Lumpy skin disease: a significant threat to Australia
Kelly J. Stanger A * and Timothy R. Bowden AA CSIRO, Australian Centre for Disease Preparedness, Geelong, Vic. 3220, Australia.

Kelly Stanger is the Group leader for the newly established Lumpy Skin Disease Research group at the Australian Centre for Disease Preparedness (ACDP). She has over 20 years of research experience, predominantly working on the production limiting diseases and disorders affecting the red meat industries. Prior to commencing the Lumpy Skin Disease research role, Kelly was the Veterinary Services manager for ACDP overseeing the operation and research activities in the animal facilities. Before joining ACDP, she was a lecturer in Cattle Medicine and Production at the University of Melbourne, completed her PhD and worked as a veterinary consultant to the sheep and beef cattle industries through her work with the Mackinnon Group. She has a keen interest in biosecurity, animal welfare and production limiting diseases of livestock. |

Tim Bowden is a Senior Research Scientist and veterinarian with over 20 years of experience in virology and molecular biology, as well as diagnostic test development and validation, focusing on the pathogenesis, diagnosis, and control of infectious animal diseases. Since completing his PhD in 2004 Tim has worked at the CSIRO Australian Centre for Disease Preparedness (ACDP) on various projects, with both domestic and international collaborators, including to enhance the diagnostic capability for diseases caused by capripoxviruses (sheeppox, goatpox and lumpy skin disease) and foot-and-mouth disease. He has broad experience with pathogenesis and vaccine efficacy trials and, prior to joining the recently established Lumpy Skin Disease Research group, was the Team Leader (Serology) within the ACDP Diagnostic Emergency Response Laboratory. |
Microbiology Australia 43(4) 186-189 https://doi.org/10.1071/MA22061
Submitted: 10 November 2022 Accepted: 6 December 2022 Published: 22 December 2022
© 2022 The Author(s) (or their employer(s)). Published by CSIRO Publishing on behalf of the ASM. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
Lumpy skin disease, a mechanically transmitted poxvirus, causes severe disease in naïve populations of cattle and buffalo. It is characterised by development of multifocal cutaneous nodules and systemic illness with significant impacts on animal health, productivity, welfare and trade. Lumpy skin disease entered South-East Asia via Myanmar (November 2020) and later Thailand (March 2021), and has spread rapidly through the region reaching Indonesia in February 2022. This article provides a brief overview of current literature and its application to the Australian context including possible routes of entry, early detection and knowledge gaps that need to be addressed to improve preparedness and response capability.
Keywords: Australia, buffalo, capripoxvirus, cattle, emergency animal disease, lumpy skin disease, poxvirus, South‐East Asia, surveillance, vector.
Introduction
Lumpy skin disease (LSD) is a severe, vector-borne disease that affects cattle and Asian water buffalo (Bubalus bubalis).1 All breeds of cattle are susceptible, but both beef and high milk producing Bos taurus animals are more susceptible than Bos indicus breeds. Lumpy skin disease virus (LSDV) is a highly host-specific member of the genus Capripoxvirus, family Poxviridae, and is closely related to sheeppox and goatpox viruses with at least 96% identity at the genome level.2,3
Lumpy skin disease usually results in medium to high morbidity (2–45%) and low mortality (<10%) in naïve populations and is characterised by the development of multifocal cutaneous skin nodules (up to 5 cm in diameter), pyrexia (fever), lymphadenopathy (enlarged lymph nodes), depression, reduced milk production and permanent hide damage.1,4 It is notifiable to the World Organisation for Animal Health (WOAH) due to its capacity for rapid transboundary spread and is considered one of the most economically important viral diseases of cattle due to its impacts on animal health, production and trade.5,6
The Australian Federal Government response to incursion is well documented.7,8 However, regaining disease free status would require at least 14 months from the detection of the last case of LSD before trade sanctions could be lifted9 having significant repercussions for the Australian economy.
Emergence of lumpy skin disease in South-East Asia
Lumpy skin disease is considered endemic to much of Africa and, prior to 2012, detection of this disease outside of endemic areas was largely confined to sporadic outbreaks in the Middle East.5,10 In the past 10 years, LSDV entered eastern Europe and was successfully controlled only after the implementation of a comprehensive and widespread vaccination program, with LSD remaining endemic in Turkey.11 Lumpy skin disease subsequently spread through India and China and more recently into South-East Asia (WAHIS (woah.org)) with outbreaks reported in Myanmar and Thailand in November 2020 and March 2021, respectively. In contrast to the sporadic and gradual spread of LSD seen historically, spread throughout South-East Asia has occurred more rapidly, with outbreaks in Malaysia (May 2021), Laos (May 2021), Singapore (March 2022) and Indonesia (February 2022) now reported12,13 (Fig. 1).
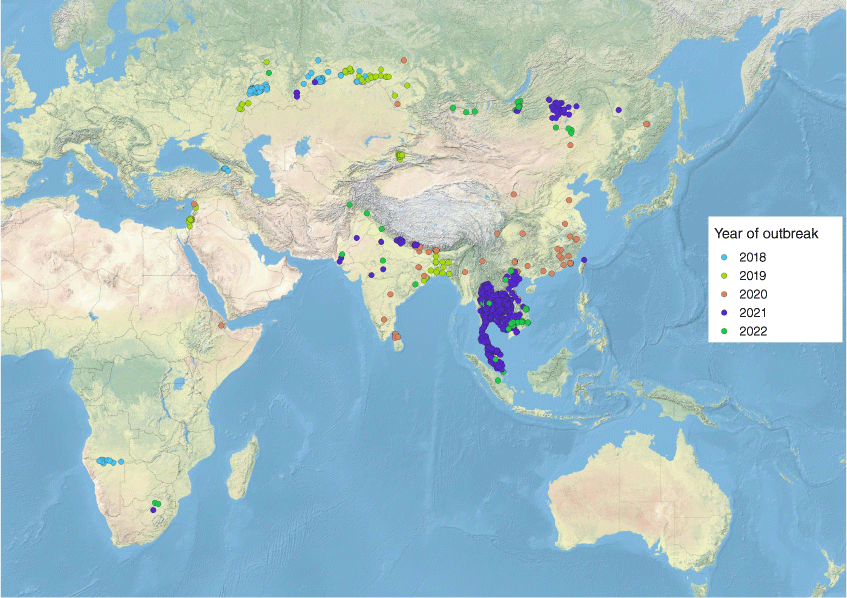
|
The accelerated spread of LSD is most likely associated with significant cross-border animal movement and insect vector activity, however questions about altered virulence characteristics of emerging strains have been raised.14 Genomic sequencing of the South-East Asian isolates have shown that some of the emerging strains are vaccine-like recombinants which have most likely resulted from suboptimal vaccine manufacturing,15 but assessment of virulence characteristics have not yet been undertaken. To our knowledge, the strain circulating in Indonesia has not yet been sequenced and so its phylogenetic origin is unknown.
However, when considering the likely routes of incursion of LSD into South-East Asia, it is feasible to expect the virus circulating within Indonesia will be closely related to either the Hong Kong (2020) or Thailand (2021) strains. Sequencing of these strains demonstrate that each is closely related to vaccine strains suggestive of recombination during vaccine manufacture.16,17 Assessment of the comparative pathogenesis of these variants has not yet been published and so any potential differences in virulence or transmission characteristics are unknown.
The current distribution of LSD within Indonesia is not well understood because early eradication and surveillance efforts have been complicated by the recent detection of foot-and-mouth disease. To date, there have been no cases of LSDV in either Papua New Guinea or Timor-Leste reported to WOAH (WAHIS (woah.org)).
Risk pathways for incursion into Australia
Since Australia does not share land borders with our near-neighbours, and with LSD being a vector-borne disease, natural pathways that facilitate the introduction of infected vectors, rather than introduction of infected animals, are considered the most likely route of incursion into Australia.7 As evidenced by the emergence of new bluetongue virus variants, there is precedent for the wind-borne introduction of infected vectors into northern Australia.18,19 Given the current trajectory of spread through South-East Asia and significant regulated and unregulated movement of animals amongst Indonesia, Papua New Guinea and Timor-Leste, northern Australia is considered most vulnerable.
This presents a major challenge in keeping LSD out of the country. Natural pathways are much more difficult to manage and monitor when compared with regulated pathways (e.g. food products). Further, predicting the most likely point of entry to ensure targeted surveillance efforts hinges on the accuracy of predictive modelling. There are many gaps in our knowledge pertaining to the climatic events and retention of vector competency through such events which generates uncertainty in the risk assessments.
What is known, however, is that outbreaks of LSD are commonly phasic and are associated with climatic conditions which favour insect activity. Typically, outbreaks occur in warm, humid and moist conditions, with transmission decreasing as ambient temperatures drop.11 For South-East Asia and northern Australia, climatic conditions favouring vector activity occur between August and April each year and so the likelihood of continued spread of LSD eastward through Indonesia, in the absence of a comprehensive vaccination program, is high.
Understanding the vectors of importance
Vector flight distance, proximity to susceptible cattle populations and likelihood of surviving long distance wind-dispersal impact risk profiling and modelling for incursion. If introduced, studies have demonstrated that a wide range of biting insects including mosquitoes, midges, biting flies and ticks are capable of mechanically transmitting LSDV, however longevity of virus survival within these vectors is variable.20–23 Ticks are unlikely to facilitate rapid spread of disease but may act as an environmental reservoir and facilitate overwintering of the virus which could impact eradication efforts.22–24
The local conditions, numbers of insects, flight distance, feeding behaviour and lifespan determine the importance of individual vector species and will be regionally specific. Australia has a diverse range of potential vectors that could aid in transmission of LSDV if incursion occurs, with some of the vector species of primary concern, given their numbers in northern Australia, being the buffalo fly (Haematobia exigua) and midges (Culicoides species) (flyboss.com.au). The role of midge species in virus uptake and onward transmission is thought to be low, but the importance of buffalo fly has not been determined. The minimal infective dose and, by extrapolation, the number of vectors required to establish infection in cattle is not well established.
Early detection, surveillance, and response
Early detection and response to an incursion of LSD in northern Australia would be challenging. There are a significant number of unmanaged feral cattle and buffalo in northern Australia and effective response efforts would be impaired by lack of access to these populations. Further, in naïve populations, detection of acute disease would form the basis of a presumptive diagnosis which, due to the presence of other diseases with similar clinical features in cattle,25 must be confirmed by laboratory diagnosis. However, during the wet season in northern Australia, physical access to managed cattle can be limited and so detection of an index case may be delayed.7
It is well recognised that large-scale vaccination of susceptible bovine populations is the most effective means to control and limit the spread of LSD.26 Currently, only live attenuated vaccines are readily available. However, although highly effective, they should be sourced from reputable vaccine manufacturers, be well characterised and shown to be free of adventitious agents before use. For Australia, as for other lumpy skin disease free countries, the pre-emptive use of LSD vaccines would result in the loss of disease-free status and have immediate impacts on trade and, as such, is unlikely to form part of any pre-incursion risk mitigation activities.
Consequently, surveillance and preparedness activities are currently focused on assisting affected near neighbouring countries to control disease outbreaks, further enhancing diagnostic assays used for surveillance activities and contributing to key knowledge gaps of significance to Australia.
Following extensive consultation between the Australian Government, State and Territory Governments, industry and non-governmental stakeholders, a National Lumpy Skin Disease Action Plan has been developed to guide and coordinate these activities.27
Conclusion
If an outbreak of lumpy skin disease were to occur in Australia, particularly in the extensive northern cattle industry, eradication would likely be challenging. There are a broad range of biting insects that would likely be able to transmit the virus within susceptible populations. Further, ticks may play a role in maintenance of the virus for extended periods. Early detection of disease may be hindered by periods of inaccessibility to northern Australian herds, especially during the wet season, and populations of unmanaged cattle and buffalo are likely to complicate eradication efforts.
However, with respect to our near neighbouring countries, LSD is confined to Indonesia with Papua New Guinea and Timor-Leste considered disease free. The reported distribution of disease within Indonesia is, however, based on the initial notification to WOAH and may not be reflective of the current status.
Should LSDV spread into Papua New Guinea and/or Timor-Leste, the most likely route of incursion into Australia would be via wind-dispersal of infected vectors into northern Australia from these countries. Risk modelling, targeted surveillance, development and validation of improved diagnostic tests, and development and assessment of vaccine candidates suitable for use in Australia are key aspects of preparedness.
Data availability
Data sharing is not applicable since no new data were generated or analysed during this study.
Conflicts of interest
The authors declare no conflicts of interest.
Declaration of funding
This research did not receive any specific funding.
Acknowledgements
We are grateful to Tristan Reid (CSIRO Australian Centre for Disease Preparedness) for his assistance in generating Fig. 1.
References
[1] Tuppurainen ESM et al. (2018) General description of lumpy skin disease. In Lumpy Skin Disease. Springer, Cham.| Crossref |
[2] Tulman, ER et al.. (2001) Genome of lumpy skin disease virus. J Virol 75, 7122–7130.
| Genome of lumpy skin disease virus.Crossref | GoogleScholarGoogle Scholar |
[3] Tulman, ER et al.. (2002) The genomes of sheeppox and goatpox viruses. J Virol 76, 6054–6061.
| The genomes of sheeppox and goatpox viruses.Crossref | GoogleScholarGoogle Scholar |
[4] Babiuk, S et al.. (2008) Quantification of lumpy skin disease virus following experimental infection in cattle. Transbound Emerg Dis 55, 299–307.
| Quantification of lumpy skin disease virus following experimental infection in cattle.Crossref | GoogleScholarGoogle Scholar |
[5] Babiuk, S et al.. (2008) Capripoxviruses: an emerging worldwide threat to sheep, goats and cattle. Transbound Emerg Dis 55, 263–272.
| Capripoxviruses: an emerging worldwide threat to sheep, goats and cattle.Crossref | GoogleScholarGoogle Scholar |
[6] Tuppureinen E, Galon N (2016) Lumpy skin disease: current situation in Europe and neighbouring regions and necessary control measures to halt the spread in south-east Europe.
| Crossref |
[7] Animal Health Australia (2022) Response strategy: Lumpy skin disease (version 5.0), 5th edn. Australian Veterinary Emergency Plan (AUSVETPLAN). Available at https://animalhealthaustralia.com.au//wp-content/uploads/2022/09/AUSVETPLAN-Manuals-Response-Lumpy-skin-disease.pdf [accessed 10 November 2022]
[8] Animal Health Australia (2022) Emergency Animal Disease Response Agreement (EADRA). Available at https://animalhealthaustralia.com.au/eadra/ [accessed 10 November 2022]
[9] World Organisation for Animal Health (2022) Terrestrial Animal Health Code. Chapter 11.9. Infection with lumpy skin disease virus. OIE, Paris. Available at https://www.woah.org/fileadmin/Home/eng/Health_standards/tahc/current/chapitre_lsd.pdf [accessed 10 November 2022]
[10] Tuppurainen, ESM et al.. (2017) Review: Capripoxvirus diseases: current status and opportunities for control. Transbound Emerg Dis 64, 729–745.
| Review: Capripoxvirus diseases: current status and opportunities for control.Crossref | GoogleScholarGoogle Scholar |
[11] (2020) Lumpy skin disease epidemiological report IV: data collection and analysis. EFSA J 18, e06010.
| Lumpy skin disease epidemiological report IV: data collection and analysis.Crossref | GoogleScholarGoogle Scholar |
[12] Azeem, S et al.. (2022) Lumpy skin disease is expanding its geographic range: a challenge for Asian livestock management and food security. Vet J 279, 105785.
| Lumpy skin disease is expanding its geographic range: a challenge for Asian livestock management and food security.Crossref | GoogleScholarGoogle Scholar |
[13] Kumar, P et al.. (2021) Emergence and transboundary spread of lumpy skin disease in South Asia. Indian J Anim Sci 91, 507–517.
| Emergence and transboundary spread of lumpy skin disease in South Asia.Crossref | GoogleScholarGoogle Scholar |
[14] Kononova, S et al.. (2021) A lumpy skin disease virus which underwent a recombination event demonstrates more aggressive growth in primary cells and cattle than the classical field isolate. Transbound Emerg Dis 68, 1377–1383.
| A lumpy skin disease virus which underwent a recombination event demonstrates more aggressive growth in primary cells and cattle than the classical field isolate.Crossref | GoogleScholarGoogle Scholar |
[15] Vandenbussche, F et al.. (2022) Recombinant LSDV strains in Asia: vaccine spillover or natural emergence? Viruses 14, 1429.
| Recombinant LSDV strains in Asia: vaccine spillover or natural emergence?Crossref | GoogleScholarGoogle Scholar |
[16] Flannery, J et al.. (2022) A novel strain of lumpy skin disease virus causes clinical disease in cattle in Hong Kong. Transbound Emerg Dis 69, e336–e343.
| A novel strain of lumpy skin disease virus causes clinical disease in cattle in Hong Kong.Crossref | GoogleScholarGoogle Scholar |
[17] Sariya, L et al.. (2022) Molecular detection and characterization of lumpy skin disease viruses from outbreaks in Thailand in 2021. Transbound Emerg Dis 69, e2145–e2152.
| Molecular detection and characterization of lumpy skin disease viruses from outbreaks in Thailand in 2021.Crossref | GoogleScholarGoogle Scholar |
[18] White, JR et al.. (2021) Bluetongue virus serotype 12 enters Australia – a further incursion of novel western lineage genome segments. J Gen Virol 102, 001536.
| Bluetongue virus serotype 12 enters Australia – a further incursion of novel western lineage genome segments.Crossref | GoogleScholarGoogle Scholar |
[19] Eagles, D et al.. (2014) Long-distance aerial dispersal modelling of Culicoides biting midges: case studies of incursions into Australia. BMC Vet Res 10, 135.
| Long-distance aerial dispersal modelling of Culicoides biting midges: case studies of incursions into Australia.Crossref | GoogleScholarGoogle Scholar |
[20] Paslaru, AI et al.. (2022) Putative roles of mosquitoes (Culicidae) and biting midges (Culicoides spp.) as mechanical or biological vectors of lumpy skin disease virus. Med Vet Entomol 36, 381–389.
| Putative roles of mosquitoes (Culicidae) and biting midges (Culicoides spp.) as mechanical or biological vectors of lumpy skin disease virus.Crossref | GoogleScholarGoogle Scholar |
[21] Sanz-Bernardo, B et al.. (2022) The acquisition and retention of lumpy skin disease virus by blood-feeding insects is influenced by the source of virus, the insect body part, and the time since feeding. J Virol 96, e00751-22.
| The acquisition and retention of lumpy skin disease virus by blood-feeding insects is influenced by the source of virus, the insect body part, and the time since feeding.Crossref | GoogleScholarGoogle Scholar |
[22] El-Ansary, RE et al.. (2022) Isolation and molecular characterization of lumpy skin disease virus from hard ticks, Rhipicephalus (Boophilus) annulatus in Egypt. BMC Vet Res 18, 302.
| Isolation and molecular characterization of lumpy skin disease virus from hard ticks, Rhipicephalus (Boophilus) annulatus in Egypt.Crossref | GoogleScholarGoogle Scholar |
[23] Tuppurainen, ESM et al.. (2011) A potential role for ixodid (hard) tick vectors in the transmission of lumpy skin disease virus in cattle. Transbound Emerg Dis 58, 93–104.
| A potential role for ixodid (hard) tick vectors in the transmission of lumpy skin disease virus in cattle.Crossref | GoogleScholarGoogle Scholar |
[24] Lubinga, JC et al.. (2013) Detection of lumpy skin disease virus in saliva of ticks fed on lumpy skin disease virus-infected cattle. Exp Appl Acarol 61, 129–138.
| Detection of lumpy skin disease virus in saliva of ticks fed on lumpy skin disease virus-infected cattle.Crossref | GoogleScholarGoogle Scholar |
[25] Department of Agriculture Fisheries and Forestry. Differential diagnoses for lumpy skin disease. https://www.agriculture.gov.au/sites/default/files/documents/lsd-differential-chart.pdf (accessed 10 November 2022)
[26] Tuppurainen, E et al.. (2021) Review: Vaccines and vaccination against lumpy skin disease. Vaccines 9, 1136.
| Review: Vaccines and vaccination against lumpy skin disease.Crossref | GoogleScholarGoogle Scholar |
[27] Department of Agriculture Fisheries and Forestry (2022) National Lumpy Skin Disease Action Plan (Department of Agriculture Fisheries and Forestry, ed.). Available at https://www.agriculture.gov.au/sites/default/files/documents/lsd-national-action-plan.pdf [accessed 10 November 2022]


