Termite–gut-associated actinomycetes as biological control agents against phytopathogen Pyrrhoderma noxium
Cherrihan Adra A , Harrchun Panchalingam A and D. İpek Kurtböke A *A School of Science, Technology and Engineering, University of the Sunshine Coast (UniSC), Maroochydore BC, Qld 4558, Australia.

Cherrihan Adra is one of the UniSC graduates with first class Honours. She also holds a BSc in the Science Program where she majored in Chemistry and minored in Microbiology. She is now continuing with this research by conducting her PhD with Dr İpek Kurtböke relating to the application of actinomycetes as biological control agents through investigating their bioactive secondary metabolites. |

Harrchun Panchalingam has recently completed his PhD under Dr Kurtböke’s supervision. He is an international student from Sri Lanka and holds BSc from Monash University Malaysia and MSc from University of Peradeniya, Sri Lanka. His PhD project involved assessment of Trichoderma and actinomycetes spp. to control Pyrrhoderma noxium infections of heritage fig trees in Brisbane. Currently he is working as research assistant at (UniSC). His research interests are biological control of plant diseases, development of biofertilizers and bioremediation of hydrocarbons. |

Dr D. İpek Kurtböke is currently a senior lecturer at the University of the Sunshine Coast (UniSC) in Australia conducting research in applied, industrial and environmental microbiology. She is an internationally reputed actinomycetologist and she has been in the field of biodiscovery since 1982 conducting research into discovery of novel and potent therapeutic compounds produced by actinomycetes in Turkey, Italy, the UK, and Australia with leading pharmaceutical companies. She has been an Executive Board member of the World Federation of Culture Collections (WFCC) since 2000, currently serving her second term as the President of the Federation. She is also one of the members of the International Committee on Taxonomy of Viruses (ICTV)’s, Bacterial Viruses Subcommittee. She has editorial duties in different journals including Marine Drugs, Diversity and Frontiers Marine Science/Marine Biotechnology. |
Microbiology Australia 43(4) 190-193 https://doi.org/10.1071/MA22052
Submitted: 6 October 2022 Accepted: 19 October 2022 Published: 7 December 2022
© 2022 The Author(s) (or their employer(s)). Published by CSIRO Publishing on behalf of the ASM. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
Brown root-rot disease is caused by phytopathogenic white-rot basidiomycete fungus, Pyyrhoderma noxium. Currently, it is causing significant issues for the Brisbane hinterland in Queensland, Australia and killing many tree species throughout the greater Metropolitan area, including park and street trees located in Shorncliffe, Taringa, New Farm, Eagle Farm, West End, Hamilton, Indooroopilly, Brisbane River, and the City Centre. Brisbane trees being attacked are figs, poinciana, jacarandas, Chinese elms, Moreton Bay eucalypts, kauris, and hoop pines and these include both public and privately owned trees. A joint study between the University of the Sunshine Coast (UniSC) and the Brisbane City Council (BCC) aimed to assess the efficacy of different biological control agents to control infections in the region. In a substudy presented here, termite gut-associated actinomycetes were evaluated to determine their antifungal abilities against the pathogen.
Keywords: actinomycetes, antifungal agents, biological control, fermentation, microbial metabolism, Pyrrhoderma noxium, Streptomyces spp., termite guts.
Introduction
Brown root-rot caused by phytopathogenic white-rot basidiomycete fungus, Pyyrhoderma noxium (P. noxium), formally known as Phellinus noxius1 is a devastating disease prevalent in many tropical and subtropical countries.2 It has caused much destruction throughout Southeast and East Asia, Oceania, the Pacific Islands, Africa, Central America, the Caribbean, and Australia.3 In fact, it has been one of the most damaging tree diseases in Taiwan,4 the Ryukyu Islands of Japan,5 Mariana Islands,6 Malaysia7 and Australia.8
This plant pathogen has a wide host range with over 260 susceptible tree species, represented by 59 families and can infect ornamental and agricultural crops as well as plantations and native forest trees.9 Within Australia, it occurs naturally throughout forests on the East Coast from Northern Queensland to Northern New South Wales where it has also caused issues for commercial plantation forests, fruit orchards and amenity plantings in urban areas.10 Identification of P. noxium causing death in avocado plantations was found in the Sunshine Coast hinterland, QLD in areas that were responsible for 50% of the total Australian avocado production.11 Additionally, it has been reported impacting Hoop Pine plantations in QLD.12 Currently, it is causing significant issues for the Brisbane area having killed many tree species throughout the greater Metropolitan area, including park and street trees located in Shorncliffe, Taringa, New Farm, Eagle Farm, West End, Hamilton, Indooroopilly, Brisbane River, and the City Centre. It has attacked many of the Brisbane trees such as figs, poinciana, jacarandas, Chinese elms, Moreton Bay eucalypts, kauris, and hoop pines and these include both public and privately owned trees.8
The infection cycle of this fungal disease begins in the roots where it commences colonisation and digestion of the tree’s wood. This degradation occurs through the production of digestive enzymes that can break down the vital components found in the cells of plants such as cellulose, hemicellulose and lignin.10 The decomposition of root-tissues leads to significantly reduced physical support and absorption of nutrients and water.2 Symptoms include discolouration, bark exudations, defoliation, chlorosis, and dieback, however, these symptoms are usually observed in the later stage of infection when it is too late for intervention.2 Transmission of this disease has two main modes: (1) through root-to-root contact with surrounding infected trees; and (2) infiltration and establishment of spores through entry points such as wounds on trees, exposed surfaces and on freshly cut tree stumps.10
Traditionally, chemical fungicides have been widely used for protection of plants against fungal pathogens;13 however, concerns surrounding the safety and environmental impacts of these chemicals has prompted investigations into alternative control methods.8 Recently, more emphasis has been placed on natural or biological approaches to controlling pests as it provides an environmentally friendly, safe, and inexpensive alternative for controlling plant pathogens. Utilisation of microorganisms and their secondary metabolites provides a promising alternative for disease prevention.14 Particularly, Streptomyces spp. are well known sources for secondary metabolism and their ability to produce various novel chemical structures with remarkable biological activities.15 Moreover, unexplored habitats and niches have attracted considerable attention in the discovery of specialised and active Streptomyces spp. such as microbiome symbionts.16
Specialised mutualistic relationships between microorganisms and hosts have seen the active culturing of beneficial microorganisms through co-evolution in exchange of bioactive small molecule.16 One such symbiosis exists between bacteria and the digestive tracts of insects, which contain communities of symbiotic and transient microorganisms.17 A relatively unexplored source is the guts of termites (Termitidae, Termitinae), which contain endosymbiotic protozoa and bacteria.18 Termites are insects belonging to the order Isoptera and predominantly feed on litter tissue and wood. It is difficult to digest lignocelluloses that are deficient in vitamins and other essential components. As a result, termites depend on beneficial symbiosis with a diverse flora of microorganisms within their hindguts for digestion and the acquisition of supplementary nutrition.19 Recent studies have revealed that actinomycetes which also cover the genus Streptomyces, were one of the dominant bacteria identified within the symbiotic lifestyle of termites.16 Actinomycetes aid termites, including nutrient recycling and exchange, as well as protecting the termites from invading pathogens.20 It has been suggested that these unexplored insect-associated actinomycetes may house rare strains with the capacity to produce complex natural products with various biological activities.21 Although plant disease management by streptomycetes have been well documented,22 investigations into the use of termite–gut-associated ones against this pathogen are yet to be explored.
In the light of the above presented information, termite–gut-associated streptomycetes were investigated for their potential control of P. noxium. Two termite gut-associated streptomycetes (USC-595B, USC-596) were selected from the UniSC’s Microbial Library (Fig. 1).23,24 First, a plate antagonism assay was performed, and all of the isolates were found to inhibit P. noxium growth (Fig. 2). Second, they were then fermented using solid-state fermentation on oatmeal agar and subsequent bioactive compound extraction was carried out as described by English et al. (2017).25 Fermentation extracts obtained from the isolates were found to display antagonism towards the pathogen following the extract activity assays and scanning electron microscopical (SEM) observations (Figs 3, 4).

|
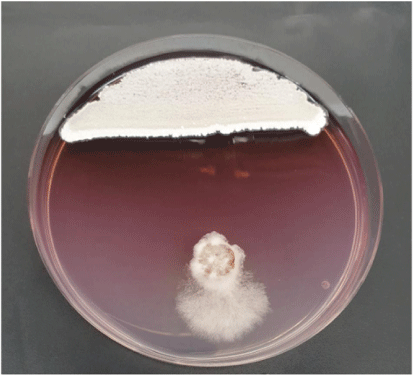
|
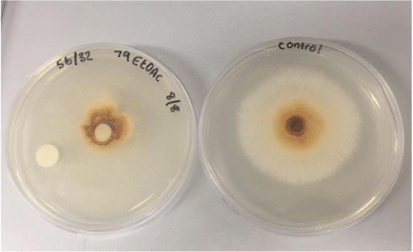
|
Structure elucidation of the bioactive compounds are underway at the UniSC. However, preliminary findings indicate that insect microbiome-symbionts might offer a feasible source for biological control of Pyrrhoderma noxium. Field trials are also underway to determine the effectivity of the isolates and their compounds under environmental conditions to complement already used Trichoderma biological control applications by the Brisbane City Council.26
Data availability
Fig. 1 contains accession numbers for the molecular data, and data is presented in the images (Figs 2–4).
Conflicts of interest
The authors declare no conflicts of interest.
Declaration of funding
The substudy presented here is part of an ongoing P. noxium management research using biological control agents jointly conducted by the UniSC and Brisbane City Council (BCC). Both parties provide cash and in-kind contributions.
Acknowledgements
The authors thank Mr Keith Foster (former Principal Arborist of the BCC) and the BCC for the funding provided. The authors also thank Dr Louisa Parkinson and Associate Professor Elizabeth Dann, QAAFI, UQ, for providing Phellinus strains used in the study as part of their collaboration with the BCC.
References
[1] Zhou, LW et al.. (2018) Taxonomy and phylogeny of Pyrrhoderma: a redefinition, the segregation of Fulvoderma, gen. nov., and identifying four new species. Mycologia 110, 872–889.| Taxonomy and phylogeny of Pyrrhoderma: a redefinition, the segregation of Fulvoderma, gen. nov., and identifying four new species.Crossref | GoogleScholarGoogle Scholar |
[2] Tsang, KSW et al.. (2020) A preliminary examination of the bacterial, archaeal, and fungal rhizosphere microbiome in healthy and Phellinus noxius-infected trees. MicrobiologyOpen 9, e1115.
| A preliminary examination of the bacterial, archaeal, and fungal rhizosphere microbiome in healthy and Phellinus noxius-infected trees.Crossref | GoogleScholarGoogle Scholar |
[3] Wu, ZC et al.. (2020) Soil is not a reservoir for Phellinus noxius. Phytopathology 110, 362–369.
| Soil is not a reservoir for Phellinus noxius.Crossref | GoogleScholarGoogle Scholar |
[4] Hsiao, WW et al.. (2019) The pathogenicity of basidiospores of Phellinus noxius which causes brown root rot disease in Taiwan. Taiwania 64, 189–194.
| The pathogenicity of basidiospores of Phellinus noxius which causes brown root rot disease in Taiwan.Crossref | GoogleScholarGoogle Scholar |
[5] Sahashi, N et al.. (2012) Brown root rot of trees caused by Phellinus noxius in the Ryukyu Islands, subtropical areas of Japan. For Pathol 42, 353–361.
| Brown root rot of trees caused by Phellinus noxius in the Ryukyu Islands, subtropical areas of Japan.Crossref | GoogleScholarGoogle Scholar |
[6] Hodges, CS and Tenorio, JA (1984) Root disease of Delonix regia and associated tree species in the Mariana Islands caused by Phellinus noxius. Plant Dis 68, 334–336.
| Root disease of Delonix regia and associated tree species in the Mariana Islands caused by Phellinus noxius.Crossref | GoogleScholarGoogle Scholar |
[7] Farid, AM et al.. (2009) Pathogenicity of Rigidoporus microporus and Phellinus noxius against four major plantation tree species in peninsular Malaysia. J Trop For Sci 21, 289–298.
[8] Schwarze, FW et al.. (2012) Evaluation of an antagonistic Trichoderma strain for reducing the rate of wood decomposition by the white rot fungus Phellinus noxius. Biol Control 61, 160–168.
| Evaluation of an antagonistic Trichoderma strain for reducing the rate of wood decomposition by the white rot fungus Phellinus noxius.Crossref | GoogleScholarGoogle Scholar |
[9] Ashiglar SM et al. (2015) Aggressive root pathogen Phellinus noxius and implications for western Pacific Islands. In Proceedings of the 62nd Annual Western International Forest Disease Work Conference (Murray M, Palacios P, comps), 8–12 September 2014, Cedar City, UT, USA. pp. 79–81.
[10] Gray P (2017) Brown root rot Phellinus noxius. pp. 1–7. https://www.northerntreecare.com.au/wp-content/uploads/2021/09/Brown-Root-Rot.pdf
[11] Dann K et al. (2011) Phellinus noxius: a basidiomycete fungus impacting productivity of Australian avocados. In 7th World Avocado Congress.
[12] Bolland, L (1984) Phellinus noxius: cause of a significant root-rot in Queensland hoop pine plantations. Aust For 47, 2–10.
| Phellinus noxius: cause of a significant root-rot in Queensland hoop pine plantations.Crossref | GoogleScholarGoogle Scholar |
[13] Ribera, J et al.. (2016) In-vitro evaluation of antagonistic Trichoderma strains for eradicating Phellinus noxius in colonised wood. J Trop For Sci 28, 457–468.
[14] Bose R et al. (2022) Biological control of forest pathogens: success stories and challenges. In Trends of Applied Microbiology for Sustainable Economy. pp. 155–184. Elsevier.
[15] Yang, Y et al.. (2019) Antagonistic activity and mechanism of an isolated Streptomyces corchorusii stain AUH-1 against phytopathogenic fungi. World J Microbiol Biotechnol 35, 145.
| Antagonistic activity and mechanism of an isolated Streptomyces corchorusii stain AUH-1 against phytopathogenic fungi.Crossref | GoogleScholarGoogle Scholar |
[16] Wu, W et al.. (2022) Isolation and identification of symbiotic actinomycetes from termites in hainan and their activity against tropical plants pathogenic fungi. Chin J Trop Crops 43, 1240–1247.
| Isolation and identification of symbiotic actinomycetes from termites in hainan and their activity against tropical plants pathogenic fungi.Crossref | GoogleScholarGoogle Scholar |
[17] Selim, MSM et al.. (2021) Secondary metabolites and biodiversity of actinomycetes. J Genet Eng Biotechnol 19, 72.
| Secondary metabolites and biodiversity of actinomycetes.Crossref | GoogleScholarGoogle Scholar |
[18] Matsui, T et al.. (2012) Antibiotics production by an actinomycete isolated from the termite gut. J Basic Microbiol 52, 731–735.
| Antibiotics production by an actinomycete isolated from the termite gut.Crossref | GoogleScholarGoogle Scholar |
[19] Krishanti, N et al.. (2018) Antimicrobial production by an actinomycetes isolated from the termite nest. J Trop Life Sci 8, 279–288.
| Antimicrobial production by an actinomycetes isolated from the termite nest.Crossref | GoogleScholarGoogle Scholar |
[20] Sujada, N et al.. (2014) Termite nests as an abundant source of cultivable actinobacteria for biotechnological purposes. Microbes Environ 29, 211–219.
| Termite nests as an abundant source of cultivable actinobacteria for biotechnological purposes.Crossref | GoogleScholarGoogle Scholar |
[21] Khucharoenphaisan, K et al.. (2012) Isolation and identification of actinomycetes from termite’s gut against human pathogen. Asian J Anim Vet Adv 7, 68–73.
| Isolation and identification of actinomycetes from termite’s gut against human pathogen.Crossref | GoogleScholarGoogle Scholar |
[22] Gomes, EdB et al.. (2018) Actinomycetes bioactive compounds: biological control of fungi and phytopathogenic insect. Afr J Biotechnol 17, 552–559.
| Actinomycetes bioactive compounds: biological control of fungi and phytopathogenic insect.Crossref | GoogleScholarGoogle Scholar |
[23] Kurtböke, DI and French, JRJ (2007) Use of phage battery to investigate the actinofloral layers of termite gut microflora. J Appl Microbiol 103, 722–734.
| Use of phage battery to investigate the actinofloral layers of termite gut microflora.Crossref | GoogleScholarGoogle Scholar |
[24] Kurtböke, DI and French, JRJ (2008) Actinobacterial resources from termite guts for regional bioindustries. Microbiol Aust 29, 42–44.
| Actinobacterial resources from termite guts for regional bioindustries.Crossref | GoogleScholarGoogle Scholar |
[25] English, AL et al.. (2017) Evaluation of fermentation conditions triggering increased antibacterial activity from a near-shore marine intertidal environment-associated Streptomyces species. Synth Syst Biotechnol 2, 28–38.
| Evaluation of fermentation conditions triggering increased antibacterial activity from a near-shore marine intertidal environment-associated Streptomyces species.Crossref | GoogleScholarGoogle Scholar |
[26] Panchalingam, H et al.. (2022) Assessing the various antagonistic mechanisms of Trichoderma strains against the brown root rot pathogen Pyrrhoderma noxium infecting heritage fig trees. J Fungi 8, 1105.
| Assessing the various antagonistic mechanisms of Trichoderma strains against the brown root rot pathogen Pyrrhoderma noxium infecting heritage fig trees.Crossref | GoogleScholarGoogle Scholar |



