Human pathogenic bacteria on fresh produce and their control using bacteriophage treatment: an E. coli example from the Sunshine Coast region
Meg Allom A , Harrchun Panchalingam A , M. Katouli A and D. İpek Kurtböke A *A School of Science, Technology and Engineering, University of the Sunshine Coast, Maroochydore BC, Qld 4558, Australia.

Meg Allom is a UniSC recent graduate with first class Honours. She also holds a BSc in the Science Program of the UniSC. Over the past 2 years she conducted research with Dr İpek Kurtböke related to the application of various actinophages. She has worked with foodborne pathogens and microbial contaminants in her studies and has a keen interest in food safety. |

Harrchun Panchalingam has recently completed his PhD under Dr Kurtböke’s supervision. He is an international student from Sri Lanka and holds BSc from Monash University Malaysia and MSc from University of Peradeniya, Sri Lanka. His PhD project involved assessment of Trichoderma and actinomycetes spp. to control Pyrrhoderma noxium infections of heritage fig trees in Brisbane. Currently he is working as research assistant at (UniSC). His research interests are biological control of plant diseases, development of biofertilisers and bioremediation of hydrocarbons. |

Associate Professor Mohammad Katouli obtained his PhD in 1980 from University of Ulster in UK. He then joined the Research and Development Department of DP Pharmaceuticals. In 1985, he took the position of the Head of Microbiology Department at the Pasteur Institute in Tehran. Between 1988 and 1998, he worked as a senior research fellow at the Microbiology and Tumour Biology Centre of the Karolinska Institute, Stockholm, Sweden. In 1998, he took a teaching and research position at University of the Sunshine Coast. His current interest is gut microbiota and the role of E. coli in pathogenesis of IBD. |

D. İpek Kurtböke is currently a senior lecturer at the University of the Sunshine Coast (UniSC) in Australia and one of the members of the Genecology Research Centre of the UniSC, conducting research in applied, industrial and environmental microbiology. She is an internationally reputed actinomycetologist and she has been in the field of biodiscovery since 1982 conducting research into discovery of novel and potent therapeutic compounds produced by actinomycetes in Turkey, Italy, the UK, and Australia with leading pharmaceutical companies. She has been an Executive Board member of the World Federation of Culture Collections (WFCC) since 2000, currently serving her second term as the President of the Federation. She is also one of the members of the International Committee on Taxonomy of Viruses (ICTV)’s, Bacterial Viruses Subcommittee. She has editorial duties in different journals including Marine Drugs, Diversity and Frontiers Marine Science/Marine Biotechnology. |
Microbiology Australia 43(4) 194-198 https://doi.org/10.1071/MA22059
Submitted: 6 October 2022 Accepted: 22 November 2022 Published: 13 December 2022
© 2022 The Author(s) (or their employer(s)). Published by CSIRO Publishing on behalf of the ASM. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
Consumers are placing increasing importance on an environmentally friendly way of food production and are turning to organically produced fruit and vegetables. Organic farming rejects the use of synthetic pesticides or fertilisers, growth promoters, antibiotics, or transgenic organisms. However, the use of manures that replace synthetic fertilisers is associated with a risk of contamination of produce with pathogenic microorganisms. There have been a considerable number of foodborne outbreaks associated with fresh produce, resulting in hospitalisations and deaths worldwide. Accordingly, bacteriophages have gained much attention as a safe, effective, and organic method for removal of pathogenic microorganisms from fresh produce. Bacteriophage treatments for control of pathogenic E. coli and other pathogens on fresh produce have several advantages over currently used treatments, including their host specificity, safety, low impact on sensory qualities of fresh produce, and their ease of isolation over other antimicrobial agents.
Keywords: antibiotic resistant pathogenic bacteria, bacteriophages, bacteriophage biocontrol, E. coli, E. coli phage, food borne pathogens, food security, fresh produce, phage inactivation.
In Australia, the production of fresh produce accounted for 7% of the gross value of agricultural production in 2017–2018.1 Australian growers are an important source of fresh produce for Australian consumption.1 Fresh produce is an essential element of a healthy diet, and is an important source of fibre, vitamins, minerals and antioxidants.2 A group of vegetables with particular importance is green leafy vegetables including silver beet, broccoli, lettuce and bok choy. These vegetables are good sources of vitamins A and B2, folic acids, various antioxidants, iron, and calcium.3
Escherichia coli may be found in various mammal digestive tracts and can be introduced into waterways and soil via faecal contamination. These bacteria are generally considered to grow poorly in the environment due to stressors such as high salinity, low moisture in soil/sand, low organic matter, low/high temperature, solar radiation and predation.4 However, studies now demonstrate that E. coli is capable of surviving for long periods of time in the environment in the presence of favourable temperatures/nutrients in the water and soil.5 This can be a serious problem for agriculture and other links in the supply chain, as well as for the safety of consumers. Contamination of fresh produce with E. coli can often occur in environmental reservoirs, including droppings from wild animals, contamination in water sources/irrigation systems, contamination from naturalised bacteria in soil, and most importantly for organic agriculture, manure/composting.6,7 This is in addition to improper post-harvest handling such as contamination by ill workers, contaminated post-harvest produce washing and contaminated work surfaces in processing facilities.8,9 Improper composting practices and water sources that are contaminated may be home to pathogenic bacteria such as E. coli O157:H7 (and other diarrheagenic E. coli), as well as Listeria spp. and Salmonella typhimurium.10,11 These reservoirs of pathogenic bacteria can harbour viable bacteria for more than 150 days in soil and 60 days on fresh produce exposed to contaminated irrigation water and improperly processed manure.7 Bacterial counts on ready to eat items that have been exposed to soil bacteria and splashes from irrigation water may fluctuate with seasonal changes.12 Microbial counts of E. coli (including pathogenic E. coli) on conventional and organic produce can vary widely and often make it hard to draw definitive conclusions.7 Contamination may also occur via damage to plants sustained in transit and agriculture, as is the case with damage to tomato plants being an important source of hard-to-treat Salmonella infection in tomatoes.13
Organically farmed produce may be subject to contamination with pathogenic bacteria present in the gut of animals when manure is applied as a soil amendment. E. coli O157:H7 from manure can survive washing steps applied post-harvest, and current processing techniques can be insufficient to protect consumers.14 Careful attention must be paid to composting processes, and environmental conditions and application times for manure must be considered to minimise growth of pathogens, especially considering organic soil amendments can contribute to the spread of antibiotic resistance genes in the environment.15,16 However, practically this may not always be possible – therefore, effective post-harvest treatments are necessary to ensure consumer safety.
Bacteriophages have gained much attention as a safe, effective, organic method for removal of pathogenic microorganisms from fresh produce.17 Bacteriophage treatments for control of pathogenic E. coli and other pathogens on fresh produce have several advantages over currently used treatments, including their host specificity, safety, low impact on sensory qualities of fresh produce, and their ease of isolation over other antimicrobial agents.18,19 Accordingly, there is a need for isolation of more bacteriophages and evaluation of their efficacy for controlling pathogenic bacteria on various green leafy vegetables, simulating real-life storage and processing conditions. There is also a need to find solutions for removal of bacteriophages from the surface of produce once the treatment is complete to reduce risk of allergic reactions and assure consumers of produce safety and quality for widespread acceptance and application of bacteriophages.
In this study, eight different polyvalent bacteriophage strains from the University of the Sunshine Coast (UniSC)-Microbial library were selected20 to produce a phage consortium and used to remove E. coli (ATCC 13706) on the sterilised21 leaf surfaces of kale, silver beet and Bok Choy freshly obtained from local supermarkets 24 h prior to the phage treatment. Phage preparation (×109 PFU/mL) was applied at different time intervals onto E. coli (×107 CFU/mL) smeared leaves at a host/phage ratio of 1:2. The phage preparation was also applied to leaf surfaces separately to determine survival over 72 h. Additionally, sterilised kale was treated with the phage preparation (×109 PFU/mL) and washed with various common household disinfectants to determine effective treatments to remove remaining phages from produce. The phage consortium was incubated at various temperatures for 1 h in triplicate to determine thermal tolerance, and at several pH levels for 2 h in triplicate to test pH tolerance. In addition, the phage consortium was tested for activity against multi-drug resistant E. coli strain ATCC BAA-196 according to the method described in Jonns et al.22
The phage consortium was shown to completely lyse E. coli on both kale and Bok Choy leaves, and almost completely lyse E. coli used to treat silver beet as well (Table 1). It is possible that the smooth surface of silver beet leaves is conducive to internalisation of the bacteria, leaving the phage unable to access and lyse the E. coli. Regardless, the E. coli on the phage-treated silver beet was reduced by several log units and almost complete lysis was achieved, indicating that leaf surface structure is not an impediment to phage activity.
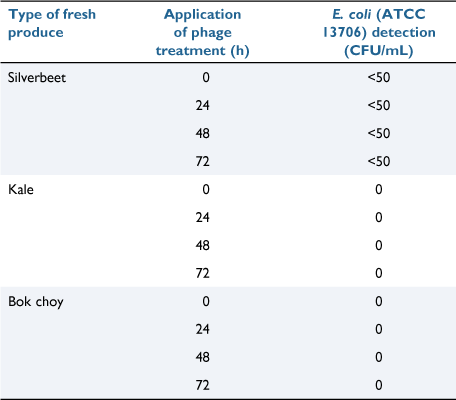
|
Several studies have confirmed the effectiveness of bacteriophage consortia in controlling E. coli and other pathogenic microbes on different types and surfaces of fresh produce, as well as meats and poultry.23–25 The bacteriophage consortium tolerated a pH range from 4 to 7 with no loss in viability and experienced a sharp drop in the lytic activity at pH 3, as well as a decrease in activity from pH 9 onwards (Fig. 1).
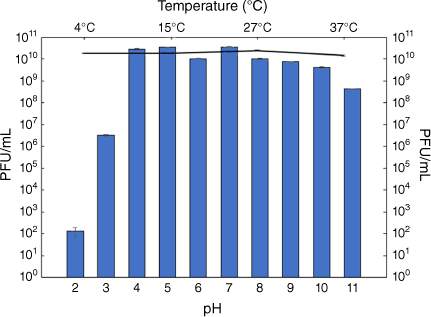
|
Other isolated bacteriophages followed this trend with peak activity between pH 3–4 and pH 9–10.26,27 Various bacteriophages (including non-E. coli bacteriophages) have been shown to vary in their stability at various pH, with many phage strains tolerating either more alkaline or more acidic conditions. It has been suggested that inactivation of phages in extreme pH conditions may be due to irreversible coagulation and precipitation.28
In terms of thermal stability, the phage consortium retained bacteriolytic activity well at all temperatures tested, with no significant differences in titre (Fig. 1). Other bacteriophages employed in reducing pathogenic bacteria on foods have been shown to be effective at low temperatures, even at 1°C.26,29 This finding indicates that this phage consortium can be used in production and processing easily, as most fresh produce would be kept in cold conditions from farm to consumer supply outlets.
Our results also indicated that the phage consortium maintained bacteriolytic activity equally on all three leaf surfaces over 72 h. This may indicate that even without the host present, the bacteriophage consortium can survive well at refrigerated temperatures, mimicking the conditions under which a commercial phage product would be applied and left on produce for treatment. Phage preparations have been shown to control various pathogenic microorganisms on various fresh produce for several days, which is in line with the performance of the phage consortium tested.30–32 The leaf surface seemed to have little bearing on the phage preparation’s bacteriolytic activity, suggesting that the phage consortium may be able to survive external stressors such as changes in pH on the leaf surface, change in temperature and desiccation of leaf surfaces. This is also in line with the phage consortium tolerating 4°C well and being able to tolerate a wide range of pH. Previous studies on phage consortia have been shown to be effective for control of E. coli on leaves such as spinach.33,34 The ability of bacteriophages to control E. coli on various leaf surfaces is very promising for their future use in controlling E. coli on fresh produce.
The phage consortium was shown to have strong lytic activity towards the host E. coli (ATCC 13706) tested, and weak lytic activity towards the multi-drug resistant E. coli (ATCC BAA-196). As a result, in the development of commercial phage products, multiple phages should be added to the final preparation of the phages to increase the likelihood of inhibition of the target bacterium and decrease the likelihood of the target bacterium developing phage resistance.23,35,36 Bacteria can gain resistance to phages like in the case of antibiotics; however, it is much easier to isolate a new virulent phage compared with discovering new and potent antibiotics. Further, compared to antibiotics, new non-toxic phages can be isolated against most target bacteria.36,37
Once the bacteriophage treatment was complete and E. coli was removed successfully, the goal was to remove the bacteriophage preparation from the leaf surfaces as well. This would allow for confidence in selling to consumers. Accordingly, leaves were treated with different disinfectants. Out of the disinfectants applied to phage treated kale, white household vinegar (4% acetic acid) was shown to be the most effective at reducing the phage titre in 30 s (Table 2).
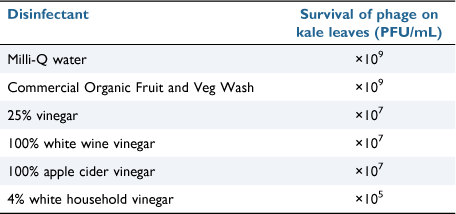
|
At a measured pH of 2.5, the effectiveness of white household vinegar in reducing phage titre is predictable, as evidenced in the pH stability study, and also previous studies showing effectiveness of the vinegar wash in reducing phage titre on the surface of strawberries.20 Diluted white household vinegar (25%) also reduced the phage titre by a moderate amount. White wine vinegar and apple cider vinegar treatments also demonstrated some reduction in phage titre, although not as pronounced as that of white household vinegar. The potential dilution of these vinegars may possibly have contributed to this result. The commercial organic fruit and vegetable wash did not show a visible reduction in phage titre. However, the wash preparation used contained mainly organic surfactants as active ingredients, perhaps suggesting that 30 s of gentle agitation may not be sufficient to use the product as intended, and a more vigorous washing step may produce better results.
In conclusion, our findings indicate that bacteriophages can be used to remove human pathogenic bacteria and once the treatment is complete, they can also be removed from fresh produce by using household white vinegar.
Data availability
Data is embedded in the text as tables and figures.
Conflicts of interest
The authors declare no conflicts of interest.
Declaration of funding
This research did not receive any specific funding.
References
[1] Department of Agriculture, Water and the Environment (2020) Vegetable industry. Farm surveys and analysis. https://www.agriculture.gov.au/abares/researchtopics/surveys/vegetables (accessed 20 February 2021)[2] Slavin, JL and Lloyd, B (2012) Health benefits of fruits and vegetables. Adv Nutr 3, 506–516.
| Health benefits of fruits and vegetables.Crossref | GoogleScholarGoogle Scholar |
[3] Randhawa MA et al. (2015) Chapter 18 - Green Leafy Vegetables: A Health Promoting Source. In Handbook of Fertility (Watson RR, ed.). pp. 205–220. Academic Press.
[4] Ishii, S and Sadowsky, MJ (2008) Escherichia coli in the environment: implications for water quality and human health. Microbes Environ 23, 101–108.
| Escherichia coli in the environment: implications for water quality and human health.Crossref | GoogleScholarGoogle Scholar |
[5] Jang, J et al.. (2017) Environmental Escherichia coli: ecology and public health implications—a review. J Appl Microbiol 123, 570–581.
| Environmental Escherichia coli: ecology and public health implications—a review.Crossref | GoogleScholarGoogle Scholar |
[6] García, A et al.. (2010) Zoonotic enterohemorrhagic Escherichia coli: a One Health perspective. ILAR J 51, 221–232.
| Zoonotic enterohemorrhagic Escherichia coli: a One Health perspective.Crossref | GoogleScholarGoogle Scholar |
[7] Maffei, DF et al.. (2016) Microbiology of organic and conventionally grown fresh produce. Braz J Microbiol 47, 99–105.
| Microbiology of organic and conventionally grown fresh produce.Crossref | GoogleScholarGoogle Scholar |
[8] Murray, K et al.. (2017) Challenges in the microbiological food safety of fresh produce: limitations of post-harvest washing and the need for alternative interventions. Food Qual Saf 1, 289–301.
| Challenges in the microbiological food safety of fresh produce: limitations of post-harvest washing and the need for alternative interventions.Crossref | GoogleScholarGoogle Scholar |
[9] Machado-Moreira, B et al.. (2019) Microbial contamination of fresh produce: what, where, and how? Compr Rev Food Sci Food Saf 18, 1727–1750.
| Microbial contamination of fresh produce: what, where, and how?Crossref | GoogleScholarGoogle Scholar |
[10] Lu, Q et al.. (2012) Land application of biosolids in the USA: a review. Appl Environ Soil Sci 2012, 201462.
| Land application of biosolids in the USA: a review.Crossref | GoogleScholarGoogle Scholar |
[11] Alegbeleye, OO et al.. (2018) Sources and contamination routes of microbial pathogens to fresh produce during field cultivation: a review. Food Microbiol 73, 177–208.
| Sources and contamination routes of microbial pathogens to fresh produce during field cultivation: a review.Crossref | GoogleScholarGoogle Scholar |
[12] Monaghan, JM and Hutchison, ML (2012) Distribution and decline of human pathogenic bacteria in soil after application in irrigation water and the potential for soil-splash-mediated dispersal onto fresh produce. J Appl Microbiol 112, 1007–1019.
| Distribution and decline of human pathogenic bacteria in soil after application in irrigation water and the potential for soil-splash-mediated dispersal onto fresh produce.Crossref | GoogleScholarGoogle Scholar |
[13] Deering, AJ et al.. (2015) Movement of Salmonella serovar Typhimurium and E. coli O157:H7 to ripe tomato fruit following various routes of contamination. Microorganisms 3, 809–825.
| Movement of Salmonella serovar Typhimurium and E. coli O157:H7 to ripe tomato fruit following various routes of contamination.Crossref | GoogleScholarGoogle Scholar |
[14] Hutchison, ML et al.. (2017) Fate of Escherichia coli O145 present naturally in bovine slurry applied to vegetables before harvest, after washing and simulated wholesale and retail distribution. J Appl Microbiol 123, 1597–1606.
| Fate of Escherichia coli O145 present naturally in bovine slurry applied to vegetables before harvest, after washing and simulated wholesale and retail distribution.Crossref | GoogleScholarGoogle Scholar |
[15] Sharma, M and Reynnells, R (2016) Importance of soil amendments: survival of bacterial pathogens in manure and compost used as organic fertilizers. Microbiol Spectr 4, 4.4.36.
| Importance of soil amendments: survival of bacterial pathogens in manure and compost used as organic fertilizers.Crossref | GoogleScholarGoogle Scholar |
[16] Lin, H et al.. (2019) Fate of tetracycline and sulfonamide resistance genes in a grassland soil amended with different organic fertilizers. Ecotoxicol Environ Saf 170, 39–46.
| Fate of tetracycline and sulfonamide resistance genes in a grassland soil amended with different organic fertilizers.Crossref | GoogleScholarGoogle Scholar |
[17] White HE, Orlova EV (2019) Bacteriophages: their structural organisation and function. In Bacteriophages - Perspectives and Future (Savva R, ed.). InTech.
[18] Carvalho CM et al. (2012) Phages as therapeutic tools to control major foodborne pathogens: Campylobacter and Salmonella. In Bacteriophages (Kurtboke I, ed.). InTech.
[19] Połaska, M and Sokołowska, B (2019) Bacteriophages—a new hope or a huge problem in the food industry. AIMS Microbiol 5, 324–346.
| Bacteriophages—a new hope or a huge problem in the food industry.Crossref | GoogleScholarGoogle Scholar |
[20] Kurtböke, Dİ et al.. (2016) Isolation and characterization of Enterobacteriaceae species infesting post-harvest strawberries and their biological control using bacteriophages. Appl Microbiol Biotechnol 100, 8593–8606.
| Isolation and characterization of Enterobacteriaceae species infesting post-harvest strawberries and their biological control using bacteriophages.Crossref | GoogleScholarGoogle Scholar |
[21] El-Tarabily, KA (2008) Promotion of tomato (Lycopersicon esculentum Mill.) plant growth by rhizosphere competent 1-aminocyclopropane-1-carboxylic acid deaminase-producing streptomycete actinomycetes. Plant Soil 308, 161–174.
| Promotion of tomato (Lycopersicon esculentum Mill.) plant growth by rhizosphere competent 1-aminocyclopropane-1-carboxylic acid deaminase-producing streptomycete actinomycetes.Crossref | GoogleScholarGoogle Scholar |
[22] Jonns, JA et al.. (2017) Streptophage-mediated control of off-flavour taint producing streptomycetes isolated from barramundi ponds. Synth Syst Biotechnol 2, 105–112.
| Streptophage-mediated control of off-flavour taint producing streptomycetes isolated from barramundi ponds.Crossref | GoogleScholarGoogle Scholar |
[23] O’Flynn, G et al.. (2004) Evaluation of a cocktail of three bacteriophages for biocontrol of Escherichia coli O157:H7. Appl Environ Microbiol 70, 3417–3424.
| Evaluation of a cocktail of three bacteriophages for biocontrol of Escherichia coli O157:H7.Crossref | GoogleScholarGoogle Scholar |
[24] Wang, L et al.. (2017) Use of bacteriophages to control Escherichia coli O157:H7 in domestic ruminants, meat products, and fruits and vegetables. Foodborne Pathog Dis 14, 483–493.
| Use of bacteriophages to control Escherichia coli O157:H7 in domestic ruminants, meat products, and fruits and vegetables.Crossref | GoogleScholarGoogle Scholar |
[25] Duc, HM et al.. (2020) Isolation, characterization and application of a polyvalent phage capable of controlling Salmonella and Escherichia coli O157:H7 in different food matrices. Food Res Int 131, 108977.
| Isolation, characterization and application of a polyvalent phage capable of controlling Salmonella and Escherichia coli O157:H7 in different food matrices.Crossref | GoogleScholarGoogle Scholar |
[26] Manohar, P et al.. (2019) Therapeutic characterization and efficacy of bacteriophage cocktails infecting Escherichia coli, Klebsiella pneumoniae, and Enterobacter species. Front Microbiol 10, 574.
| Therapeutic characterization and efficacy of bacteriophage cocktails infecting Escherichia coli, Klebsiella pneumoniae, and Enterobacter species.Crossref | GoogleScholarGoogle Scholar |
[27] Yazdi, M et al.. (2020) Isolation, characterization and genomic analysis of a novel bacteriophage VB_EcoS-Golestan infecting multidrug-resistant Escherichia coli isolated from urinary tract infection. Sci Rep 10, 7690.
| Isolation, characterization and genomic analysis of a novel bacteriophage VB_EcoS-Golestan infecting multidrug-resistant Escherichia coli isolated from urinary tract infection.Crossref | GoogleScholarGoogle Scholar |
[28] Jończyk, E et al.. (2011) The influence of external factors on bacteriophages—review. Folia Microbiol (Praha) 56, 191–200.
| The influence of external factors on bacteriophages—review.Crossref | GoogleScholarGoogle Scholar |
[29] Sillankorva, SM et al.. (2012) Bacteriophages and their role in food safety. Int J Microbiol 2012, 863945.
| Bacteriophages and their role in food safety.Crossref | GoogleScholarGoogle Scholar |
[30] Guenther, S et al.. (2009) Virulent bacteriophage for efficient biocontrol of Listeria monocytogenes in ready-to-eat foods. Appl Environ Microbiol 75, 93–100.
| Virulent bacteriophage for efficient biocontrol of Listeria monocytogenes in ready-to-eat foods.Crossref | GoogleScholarGoogle Scholar |
[31] Sharma, M et al.. (2009) Effectiveness of bacteriophages in reducing Escherichia coli O157:H7 on fresh-cut cantaloupes and lettuce. J Food Prot 72, 1481–1485.
| Effectiveness of bacteriophages in reducing Escherichia coli O157:H7 on fresh-cut cantaloupes and lettuce.Crossref | GoogleScholarGoogle Scholar |
[32] Kazi, M and Annapure, US (2016) Bacteriophage biocontrol of foodborne pathogens. J Food Sci Technol 53, 1355–1362.
| Bacteriophage biocontrol of foodborne pathogens.Crossref | GoogleScholarGoogle Scholar |
[33] Boyacioglu, O et al.. (2013) Biocontrol of Escherichia coli O157: H7 on fresh-cut leafy greens. Bacteriophage 3, e24620.
| Biocontrol of Escherichia coli O157: H7 on fresh-cut leafy greens.Crossref | GoogleScholarGoogle Scholar |
[34] Snyder, AB et al.. (2016) Developing and optimizing bacteriophage treatment to control enterohemorrhagic Escherichia coli on fresh produce. Int J Food Microbiol 236, 90–97.
| Developing and optimizing bacteriophage treatment to control enterohemorrhagic Escherichia coli on fresh produce.Crossref | GoogleScholarGoogle Scholar |
[35] Tanji, Y et al.. (2004) Toward rational control of Escherichia coli O157:H7 by a phage cocktail. Appl Microbiol Biotechnol 64, 270–274.
| Toward rational control of Escherichia coli O157:H7 by a phage cocktail.Crossref | GoogleScholarGoogle Scholar |
[36] Loc-Carrillo, C and Abedon, ST (2011) Pros and cons of phage therapy. Bacteriophage 1, 111–114.
| Pros and cons of phage therapy.Crossref | GoogleScholarGoogle Scholar |
[37] Kim, B-o et al.. (2019) Phage-derived antibacterials: harnessing the simplicity, plasticity, and diversity of phages. Viruses 11, 268.
| Phage-derived antibacterials: harnessing the simplicity, plasticity, and diversity of phages.Crossref | GoogleScholarGoogle Scholar |


