain to Tasmania during the long periods the land bridge was open. Where are they now? The most southern records of K. cognatus in the ANIC are from near Omeo (650 m a.s.l), Mt. Ewan, and Ensay North (300 m a.s.l) in Victoria which are roughly 50 km further south than our most southern samples. We have not verified the identification of these samples. But in combination with further collections at those sites, they would be of great interest to determine whether the distribution of K. cognatus does in fact still extend into the southern lowlands. Revisiting insect collections from the far southeast and at locations where other Bassian fauna are found is recommended to determine the whereabouts of the ancestors of K. tasmanicus.
Kosciuscola tasmanicus
Kosciuscola tasmanicus is the only member of the genus known from Tasmania and was described as a separate species due to this geographic isolation (Rehn 1957). Our phylogeny recovered it as a well-supported monophyletic group that split from a K. cognatus (NSW & Vic Clade) ancestor (Figs. 3, 5d). We suggest that during the relatively cool conditions of the Pleistocene, the ancestral K. cognatus expanded its range to the south when the land bridge between the mainland and Tasmania was open (Lambeck and Chappell 2001) and a subsequent separation of Tasmania from the mainland led to allopatric speciation. The metallic snow skink (Carinscincus metallicus) provides a clear example of a land bridge distribution in which there are populations on the chain of Bass Strait islands between Tasmania’s north-eastern cape and Victoria’s Wilson’s Promontory (McCoull 2000; Kreger et al. 2020). We are not aware of Kosciuscola on Bass Strait islands but suggest that they could be important targets for future sampling. If Kosciuscola took the same route to Tasmania as Carinascincus then, once on Tasmania, they may have quickly expanded into empty niches that became available after the retreat of extensive glaciation across the island. Our samples are restricted to the central plateau and showed no strong structure, a pattern concordant with the largely contiguous geography of Tasmania’s central plateau, Our sampling may be an incomplete treatment of the species distribution; however, we have searched many Tasmanian mountains and neither our sampling trips nor any historical records we know of place Kosciuscola outside the central plateau.
Conservation implications
The extent of the area in the Australian Alps with the cool, wet conditions to which the modern biota are accustomed is shrinking (Parida et al. 2015). The winter snowpack is thinning and the snow line is receding as climate change progresses quickly in the region (Hennessy et al. 2007). Predictions suggest that the alps will have little snow by 2070, less than 50 years from now (Hughes 2003, 2011). Much of the flora and fauna of the Australian Alps is vulnerable to climate change with experts predicting shifts in vegetation cover, and the abundances and elevational ranges for many species over the next 25 years, sighting winners, losers and substantial uncertainty (Camac et al. 2020). The strong effects of climate change have been evident for several decades (Annandale and Kirkpatrick 2017; Kirkpatrick et al. 2019; Karpala 2020), and have recently been brought into sharp relief by Australia’s mega-fires in the summer of 2019–2020 in which large stretches of alpine and subalpine habitat burned (Wintle et al. 2020). Despite the vast majority of Australia’s alpine and sub-alpine habitat being contained in the protection of National Parks, the effects of climate change are unfortunately exacerbated by several other, compounding threats. Habitat damage by feral horses and human development, predation by cats, foxes, competition from weeds and disease from fungal pathogens are all significant threats to alpine biota in their own right, each to a greater or lesser degree when considered on a species-by-species basis (Driscoll et al. 2019; Hoffmann et al. 2019). Add those threats to both the challenges in overcoming geopolitical boundaries across four states and territories, and an overall desperate lack of basic knowledge on most species in the region and the outlook for life in the Australian Alps is currently bleak, representing a formidable conservation challenge (Williams et al. 2015). So, what do we need?
Kosciuscola probably consists of not 5 but up to 14 species, all of which are flightless, and therefore probably short-range endemics. There are several actions required immediately to assess their vulnerability to the suite of threats in the alps. A taxonomic revision of the genus is needed to name the new species so their conservation status can be formally assessed (Muschett 2016). To understand their vulnerability to climate change, building on Slatyer et al. (2014), knowledge about their susceptibility to high and low temperatures, and to desiccation is required across their lifecycle, especially for the egg stage, which is potentially vulnerable to freezing in low snow years when insulation in the subnivium is compromised. To conduct precise species distribution modelling to identify potential refugia, we need data on growth rate at different temperatures (Elith et al. 2010; Guisan et al. 2013). With this knowledge, we can determine what kinds of conservation actions will be optimal for Kosciuscola, such as assisted migration (if suitable sites exist), ex situ conservation (if resources are available long-term) or assisted evolution (if not prohibitively expensive due to massive genome size). Ecologically, we suspect that the skyhoppers are similar to the bogong moths in terms of making critical nutrients available to the predators (Green et al. 2020), and also that the plant communities are likely shaped by their relentless herbivory. We predict that population crashes in Kosciuscola such as we have recently seen in bogong moths (Green et al. 2020) would have far-reaching effects on the Australian Alps ecosystems. While the empirical data listed above are generated to determine the nuanced vulnerabilities of the skyhoppers, we urge ecosystem-wide approaches to maintaining biodiversity in the Australian Alps, especially action on climate change.
Ours and many studies before ours show clearly that the Australian Alps harbour a great diversity of surprisingly old species across the ranges, and that the current set of described species is certainly an underestimate of the true diversity of the region (Hatley and Murphy 2016; Haines et al. 2017). To protect the species we know, and those yet to be described, we need to identify priority actions, areas and taxa. One critical next step toward setting the right management targets is to synthesise all the phylogeographic studies so far conducted in the Australian Alps to identify common patterns and genetic refugia. We must gather the data we have to guide the difficult but necessary decisions we must make now about what actions to take. We also need brave management and bold conservation practitioners to be given the resources to implement it (Redding and Mooers 2006; Nicotra et al. 2015). For the Australian Alps as a whole, the strongest possible action on climate change (net negative) is required with the greatest of urgency if the current assemblage of native biota is to persist unchanged for the next 100 years. In addition, it is also clear that the compounding threats of feral species, development, and disease must be brought under careful control to maintain current levels of biodiversity in the region (Brannelly et al. 2015; Driscoll et al. 2019). Most Australia’s alpine regions are already contained within National Parks so the opportunity to act to remove compounding threats and bend the curve on greenhouse gas emissions to cool the mountains is already available to all governments where the will exists.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgements
We thank You Ning Su from the ANIC for support in providing access to the collection for location information and specimen verification. This project was funded through a grant from the Hermon Slade Foundation, HSF 13-9: Speciation in the mountains: phylogenomics of the alpine grasshopper genus Kosciuscola. Tasmania permit: DPIPWE FA13927, NSW National Parks and Wildlife Permit SL100763, DEPI Victoria Permit 10 006 742, License to collect in ACT (no permit number provided). We thank Texas A&M AgriLife Genomics and Bioinformatics Service for data generation and retrieval.
References
Annandale, B., and Kirkpatrick, J. B. (2017). Diurnal to decadal changes in the balance between vegetation and bare ground in Tasmanian fjaeldmark. Arctic, Antarctic, and Alpine Research 49, 473–486.| Diurnal to decadal changes in the balance between vegetation and bare ground in Tasmanian fjaeldmark.Crossref | GoogleScholarGoogle Scholar |
Atkins, Z. S., Amor, M. D., Clemann, N., Chapple, D. G., While, G. M., Gardner, M. G., Haines, M. L., Harrisson, K. A., Schroder, M., and Robert, K. A. (2020). Allopatric divergence drives the genetic structuring of an endangered alpine endemic lizard with a sky-island distribution. Animal Conservation 23, 104–118.
| Allopatric divergence drives the genetic structuring of an endangered alpine endemic lizard with a sky-island distribution.Crossref | GoogleScholarGoogle Scholar |
Baker, C. H., Graham, G. C., Scott, K. D., Cameron, S. L., Yeates, D. K., and Merritt, D. J. (2008). Distribution and phylogenetic relationships of Australian glow-worms Arachnocampa (Diptera, Keroplatidae). Molecular Phylogenetics and Evolution 48, 506–514.
| Distribution and phylogenetic relationships of Australian glow-worms Arachnocampa (Diptera, Keroplatidae).Crossref | GoogleScholarGoogle Scholar | 18583158PubMed |
Bell, N., Griffin, P. C., Hoffmann, A. A., and Miller, A. D. (2018). Spatial patterns of genetic diversity among Australian alpine flora communities revealed by comparative phylogenomics. Journal of Biogeography 45, 177–189.
| Spatial patterns of genetic diversity among Australian alpine flora communities revealed by comparative phylogenomics.Crossref | GoogleScholarGoogle Scholar |
Bensasson, D., Petrov, D. A., Zhang, D.-X., Hartl, D. L., and Hewitt, G. M. (2001). Genomic gigantism: DNA loss is slow in mountain grasshoppers. Molecular Biology and Evolution 18, 246–253.
| Genomic gigantism: DNA loss is slow in mountain grasshoppers.Crossref | GoogleScholarGoogle Scholar | 11158383PubMed |
Bouckaert, R., Vaughan, T. G., Barido-Sottani, J., Duchêne, S., Fourment, M., Gavryushkina, A., Heled, J., Jones, G., Kühnert, D., Maio, N. D., Matschiner, M., Mendes, F. K., Müller, N. F., Ogilvie, H. A., Plessis, L., du, Popinga, A., Rambaut, A., Rasmussen, D., Siveroni, I., Suchard, M. A., Wu, C.-H., Xie, D., Zhang, C., Stadler, T., and Drummond, A. J. (2019). BEAST 2.5: An advanced software platform for Bayesian evolutionary analysis. PLoS Computational Biology 15, e1006650.
| BEAST 2.5: An advanced software platform for Bayesian evolutionary analysis.Crossref | GoogleScholarGoogle Scholar | 30958812PubMed |
Brannelly, L. A., Hunter, D. A., Lenger, D., Scheele, B. C., Skerratt, L. F., and Berger, L. (2015). Dynamics of chytridiomycosis during the breeding season in an Australian alpine amphibian. PLoS ONE 10, e0143629.
| Dynamics of chytridiomycosis during the breeding season in an Australian alpine amphibian.Crossref | GoogleScholarGoogle Scholar | 26629993PubMed |
Camac, J. S., Umbers, K. D. L., Morgan, J. W., Geange, S. R., Hanea, A., Slatyer, R. A., McDougall, K. L., Venn, S. E., Vesk, P. A., Hoffmann, A. A., and Nicotra, A. B. (2020). Predicting species and community responses to global change in Australian mountain ecosystems using structured expert judgement. bioRxiv , .
| Predicting species and community responses to global change in Australian mountain ecosystems using structured expert judgement.Crossref | GoogleScholarGoogle Scholar |
Campbell, N. A., and Dearn, J. M. (1980). Altitudinal variation in and morphological divergence between three related species of grasshopper Praxibulus sp., Kosciuscola cognatus and Kosciuscola usitatus (Orthoptera: Acrididae). Australian Journal of Zoology 28, 103–118.
| Altitudinal variation in and morphological divergence between three related species of grasshopper Praxibulus sp., Kosciuscola cognatus and Kosciuscola usitatus (Orthoptera: Acrididae).Crossref | GoogleScholarGoogle Scholar |
Chifman, J., and Kubatko, L. (2014). Quartet inference from SNP data under the coalescent model. Bioinformatics 30, 3317–3324.
| Quartet inference from SNP data under the coalescent model.Crossref | GoogleScholarGoogle Scholar | 25104814PubMed |
Driscoll, D. A., Worboys, G. L., Allan, H., Banks, S. C., Beeton, N. J., Cherubin, R. C., Doherty, T. S., Finlayson, C. M., Green, K., Hartley, R., Hope, G., Johnson, C. N., Lintermans, M., Mackey, B., Paull, D. J., Pittock, J., Porfirio, L. L., Ritchie, E. G., Sato, C. F., Scheele, B. C., Slattery, D. A., Venn, S., Watson, D., Watson, M., and Williams, R. M. (2019). Impacts of feral horses in the Australian Alps and evidence-based solutions. Ecological Management & Restoration 20, 63–72.
| Impacts of feral horses in the Australian Alps and evidence-based solutions.Crossref | GoogleScholarGoogle Scholar |
Drummond, A. J., and Rambaut, A. (2007). BEAST: Bayesian evolutionary analysis by sampling trees. BMC Evolutionary Biology 7, 214.
| BEAST: Bayesian evolutionary analysis by sampling trees.Crossref | GoogleScholarGoogle Scholar | 17996036PubMed |
Dunn, K. L. (2019). Oreixenica latialis (Lepidoptera: Nymphalidae: Satyrinae) on Mount Buffalo, Victoria: field notes on its local distribution and adult behaviour to assist future conservation work. Calodema 724, 1–24.
Eaton, D. A. R. (2014). PyRAD: assembly of de novo RADseq loci for phylogenetic analyses. Bioinformatics 30, 1844–1849.
| PyRAD: assembly of de novo RADseq loci for phylogenetic analyses.Crossref | GoogleScholarGoogle Scholar |
Edgar, R. C. (2004). MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Research 32, 1792–1797.
| MUSCLE: multiple sequence alignment with high accuracy and high throughput.Crossref | GoogleScholarGoogle Scholar | 15034147PubMed |
Elith, J., Kearney, M., and Phillips, S. (2010). The art of modelling range-shifting species. Methods in Ecology and Evolution 1, 330–342.
| The art of modelling range-shifting species.Crossref | GoogleScholarGoogle Scholar |
Endo, Y., Nash, M., Hoffmann, A. A., Slatyer, R., and Miller, A. D. (2015). Comparative phylogeography of alpine invertebrates indicates deep lineage diversification and historical refugia in the Australian Alps. Journal of Biogeography 42, 89–102.
| Comparative phylogeography of alpine invertebrates indicates deep lineage diversification and historical refugia in the Australian Alps.Crossref | GoogleScholarGoogle Scholar |
Filshie, B. K., Day, M. F., and Mercer, E. H. (1975). Colour and colour change in the grasshopper, Kosciuscola tristis. Journal of Insect Physiology 21, 1763–1770.
| Colour and colour change in the grasshopper, Kosciuscola tristis.Crossref | GoogleScholarGoogle Scholar |
Furse, J. M., and Coughran, J. (2011). An assessment of the distribution, biology, threatening processes and conservation status of the freshwater crayfish, genus Euastacus (Decapoda, Parastacidae), in continental Australia. I. Biological background and current status. New Frontiers in Crustacean Biology , 241–252.
| An assessment of the distribution, biology, threatening processes and conservation status of the freshwater crayfish, genus Euastacus (Decapoda, Parastacidae), in continental Australia. I. Biological background and current status.Crossref | GoogleScholarGoogle Scholar |
Gallagher, S. J., Greenwood, D. R., Taylor, D., Smith, A. J., Wallace, M. W., and Holdgate, G. R. (2003). The Pliocene climatic and environmental evolution of southeastern Australia: evidence from the marine and terrestrial realm. Palaeogeography, Palaeoclimatology, Palaeoecology 193, 349–382.
| The Pliocene climatic and environmental evolution of southeastern Australia: evidence from the marine and terrestrial realm.Crossref | GoogleScholarGoogle Scholar |
Geiser, F., and Broome, L. S. (1991). Hibernation in the mountain pygmy possum Burramys parvus (Marsupialia). Journal of Zoology 223, 593–602.
| Hibernation in the mountain pygmy possum Burramys parvus (Marsupialia).Crossref | GoogleScholarGoogle Scholar |
Goodwin, E. K., Rader, R., Encinas-Viso, F., and Saunders, M. E. (2021). Weather conditions affect the visitation frequency, richness and detectability of insect flower visitors in the Australian alpine zone. Environmental Entomology 50, 348–358.
| Weather conditions affect the visitation frequency, richness and detectability of insect flower visitors in the Australian alpine zone.Crossref | GoogleScholarGoogle Scholar | 33479744PubMed |
Green, K., and Osborne, M. J. (1994). ‘Wildlife of the Australian snow-country’. (Reed Books: Chatswood.)
Green, K., and Slatyer, R. (2019). Arthropod community composition along snowmelt gradients in snowbeds in the Snowy Mountains of south-eastern Australia. Austral Ecology 45, 144–157.
| Arthropod community composition along snowmelt gradients in snowbeds in the Snowy Mountains of south-eastern Australia.Crossref | GoogleScholarGoogle Scholar |
Green, K., Caley, P., Baker, M., Dreyer, D., Wallace, J., and Warrant, E. (2020). Australian Bogong moths Agrotis infusa (Lepidoptera: Noctuidae), 1951–2020: decline and crash. Austral Entomology 60, 66–81.
| Australian Bogong moths Agrotis infusa (Lepidoptera: Noctuidae), 1951–2020: decline and crash.Crossref | GoogleScholarGoogle Scholar | 33777401PubMed |
Griffin, P. C., and Hoffmann, A. A. (2014). Limited genetic divergence among Australian alpine Poa tussock grasses coupled with regional structuring points to ongoing gene flow and taxonomic challenges. Annals of Botany 113, 953–965.
| Limited genetic divergence among Australian alpine Poa tussock grasses coupled with regional structuring points to ongoing gene flow and taxonomic challenges.Crossref | GoogleScholarGoogle Scholar | 24607721PubMed |
Guisan, A., Tingley, R., Baumgartner, J. B., Naujokaitis‐Lewis, I., Sutcliffe, P. R., Tulloch, A. I. T., Regan, T. J., Brotons, L., McDonald‐Madden, E., Mantyka‐Pringle, C., Martin, T. G., Rhodes, J. R., Maggini, R., Setterfield, S. A., Elith, J., Schwartz, M. W., Wintle, B. A., Broennimann, O., Austin, M., Ferrier, S., Kearney, M. R., Possingham, H. P., and Buckley, Y. M. (2013). Predicting species distributions for conservation decisions. Ecology Letters 16, 1424–1435.
| Predicting species distributions for conservation decisions.Crossref | GoogleScholarGoogle Scholar | 24134332PubMed |
Haines, M. L., Stuart-Fox, D., Sumner, J., Clemann, N., Chapple, D. G., and Melville, J. (2017). A complex history of introgression and vicariance in a threatened montane skink (Pseudemoia cryodroma) across an Australian sky island system. Conservation Genetics 18, 939–950.
| A complex history of introgression and vicariance in a threatened montane skink (Pseudemoia cryodroma) across an Australian sky island system.Crossref | GoogleScholarGoogle Scholar |
Hatley, J., and Murphy, N. P. (2016). Trouble at the top? Restricted distribution and extreme population isolation in an alpine crustacean assemblage with unexpected lineage diversity. Freshwater Biology 61, 1891–1904.
| Trouble at the top? Restricted distribution and extreme population isolation in an alpine crustacean assemblage with unexpected lineage diversity.Crossref | GoogleScholarGoogle Scholar |
Hennessy, K., Fitzharris, B., Bates, B. C., Harvey, N., Howden, S. M., Hughes, L., Salinger, J., and Warrick, R. (2007). Australia and New Zealand. Climate Change 2007: Impacts, Adaptation and Vulnerability. Contribution of Working Group II to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge, UK.
Hoffmann, A. A., Rymer, P. D., Byrne, M., Ruthrof, K. X., Whinam, J., McGeoch, M., Bergstrom, D. M., Guerin, G. R., Sparrow, B., Joseph, L., Hill, S. J., Andrew, N. R., Camac, J., Bell, N., Riegler, M., Gardner, J. L., and Williams, S. E. (2019). Impacts of recent climate change on terrestrial flora and fauna: Some emerging Australian examples. Austral Ecology 44, 3–27.
| Impacts of recent climate change on terrestrial flora and fauna: Some emerging Australian examples.Crossref | GoogleScholarGoogle Scholar |
Holdgate, G. R., Wallace, M. W., Gallagher, S. J., Wagstaff, B. E., and Moore, D. (2008). No mountains to snow on: major post-Eocene uplift of the East Victoria Highlands; evidence from Cenozoic deposits. Australian Journal of Earth Sciences 55, 211–234.
| No mountains to snow on: major post-Eocene uplift of the East Victoria Highlands; evidence from Cenozoic deposits.Crossref | GoogleScholarGoogle Scholar |
Hughes, L. (2003). Climate change and Australia: Trends, projections and impacts. Austral Ecology 28, 423–443.
| Climate change and Australia: Trends, projections and impacts.Crossref | GoogleScholarGoogle Scholar |
Hughes, L. (2011). Climate change and Australia: key vulnerable regions. Regional Environmental Change 11, 189–195.
| Climate change and Australia: key vulnerable regions.Crossref | GoogleScholarGoogle Scholar |
Hunter, D., Marantelli, G., McFadden, M., Harlow, P., Scheele, B., and Pietsch, R. (2010). Assessment of re-introduction methods for the Southern Corroboree Frog in the Snowy Mountains region of Australia. In ‘Global Reintroduction perspectives 2010’. (Ed P. S. Soorae.) pp. 72–76. (IUCN Re-introduction Specialist Group: Abu Dhabi, UAE.)
Karpala, T. (2020). How are Australian alpine ecosystems responding to climate change? Measuring shrubs as bioindicators of change, Victorian Alps, Australia. PhD Thesis, Deakin University Melbourne. Available at http://dro.deakin.edu.au/view/DU:30139127 [Accessed 1 February 2021]
Key, K. H. L., and Day, M. F. (1954a). A temperature-controlled physiological colour response in the grasshopper, Kosciuscola tristis Sjöst. (Orthoptera: Acrididae). Australian Journal of Zoology 2, 309–339.
| A temperature-controlled physiological colour response in the grasshopper, Kosciuscola tristis Sjöst. (Orthoptera: Acrididae).Crossref | GoogleScholarGoogle Scholar |
Key, K. H. L., and Day, M. F. (1954b). The physiological mechanism of colour change in the grasshopper, Kosciuscola tristis Sjöst. (Orthoptera: Acrididae). Australian Journal of Zoology 2, 340–363.
| The physiological mechanism of colour change in the grasshopper, Kosciuscola tristis Sjöst. (Orthoptera: Acrididae).Crossref | GoogleScholarGoogle Scholar |
Key, K. L. (1989). Revision of the genus Praxibulus (Orthoptera: Acrididae). Invertebrate Systematics 3, 1–121.
| Revision of the genus Praxibulus (Orthoptera: Acrididae).Crossref | GoogleScholarGoogle Scholar |
Kirkpatrick, J. B., Deane, A., and Parry, J. (2019). The dynamics of rush circles in subalpine grassland. Australian Journal of Botany 67, 335–340.
| The dynamics of rush circles in subalpine grassland.Crossref | GoogleScholarGoogle Scholar |
Knowles, L. L. (2000). Tests of Pleistocene speciation in montane grasshoppers (genus Melanoplus) from the sky islands of Western North America. Evolution 54, 1337–1348.
| Tests of Pleistocene speciation in montane grasshoppers (genus Melanoplus) from the sky islands of Western North America.Crossref | GoogleScholarGoogle Scholar | 11005300PubMed |
Knowles, L. L. (2001). Genealogical portraits of speciation in montane grasshoppers (genus Melanoplus) from the sky islands of the Rocky Mountains. Proceedings of the Royal Society of London. Series B: Biological Sciences 268, 319–324.
| Genealogical portraits of speciation in montane grasshoppers (genus Melanoplus) from the sky islands of the Rocky Mountains.Crossref | GoogleScholarGoogle Scholar | 11217904PubMed |
Kreger, K. M., Shaban, B., Wapstra, E., and Burridge, C. P. (2020). Phylogeographic parallelism: Concordant patterns in closely related species illuminate underlying mechanisms in the historically glaciated Tasmanian landscape. Journal of Biogeography 47, 1674–1686.
| Phylogeographic parallelism: Concordant patterns in closely related species illuminate underlying mechanisms in the historically glaciated Tasmanian landscape.Crossref | GoogleScholarGoogle Scholar |
Lambeck, K., and Chappell, J. (2001). Sea level change through the last glacial cycle. Science 292, 679–686.
| Sea level change through the last glacial cycle.Crossref | GoogleScholarGoogle Scholar | 11326090PubMed |
Lanfear, R., Frandsen, P. B., Wright, A. M., Senfeld, T., and Calcott, B. (2017). PartitionFinder 2: New methods for selecting partitioned models of evolution for molecular and morphological phylogenetic analyses. Molecular Biology and Evolution 34, 772–773.
| PartitionFinder 2: New methods for selecting partitioned models of evolution for molecular and morphological phylogenetic analyses.Crossref | GoogleScholarGoogle Scholar | 28013191PubMed |
Leaché, A. D., Banbury, B. L., Felsenstein, J., de Oca, A., nieto-Montes, , and Stamatakis, A. (2015). Short Tree, Long Tree, Right Tree, Wrong Tree: New acquisition bias corrections for inferring SNP phylogenies. Systematic Biology 64, 1032–1047.
| Short Tree, Long Tree, Right Tree, Wrong Tree: New acquisition bias corrections for inferring SNP phylogenies.Crossref | GoogleScholarGoogle Scholar | 26227865PubMed |
McCoull, C. J. (2000). Geographic variation and adaptation in the Tasmanian metallic skink (Niveoscincus metallicus). Phd, University of Tasmania. Available at https://eprints.utas.edu.au/20485/ [Accessed 24 February 2021]
Milla, L., and Encinas-Viso, F. (2020). Plant-pollinator communities in the Australian Alps. Australasian Plant Conservation: Journal of the Australian Network for Plant Conservation 28, 13–16.
Miller, M. A., Pfeiffer, W., and Schwartz, T. (2012). The CIPRES science gateway: enabling high-impact science for phylogenetics researchers with limited resources. In ‘Proceedings of the 1st Conference of the Extreme Science and Engineering Discovery Environment: Bridging from the eXtreme to the campus and beyond’. XSEDE ’12. pp. 1–8. (Association for Computing Machinery: New York, NY, USA.)
| Crossref |
Mitrovski, P., Heinze, D. A., Broome, L., Hoffmann, A. A., and Weeks, A. R. (2007). High levels of variation despite genetic fragmentation in populations of the endangered mountain pygmy-possum, Burramys parvus, in alpine Australia. Molecular Ecology 16, 75–87.
| High levels of variation despite genetic fragmentation in populations of the endangered mountain pygmy-possum, Burramys parvus, in alpine Australia.Crossref | GoogleScholarGoogle Scholar | 17181722PubMed |
Muschett, G. (2016). Evolution of aggressive behaviour in the Australian alpine grasshopper genus Kosciuscola. PhD Thesis, Macquarie University Sydney. Available at https://trove.nla.gov.au/version/249036968 [Accessed 31 May 2020]
Muschett, G., Umbers, K. D. L., and Herberstein, M. E. (2017). Within-season variability of fighting behaviour in an Australian alpine grasshopper. PLoS ONE 12, e0171697.
| Within-season variability of fighting behaviour in an Australian alpine grasshopper.Crossref | GoogleScholarGoogle Scholar | 28403243PubMed |
Mynott, J. H. (2015). Mitochondrial DNA allows the association of life stages to facilitate species recognition and delimitation in Australian stoneflies (Plecoptera: Gripopterygidae: Newmanoperla). Invertebrate Systematics 29, 223–238.
| Mitochondrial DNA allows the association of life stages to facilitate species recognition and delimitation in Australian stoneflies (Plecoptera: Gripopterygidae: Newmanoperla).Crossref | GoogleScholarGoogle Scholar |
icotra, A. B., Beever, E. A., Robertson, A. L., Hofmann, G. E., and O’Leary, J. (2015). Assessing the components of adaptive capacity to improve conservation and management efforts under global change. Conservation Biology 29, 1268–1278.
| Assessing the components of adaptive capacity to improve conservation and management efforts under global change.Crossref | GoogleScholarGoogle Scholar |
Parida, M., Hoffmann, A. A., and Hill, M. P. (2015). Climate change expected to drive habitat loss for two key herbivore species in an alpine environment. Journal of Biogeography 42, 1210–1221.
| Climate change expected to drive habitat loss for two key herbivore species in an alpine environment.Crossref | GoogleScholarGoogle Scholar |
Pepper, M., Sumner, J., Brennan, I. G., Hodges, K., Lemmon, A. R., Lemmon, E. M., Peterson, G., Rabosky, D. L., Schwarzkopf, L., Scott, I. A. W., Shea, G., and Keogh, J. S. (2018). Speciation in the mountains and dispersal by rivers: Molecular phylogeny of Eulamprus water skinks and the biogeography of Eastern Australia. Journal of Biogeography 45, 2040–2052.
| Speciation in the mountains and dispersal by rivers: Molecular phylogeny of Eulamprus water skinks and the biogeography of Eastern Australia.Crossref | GoogleScholarGoogle Scholar |
Rambaut, A., and Drummond, A. J. (2003). Tracer: MCMC trace analysis tool (Version 1.7.1). Available at http://tree.bio.ed.ac.uk/software/tracer/ [Accessed 16 June 2021]
Redding, D. W., and Mooers, A. Ø. (2006). Incorporating evolutionary measures into conservation prioritization. Conservation Biology 20, 1670–1678.
| Incorporating evolutionary measures into conservation prioritization.Crossref | GoogleScholarGoogle Scholar | 17181802PubMed |
Rehn, J. A. G. (1957). ‘The grasshoppers and locusts (Acridoidea) of Australia. Family Acrididae: Subfamily Cyrtacanthacrldinae tribes Oxyini. Spathosternini. and Praxibulini’. (CSIRO: Melbourne.)
Rutter, N. J., Mynott, J. H., Howell, T. J., Stukas, A. A., Pascoe, J. H., Bennett, P. C., and Murphy, N. P. (2021). Buzzing with possibilities: Training and olfactory generalization in conservation detection dogs for an endangered stonefly species. Aquatic Conservation: Marine and Freshwater Ecosystems 31, 984–989.
| Buzzing with possibilities: Training and olfactory generalization in conservation detection dogs for an endangered stonefly species.Crossref | GoogleScholarGoogle Scholar |
Sjöstedt, Y. (1934). Neue australische Acrididen. Arkiv för Zoology 26 A, 1–9.
Slatyer, R. A., Nash, M. A., Miller, A. D., Endo, Y., Umbers, K. D. L., and Hoffmann, A. A. (2014). Strong genetic structure corresponds to small-scale geographic breaks in the Australian alpine grasshopper Kosciuscola tristis. BMC Evolutionary Biology 14, 204.
| Strong genetic structure corresponds to small-scale geographic breaks in the Australian alpine grasshopper Kosciuscola tristis.Crossref | GoogleScholarGoogle Scholar | 25273226PubMed |
Slatyer, R. A., Nash, M. A., and Hoffmann, A. A. (2016). Scale-dependent thermal tolerance variation in Australian mountain grasshoppers. Ecography 39, 572–582.
| Scale-dependent thermal tolerance variation in Australian mountain grasshoppers.Crossref | GoogleScholarGoogle Scholar |
Song, H., Mariño-Pérez, R., Woller, D. A., and Cigliano, M. M. (2018). Evolution, Diversification, and Biogeography of Grasshoppers (Orthoptera: Acrididae). Insect Systematics and Diversity 2, 1–25.
| Evolution, Diversification, and Biogeography of Grasshoppers (Orthoptera: Acrididae).Crossref | GoogleScholarGoogle Scholar |
Stamatakis, A. (2014). RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 30, 1312–1313.
| RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies.Crossref | GoogleScholarGoogle Scholar | 24451623PubMed |
Stamatakis, A., Hoover, P., and Rougemont, J. (2008). A rapid bootstrap algorithm for the RAxML web servers. Systematic Biology 57, 758–771.
| A rapid bootstrap algorithm for the RAxML web servers.Crossref | GoogleScholarGoogle Scholar | 18853362PubMed |
Tatarnic, N. J., Umbers, K. D. L., and Song, H. (2013). Molecular phylogeny of the Kosciuscola grasshoppers endemic to the Australian alpine and montane regions. Invertebrate Systematics 27, 307–316.
| Molecular phylogeny of the Kosciuscola grasshoppers endemic to the Australian alpine and montane regions.Crossref | GoogleScholarGoogle Scholar |
Umbers, K. D. L. (2011). Turn the temperature to turquoise: cues for colour change in the male chameleon grasshopper (Kosciuscola tristis) (Orthoptera: Acrididae). Journal of Insect Physiology 57, 1198–1204.
| Turn the temperature to turquoise: cues for colour change in the male chameleon grasshopper (Kosciuscola tristis) (Orthoptera: Acrididae).Crossref | GoogleScholarGoogle Scholar |
Umbers, K. D. L., Tatarnic, N. J., and Herberstein, M. E. (2012). Ferocious fighting between male grasshoppers. PLoS ONE 7, e49600.
| Ferocious fighting between male grasshoppers.Crossref | GoogleScholarGoogle Scholar |
Umbers, K. D. L., Herberstein, M. E., and Madin, J. S. (2013). Colour in insect thermoregulation: empirical and theoretical tests in the colour-changing grasshopper, Kosciuscola tristis. Journal of Insect Physiology 59, 81–90.
| Colour in insect thermoregulation: empirical and theoretical tests in the colour-changing grasshopper, Kosciuscola tristis.Crossref | GoogleScholarGoogle Scholar |
Umbers, K. D. L., Riley, J. L., Kelly, M. B. J., Taylor‐Dalton, G., Lawrence, J. P., and Byrne, P. G. (2020). Educating the enemy: Harnessing learned avoidance behavior in wild predators to increase survival of reintroduced southern corroboree frogs. Conservation Science and Practice 2, e139.
| Educating the enemy: Harnessing learned avoidance behavior in wild predators to increase survival of reintroduced southern corroboree frogs.Crossref | GoogleScholarGoogle Scholar |
Vaidya, G., Lohman, D. J., and Meier, R. (2011). SequenceMatrix: concatenation software for the fast assembly of multi-gene datasets with character set and codon information. Cladistics 27, 171–180.
| SequenceMatrix: concatenation software for the fast assembly of multi-gene datasets with character set and codon information.Crossref | GoogleScholarGoogle Scholar |
Williams, R. J., Wahren, C.-H., Stott, K. A. J., Camac, J. S., White, M., Burns, E., Harris, S., Nash, M., Morgan, J. W., Venn, S., Papst, W. A., and Hoffmann, A. A. (2015). An International Union for the Conservation of Nature Red List ecosystems risk assessment for alpine snow patch herbfields, South-Eastern Australia. Austral Ecology 40, 433–443.
| An International Union for the Conservation of Nature Red List ecosystems risk assessment for alpine snow patch herbfields, South-Eastern Australia.Crossref | GoogleScholarGoogle Scholar |
Wintle, B. A., Legge, S., and Woinarski, J. C. Z. (2020). After the megafires: What next for Australian wildlife? Trends in Ecology & Evolution 35, 753–757.
| After the megafires: What next for Australian wildlife?Crossref | GoogleScholarGoogle Scholar |
Yadav, S., Stow, A., and Dudaniec, R. Y. (2020). Microgeographic adaptation corresponds with elevational distributions of congeneric montane grasshoppers. Molecular Ecology 30, 481–498.
| Microgeographic adaptation corresponds with elevational distributions of congeneric montane grasshoppers.Crossref | GoogleScholarGoogle Scholar | 33217095PubMed |





