The Australian Gonococcal Surveillance Programme 1979–2017
Monica M Lahra A B F , CR Robert George A C and David M Whiley D EA WHO Collaborating Centre for STD, Microbiology Department, New South Wales Health Pathology East, Prince of Wales Hospital, Sydney, NSW 2031, Australia
B School of Medical Sciences, The University of New South Wales, Sydney, NSW 2052, Australia
C Centre for Infectious Diseases and Microbiology Laboratory Services, NSW Health Pathology, Sydney, NSW, Australia
D Faculty of Medicine, UQ Centre for Clinical Research, The University of Queensland, Herston, Qld 4029, Australia
E Pathology Queensland, Microbiology Department, Herston, Qld 4029, Australia
F Email: Monica.Lahra@health.nsw.gov.au
Microbiology Australia 38(4) 175-179 https://doi.org/10.1071/MA17062
Published: 2 November 2017
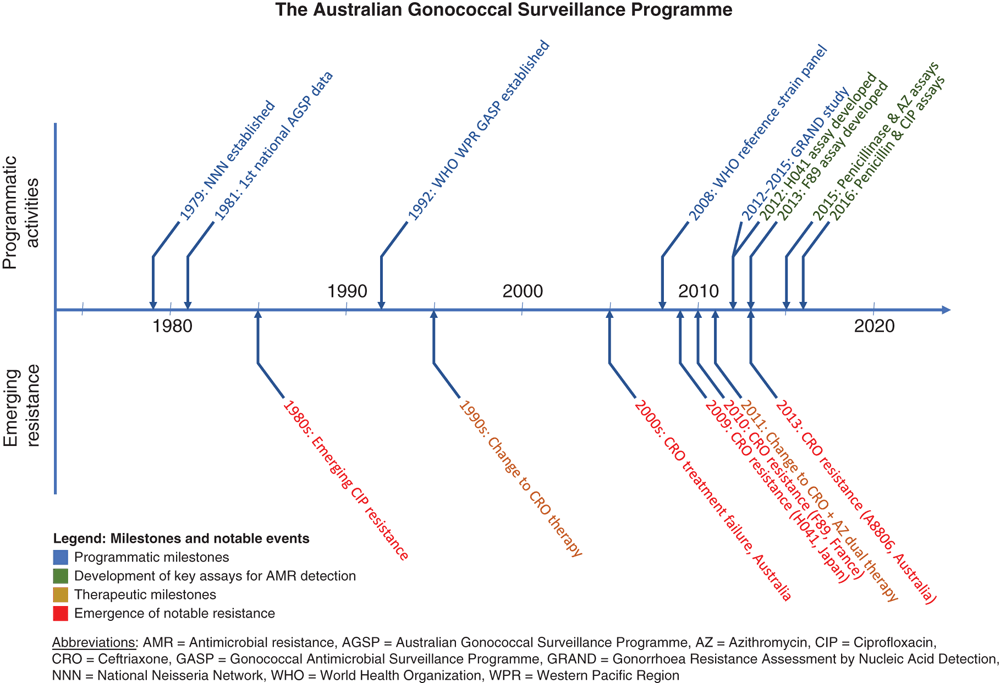
Neisseria gonorrhoeae (NG) is an important human bacterial pathogen responsible for more than 78 million infections per annum globally1. In Australia and elsewhere, NG infection rates are increasing and, critically, antimicrobial resistance (AMR) in NG poses a substantial threat to health security2. In response, the Australian Gonococcal Surveillance Programme (AGSP) was established in 1979, and has steadfastly evolved since that time to meet the challenges of continuously emerging AMR (Figure 1).
Effective antibiotic treatment for gonorrhoea is critical for gonococcal disease control, minimising sequelae, reducing HIV transmission3, and NG elimination and eradication efforts (theoretically possible as there is no external host). Heightened concerns regarding NG AMR are based on the fact that NG has acquired resistance to all of the antibiotics used as treatment, and has maintained resistance to these various antibiotics even in the absence of selection pressure4. The current first line treatment recommended for gonorrhoea in urban Australia, Europe, the United States of America, the United Kingdom, in many countries in the Asia Pacific, and elsewhere, is ceftriaxone and azithromycin. The addition of azithromycin to ceftriaxone monotherapy in recent years was in response to global trends of increasing ceftriaxone MIC values5, increasing reports of treatment failures using ceftriaxone monotherapy, and the emergence of the ceftriaxone resistant strains from Japan in 2010 (the H041 strain)5, followed by France then Spain (the F89 strain)6,7, and then Australia (the A8806 strain)8. The move to dual therapy was a speculative bid, without a strong evidence base, to delay and prevent widespread development and expansion of NG AMR to ceftriaxone in the absence of an ideal alternate treatment5. Unfortunately with recent reports of increasing azithromycin resistance (detailed below) this particular dual treatment strategy will need review.
The World Health Organization (WHO) recommends that treatment regimens for sexually transmitted diseases have an efficacy of at least 95%, and that the threshold for treatment change (5% resistance) be informed by local surveillance data5. Globally there are, however, serious gaps in NG AMR surveillance, and least represented are countries in the WHO African Region and Eastern Mediterranean Region2. Further complicating NG disease control are a number of factors including a high proportion of asymptomatic infections, particularly in women, and in pharyngeal and rectal infections5; increasing rates of AMR4; and the lack of a vaccine for gonorrhoea9. Notwithstanding these issues, the use of disease prevention messages, sensitive and specific tests for diagnosis, high risk patient screening, follow up and treatment of partners and contacts, and monitoring AMR by surveillance to inform treatment guidelines, remain key components of the WHO strategies for NG disease control5.
In 2014, the WHO published the first global report on the status of AMR bacteria of international concern including NG10. Concerningly, data were available from only 22% of the Member States (42/194), and the available data indicated that the emergence of NG AMR resistance to the extended spectrum cephalosporin antibiotics was more rapid than the development of alternate strategies for treatment which will almost certainly impact on disease burden. Critically, the report highlighted that data were lacking from countries and areas where disease burden was highest, and information to inform treatment and control strategies is most needed. Building capacity for surveillance was a primary recommendation of this report, as was the maintenance, enhancement and expansion of existing surveillance systems so as to provide additional evidence to underpin strategies for NG control10.
The AGSP was established in 1979 and is recognised as the longest running, and most comprehensive, programme of its kind. The need for NG AMR surveillance was recognised in Australia in the 1970s, and through leadership in the medical and scientific community across the country the Australian National Neisseria Network (NNN) was established. The first steps towards the establishment of the AGSP, which was the NNN’s first AMR programme, was to develop an agreed methodology of MIC determination for NG AMR testing, and to gauge its reliability11. This was achieved over a period of 18 months and included cross jurisdictional assessment of the testing systems11. Integral to the AGSP is the programme specific quality assurance surveys that are part of the NNN strategy to ensure data quality and to monitor proficiency in the NNN12. This networked approach across the jurisdictions of the AGSP continues to be coordinated by the NNN Coordinating Laboratory in Sydney, NSW, which was designated a WHO Collaborating Centre in 1998, following the successful establishment of the WHO Gonococcal Antimicrobial Surveillance Programme for the Western Pacific Region in 1992.
At the time of establishment of the NNN in 1979 a primary concern was the emergence of NG resistant to penicillin, which was the recommended treatment at that time. The AGSP data were first reported in Communicable Diseases Intelligence (CDI) in 198113, and since then, quarterly and annual reports have been published continuously. Initially, only susceptibility to the penicillins was reported, and other antibiotics were introduced subsequently. These data have been pivotal in informing changes in NG treatment in urban Australia, from penicillin to ciprofloxacin in the 1980s, then ciprofloxacin to ceftriaxone in the late 1990s; and also enabling the maintenance of an oral based treatment strategy in remote Australia.
Now, 38 years on, the NNNs Australian Gonococcal Surveillance Programme is the longest running continuously reporting NG AMR programme and the most comprehensive of its kind. The Programmes of the NNN now include the AGSP and the Australian Meningococcal Surveillance Programmes. These NNN programmes continue to be supported by the Australian Government Department of Health, New South Wales Health Pathology, and by the jurisdictional authorities responsible for the Reference Laboratories in each State and Territory.
Since its establishment, the NNN has achieved many notable outcomes. Amongst the most significant achievements is building and maintaining of the Network itself, and the strength of its collaboration which has been maintained over time. The Network comprises seven laboratories, and academic partners, which test and report on more than 6500 NG clinical samples per year. This equates to about 1/3 of all infecting strains nationally, annually, with the remainder diagnosed by nucleic acid amplification testing. The NNN works closely with local and national health, academic and research partners, and international partners including the WHO at the Regional Offices and Headquarters.
The NNN reports NG antimicrobial susceptibility data for remote regions of Australia as well for urban regions, as the disease epidemiology for remote, indigenous populations differs vastly from the urban settings. Overall, in 2015, the rate of notification of NG infections in the Aboriginal and Torres Strait Islander population was 10 times that in the non-Indigenous population (625.6 per 100 000 compared with 62.4 per 100 000). However, over the period 2011–2015, the notification rate of NG decreased by 22% in the Indigenous population, compared with a 94% increase in the non-indigenous population14. Whilst the NG disease rates are very high in remote regions with a predominant Aboriginal and Torres Strait Islander population, the AGSP data show surprisingly low rates of AMR. This has enabled a combination of penicillin, azithromycin and probenecid to be continued as the recommended treatment. A significant benefit to this strategy is that it is oral and easier to use and administer in remote settings. Thus, in addition to providing continuous national trend data for NG AMR over the past 36 years, the regional specific AGSP data are used to inform two treatment guidelines – for urban and for remote settings15.
Recent years have been particularly interesting in terms of NG disease and NG AMR. In Australia over the period 2011–2015, rates of NG disease notifications almost doubled in urban populations14, and a coincident rise in the proportion of NG strains with elevated MIC values to ceftriaxone was reported, peaking at 8.8% in 201316. Further, in 2013, the A8806 strain was identified with the highest ceftriaxone MIC ever reported in Australia, and concerning key genetic similarities to the H041 strain reported in Japan8. In 2014, Australia followed the UK, the USA and Europe in moving from ceftriaxone monotherapy to dual treatment with ceftriaxone and azithromycin. The introduction of dual therapy was followed in 2015 and 2016 with a decrease in proportions of strains nationally with decreased susceptibility to ceftriaxone17. Until 2016, NG azithromycin resistance in Australia was relatively low, averaging approximately 3% nationally. However, in the first quarter of 2016, in the state of South Australia, azithromycin resistance significantly increased (P < 0.0001) from <5% in the latter half of 2015 to 30% in the first quarter of 2016. Resistance remained high through 2016, with the overall proportion of azithromycin resistant NG strains in South Australia in 2016 reported as 19.5%17. By the end of the second quarter of 2017 most states reported isolates with resistance to azithromycin, and the overall incidence of azithromycin resistance, nationally, by the end of quarter 2, 2017 was 11.0%, more than double the proportion of azithromycin resistance reported nationally for 2016 (5.0%), and more than four times the level of azithromycin resistance reported in Australia for 2013–2015 (2.1–2.6%)18. With the azithromycin resistance levels now greater than the WHO threshold of 5%, consideration is being given to the next therapeutic recommendations for NG but the direction remains unclear, and heightened surveillance activities are in place.
Since its foundation, the NNN has actively engaged in collaborative research programmes including most recently the Gonorrhoea Resistance Assessment via Nucleic Acid Detection (GRAND) study funded by the NHMRC from 2012 to 2015. The GRAND project represents a highly successful collaboration between the NNN and scientists, clinicians and academics throughout Australia. Key outcomes include the development of new molecular tools for NG AMR surveillance. These include assays for the detection of penicillin, ciprofloxacin and azithromycin resistance19–21, which have improved understanding of NG and associated AMR in the Australian population, enabled molecular surveillance of penicillin resistance in NG from remote Australia and translation of new methods into routine practice. Notable publications from GRAND include The Molecular Epidemiology and Antimicrobial Resistance of Neisseria gonorrhoeae in Australia: A Nationwide Cross-Sectional Study, 2012, published in Clinical Infectious Diseases and Molecular Antimicrobial Resistance Surveillance for Neisseria gonorrhoeae, Northern Territory, Australia being the final GRAND article and recently published in Emerging Infectious Diseases22,23.
A second study, the GRAND2 Study, funded via the NHMRC from 2017 to 2020, will be exploring alternative treatment options for gonorrhoea. Via the GRAND study, we developed a PCR test that has 99% accuracy for predicting NG ciprofloxacin susceptibility. The GRAND2 will assess the performance of this test in facilitating an individualised treatment approach for gonorrhoea, whereby patients are treated on the basis of the ciprofloxacin PCR test. If successful, the approach will facilitate broader use of oral antibiotics for gonorrhoea treatment; enable partner delivered patient therapy; and spare unnecessary use of ceftriaxone.
The NNN has worked collaboratively to monitor NG disease epidemiology and NG AMR in Australia for the past 36 years. The need for close and continued monitoring of gonococcal AMR is clearly evident, and the work of the NNN is critical for clinical management in the Australian setting; detection and response to resistant strains; and to continue to provide data for treatment guidelines.
Acknowledgements
The authors recognise and acknowledge the contribution of the many scientists, researchers and clinicians in the establishment, development and maintenance of the NNN, particularly Professor John Tapsall AM who led the establishment of the NNN and engaged, mentored and supported many in the field nationally and internationally for more than 30 years.
References
[1] World Health Organization (2016) Sexually transmitted infections (STIs). http://www.who.int/mediacentre/factsheets/fs110/en/ (accessed 30 August 2017).[2] Wi, T. et al. (2017) Antimicrobial resistance in Neisseria gonorrhoeae: global surveillance and a call for international collaborative action. PLoS Med. 14, e1002344.
| Antimicrobial resistance in Neisseria gonorrhoeae: global surveillance and a call for international collaborative action.Crossref | GoogleScholarGoogle Scholar |
[3] Craib, K.J. et al. (1995) Rectal gonorrhoea as an independent risk factor for HIV infection in a cohort of homosexual men. Sex. Transm. Infect. 71, 150–154.
| Rectal gonorrhoea as an independent risk factor for HIV infection in a cohort of homosexual men.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK2Mzmt1altQ%3D%3D&md5=e2e8c95ba07d8892d0f5478f63b369d5CAS |
[4] Goire, N. et al. (2014) Molecular approaches to enhance surveillance of gonococcal antimicrobial resistance. Nat. Rev. Microbiol. 12, 223–229.
| Molecular approaches to enhance surveillance of gonococcal antimicrobial resistance.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXitFamtbw%3D&md5=ccd36db6b924c3611a4f0f62500612f3CAS |
[5] World Health Organization (2016) WHO guidelines for the treatment of Neisseria gonorrhoeae.
[6] Ohnishi, M. et al. (2011) Is Neisseria gonorrhoeae initiating a future era of untreatable gonorrhea?: detailed characterization of the first strain with high-level resistance to ceftriaxone. Antimicrob. Agents Chemother. 55, 3538–3545.
| Is Neisseria gonorrhoeae initiating a future era of untreatable gonorrhea?: detailed characterization of the first strain with high-level resistance to ceftriaxone.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXos1Ortbc%3D&md5=f15a57a952925d46f8688714eb03ac4fCAS |
[7] Unemo, M. et al. (2012) High-level cefixime- and ceftriaxone-resistant Neisseria gonorrhoeae in France: novel penA mosaic allele in a successful international clone causes treatment failure. Antimicrob. Agents Chemother. 56, 1273–1280.
| High-level cefixime- and ceftriaxone-resistant Neisseria gonorrhoeae in France: novel penA mosaic allele in a successful international clone causes treatment failure.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XjsVKlsL8%3D&md5=6d09e3fa70803c4c4278ab5e3d663e1dCAS |
[8] Lahra, M.M. et al. (2014) A new multidrug-resistant strain of Neisseria gonorrhoeae in Australia. N. Engl. J. Med. 371, 1850–1851.
| A new multidrug-resistant strain of Neisseria gonorrhoeae in Australia.Crossref | GoogleScholarGoogle Scholar |
[9] Ison, C.A. et al. (2013) Current and future treatment options for gonorrhoea. Sex. Transm. Infect. 89, iv52–iv56.
| Current and future treatment options for gonorrhoea.Crossref | GoogleScholarGoogle Scholar |
[10] World Health Organization (2014) Antimicrobial resistance: global report on surveillance. Geneva, Switzerland.
[11] Tapsall, J.W. et al. (1984) Penicillin sensitivity of gonococci in Australia: development of Australian Gonococcal Surveillance Programme. Members of the Australian Gonococcal Surveillance Programme. Br. J. Vener. Dis. 60, 226–230.
[12] Tapsall, J.W. (1990) Use of a quality assurance scheme in a long-term multicentric study of antibiotic susceptibility of Neisseria gonorrhoeae. Genitourin. Med. 66, 8–13.
| 1:STN:280:DyaK3c7osFKrtQ%3D%3D&md5=55e108cef64b65dd8878e6fd9cecb528CAS |
[13] Australian Gonococcal Surveillance Programme (1981) Gonococcal surveillance – Australia (July–September 1981). Commun. Dis. Intell. 25, 2–3.
[14] The Kirby Institute (2016) HIV, viral hepatitis and sexually transmissible infections in Australia Annual Surveillance Report 2016. https://kirby.unsw.edu.au/sites/default/files/kirby/report/SERP_Annual-Surveillance-Report-2016_UPD170627.pdf
[15] Speers, D.J. et al. (2014) Non-culture Neisseria gonorrhoeae molecular penicillinase production surveillance demonstrates the long-term success of empirical dual therapy and informs gonorrhoea management guidelines in a highly endemic setting. J. Antimicrob. Chemother. 69, 1243–1247.
| Non-culture Neisseria gonorrhoeae molecular penicillinase production surveillance demonstrates the long-term success of empirical dual therapy and informs gonorrhoea management guidelines in a highly endemic setting.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXls1OgtLs%3D&md5=eefab7fdc5fe0271cec2ace7e41aa137CAS |
[16] Lahra, M.M. (2015) Australian Gonococcal Surveillance Programme. Australian Gonococcal Surveillance Programme annual report, 2013. Commun. Dis. Intell. Q. Rep. 39, E137–E145.
[17] Lahra, M.M. and Enriquez, R.P. () Australian Gonococcal Surveillance Programme. Annual report 2016. Commun. Dis. Intell., , .
[18] Lahra, M.M. and Enriquez, R.P. () Australian Gonococcal Surveillance Programme. Gonococcal surveillance – Australia (April–July 2017). Commun. Dis. Intell., , .
[19] Goire, N. et al. (2011) Enhancing gonococcal antimicrobial resistance surveillance: a real-time PCR assay for detection of penicillinase-producing Neisseria gonorrhoeae by use of noncultured clinical samples. J. Clin. Microbiol. 49, 513–518.
| Enhancing gonococcal antimicrobial resistance surveillance: a real-time PCR assay for detection of penicillinase-producing Neisseria gonorrhoeae by use of noncultured clinical samples.Crossref | GoogleScholarGoogle Scholar |
[20] Buckley, C. et al. (2016) A real-time PCR assay for direct characterization of the Neisseria gonorrhoeae GyrA 91 locus associated with ciprofloxacin susceptibility. J. Antimicrob. Chemother. 71, 353–356.
| A real-time PCR assay for direct characterization of the Neisseria gonorrhoeae GyrA 91 locus associated with ciprofloxacin susceptibility.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC28XjslWjtL4%3D&md5=a1cb1a6e9cba10e565c27e5124ed7900CAS |
[21] Trembizki, E. et al. (2015) Direct real-time PCR-based detection of Neisseria gonorrhoeae 23S rRNA mutations associated with azithromycin resistance. J. Antimicrob. Chemother. 70, 3244–3249.
| 1:CAS:528:DC%2BC2MXitVyku77J&md5=39bb6bfecdb3b90f812d55be6ebeb592CAS |
[22] Whiley, D.M. et al. (2017) Molecular antimicrobial resistance surveillance for Neisseria gonorrhoeae, Northern Territory, Australia. Emerg. Infect. Dis. 23, 1478–1485.
| Molecular antimicrobial resistance surveillance for Neisseria gonorrhoeae, Northern Territory, Australia.Crossref | GoogleScholarGoogle Scholar |
[23] Trembizki, E. et al. (2016) The molecular epidemiology and antimicrobial resistance of Neisseria gonorrhoeae in Australia: a nationwide cross-sectional study, 2012. Clin. Infect. Dis. 63, 1591–1598.
| The molecular epidemiology and antimicrobial resistance of Neisseria gonorrhoeae in Australia: a nationwide cross-sectional study, 2012.Crossref | GoogleScholarGoogle Scholar |
Biographies
Professor Monica Lahra is the Medical Director, Division of Bacteriology, and Director of the World Health Organization Collaborating Centre for STD, Sydney, based in the Department of Microbiology, New South Wales Health Pathology at The Prince of Wales Hospital in Sydney. Prof Lahra is in charge of diagnostic bacteriology, and the operations and programmes of the WHO CC. This role includes the responsibility for national and international AMR surveillance networks. Prof Lahra is the author of more than 100 papers in peer-reviewed journals, and has participated in the preparation of technical documents on antimicrobial resistance surveillance and treatment guidelines in the role of a Temporary Advisor for the World Health Organization.
Dr Robert George is a Microbiology Registrar based at the Institute of Clinical Pathology and Medical Research, NSW Health Pathology. He has worked with the WHO Collaborating Centre for Sexually Transmitted Diseases, and previously completed a Doctor of Philosophy at the University of Queensland where he worked on spatial modelling.
Associate Professor David Whiley is a principal research fellow at the Faculty of Medicine, UQ Centre for Clinical Research, The University of Queensland and research scientist at Pathology Queensland. Much of his work is aimed at enhancing the capacity of clinical laboratories to diagnose, identify and characterise pathogens, with a particular research emphasis on sexually transmitted infections and antimicrobial resistance. He has authored 148 articles to date and currently holds an NHMRC Career development Fellowship level 2. In recent years A/Prof Whiley has been leading the NHMRC-funded Gonorrhoea Resistance Assessment via Nucleic Acid Detection (GRAND) studies that aim to enhance gonorrhoea antimicrobial resistance surveillance and treatment strategies, particularly in remote settings.