The role of microbiology in gonococcal control in the West: helping to understand the enemy
David J SpeersDepartment of Microbiology
PathWest Laboratory Medicine WA
Queen Elizabeth II Medical Centre
Hospital Avenue
Nedlands, WA 6009, Australia
Tel: +61 8 9346 2197
Fax: 08 9346 3960
Email: david.speers@health.wa.gov.au
Microbiology Australia 38(4) 171-174 https://doi.org/10.1071/MA17061
Published: 31 October 2017
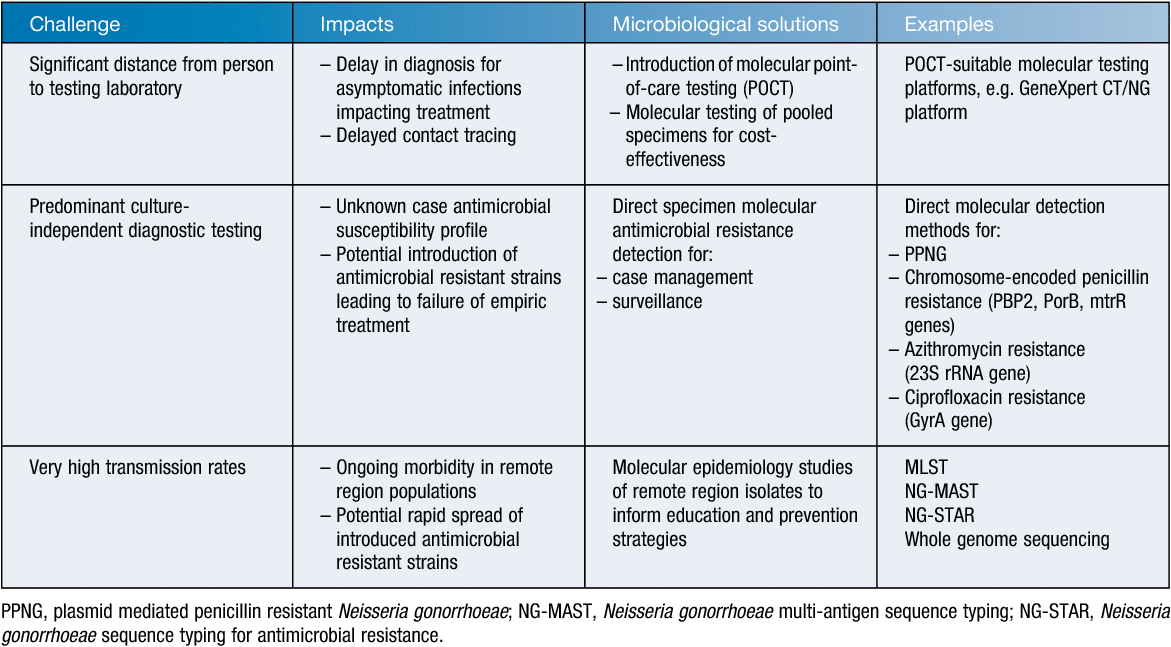
Western Australia (WA), Australia’s largest state by area, has one of the highest notification rates of gonorrhoea in the world. This is likely a reflection of the challenges of providing health services over a vast remote area combined with a unique set of sociocultural aspects. Despite this, microbiology can play a pivotal role in the public health management of gonorrhoea even if the primary health services are thousands of kilometres away from the laboratory. However, it requires new approaches to how diagnostic testing and laboratory surveillance are conducted and the repurposing of existing technologies to cater for novel demands. In this article I describe some of the microbiological approaches that have been undertaken in WA to help address the public health challenge of gonorrhoea. That is, facilitating the appropriate antimicrobial management of gonorrhoea in an era of increasing resistance to prevent treatment failure, timely provision of an accurate diagnosis to inform appropriate treatment, and providing molecular insights to better understand gonococcal transmission (Table 1).
|
Mitigating antimicrobial resistance
Neisseria gonorrhoeae has shown a remarkable capacity to become resistant to the antimicrobial agents employed to control it. With an estimated 78 million new cases each year and the emergence of ceftriaxone and azithromycin resistance in many countries around the world1, including Australia2,3, N. gonorrhoeae has earned its World Health Organization and Centers for Disease Control and Prevention designation as an urgent antimicrobial resistance threat. It is critical to delay the introduction of multiresistant N. gonorrhoeae strains into remote WA, with its high rate of gonorrhoea and more limited access to healthcare. Australia, like most of the world, has transitioned to a combination of ceftriaxone and azithromycin4,5 for empiric gonorrhoea therapy but the emerging resistance to extended spectrum cephalosporins6 and the increasing prevalence of low level azithromycin resistance in Australia3 is a reminder that resistance is an inevitable consequence of widespread use of these agents. The incursion of ceftriaxone- and/or azithromycin-resistant gonococci into remote WA communities would create an enormous public health challenge.
There are few places in the world where penicillins remain the first-line treatment for gonorrhoea, but remote WA is one such place. Despite gonorrhoea being highly endemic in the remote Kimberley, Pilbara, Midwest and Goldfields regions of WA, oral combinations of amoxicillin, probenecid and azithromycin have been used for many years for the syndromic management of gonorrhoea, based on the relatively high co-occurrence of N. gonorrhoeae and Chlamydia trachomatis infection and the relative susceptibility of N. gonorrhoeae to penicillin and azithromycin7. This has avoided the logistic and cultural issues of administering intramuscular injections in less accessible regions. As long as the combination azithromycin, amoxicillin and probenecid remains effective for locally acquired uncomplicated gonorrhoea in these remote regions its use will limit the exposure of remote WA region N. gonorrhoeae strains to third-generation cephalosporins, potentially delaying the establishment of resistance. To ensure this relative susceptibility to penicillins remains the case ongoing surveillance for antimicrobial resistance is essential, but these efforts are hampered by the low culture confirmation rates of gonorrhoea notifications from remote region WA, recorded at 14% in 20168. Therefore, molecular surveillance for antimicrobial resistance in these regions would be extremely helpful.
Penicillin resistance (MIC ≥ 1 mg/L) in the remote WA regions is almost all due to penicillinase producing N. gonorrhoeae (PPNG), which harbours a 7426 bp multicopy TEM plasmid9. In 2016 no isolate from the remote WA regions exhibited chromosomally mediated penicillin resistance8. This provides a unique opportunity to monitor penicillin resistance in gonococci from remote WA regions by using real-time PCR to detect this plasmid on N. gonorrhoeae positive nucleic acid extracts, thereby not requiring viable cultures. This surveillance has been performed on all N. gonorrhoeae positive nucleic acid extracts at the PathWest Laboratory Medicine WA Queen Elizabeth II Laboratory, which receives most of the N. gonorrhoeae specimens from remote WA regions, since 201210.
PPNG rates remained very low and below the WHO empiric therapy standard of 5% resistance in all remote WA regions6 until 2016 when the PPNG molecular detection rate increased in the Mid West region from 4.7% of 43 specimens in 2015 to 23% of 121 specimens tested in 2016. This was not apparent from phenotypic susceptibility testing as only two of the 18 Mid West isolates in 2016 were PPNG8. On further investigation an incursion of PPNG around Geraldton, the major regional centre of the Mid West, had occurred. In response to this the regional public health unit was notified and empiric prescribing for locally acquired uncomplicated gonorrhoea was changed from ZAP (azithromycin, amoxycillin, probenecid) packs to intramuscular ceftriaxone, in combination with azithromycin. In terms of laboratory response to this incursion reporting of the PPNG result in combination with the N. gonorrhoeae PCR detection will soon commence, together with investigation into a non-culture ciprofloxacin detection method to be run in parallel with PPNG detection, to allow the safe use of these agents when appropriate. Ciprofloxacin resistance occurred in 4.5% of remote WA region isolates in 20168.
Timely provision of accurate diagnosis
Making the correct diagnosis is the cornerstone of appropriate treatment and microbiology has a central role in ensuring practical yet accurate rapid diagnostic tests for N. gonorrhoeae detection. For diseases like gonorrhoea in WA, where the nearest tertiary molecular laboratory may be over 3000 km away, this is best achieved by molecular point of care testing (POCT). The Test, Treat ANd GO (TTANGO) trial11 was a randomised controlled trial by the Kirby Institute, UNSW, with a cross-over design conducted from 2013 to 2015 to explore the benefits of molecular POCT for N. gonorrhoeae and C. trachomatis as part of routine clinical care in 12 remote Australian Aboriginal primary health services, the first study of its kind. Overall N. gonorrhoeae diagnostic concordance was 99.9% for the 2486 samples between the GeneXpert CT/NG® (Cepheid, Sunnyvale, CA) molecular POCT compared to reference laboratory testing12. TTANGO demonstrated that the introduction of molecular POCT for N. gonorrhoeae and C. trachomatis in the remote clinic setting requires considerable planning, attention to quality control and changes to clinical guidelines. However, the results are highly accurate with a range of benefits including more timely and targeted prescribing, more efficient contact tracing and reduced staff time spent in contact tracing. Following this pilot study TTANGO 2 commenced in 2016 as an expanded program to a wider network of remote health services (including 15 sites in WA) over a 5-year period. The overall aim of TTANGO2 is to determine whether molecular POCT combined with increased testing is a sustainable and useful strategy for N. gonorrhoeae and C. trachomatis disease control in remote communities.
One of the major drawbacks to molecular POCT in the clinic situation is affordability due to the current lack of a Medicare rebate. One option to decrease the reagent costs of molecular POCT is specimen pooling. A high prevalence cohort over 100 symptomatic men who have sex with men and contacts of those with known gonorrhoea attending an inner city sexual health clinic was chosen for a prospective comparison of the GeneXpert CT/NG® testing of pharyngeal and rectal swabs pooled with urine samples to testing of individual specimens by routine laboratory methods13. The pooled testing was found to be 100% concordant for N. gonorrhoeae detection when compared to individual specimen testing, showing this method to be potentially useful as a POCT for screening such high risk groups.
Understanding the enemy
With the recent incursion of an antimicrobial resistant N. gonorrhoeae strain into Geraldton in the Mid West region of WA it is important to understand the transmission networks locally, between remote regions, and between remote and more populated regions of WA. An understanding of the transmission pathways could also provide insights into the driving factors for the high N. gonorrhoeae transmission rates in WA. Microbiology can provide a better understanding of N. gonorrhoeae transmission through molecular epidemiological studies, thereby highlighting where interventions may have the greatest impact on transmission. To investigate the N. gonorrhoeae epidemiology in WA pulse-field gel electrophoresis (PFGE) and N. gonorrhoeae multi-antigen sequence typing (NG-MAST) was performed on 128 consecutive WA N. gonorrhoeae isolates cultured between January 2011 and December 201314. A total of 67 distinct PFGE pulsotypes were identified with 59 NG-MAST sequence types but no predominant NG-MAST or PFGE types were found, highlighting the diversity of N. gonorrhoeae strains within WA. However, when the isolates were classified based on the more conserved tbpB gene to more clearly demonstrate linkages over time the presence of four large clusters, which accounted for 67% percent of all isolates, were identified. This suggests that the majority of the isolates originated from independent, small sexual networks with an infrequent interchange between other communities and regions. In terms of the N. gonorrhoeae genomogroups, some genomogroups were present throughout the study period, some disappeared and others were introduced demonstrating how the composition of the gonococcal population in remote regions is ever changing due to, as yet, unknown selection forces. It also suggests that gonococcal epidemiology in remote region WA is quite different to the major population centres and, in some cases, geographically restricted.
To further explore the gonococcal epidemiology of remote region WA core genome phylogeny with MLST was performed on over 50 WA isolates and compared to an international N. gonorrhoeae collection15. Two distinct population groups were found among the remote region isolates that carried no chromosomal antimicrobial resistance genotypes. In contrast, most isolates from the metropolitan region of Perth belonged to population groups that were globally distributed and were frequently multi-drug resistant, suggesting these isolates had been introduced through international travel. This data is consistent with independent gonococcal transmission networks in remote WA regions curtailing the incursion of antimicrobial resistant strains and resulting in a distinct population structure of N. gonorrhoeae, which may explain the failure to establish antimicrobial resistant strains in remote WA. However, with the circulation of remote region population group isolates we may now see more widespread dissemination of the PPNG strain that appeared around Geraldton in 2016. Moreover, this information emphasises the fact that the public health measures employed to combat remote region gonococcal transmission must be culturally appropriate and relevant. Further interrogation of the sequence data is underway to identify novel markers linked to antimicrobial resistance suitable for PCR detection at the time of N. gonorrhoeae detection that may be able to rapidly identify gonorrhoea cases with and without antimicrobial resistance beyond PPNG.
In summary, microbiology has much to contribute to the public health management of gonorrhoea by identifying features of the pathogen and the host-pathogen interaction that can be exploited for the public health management of N. gonorrhoeae. An appreciation of these differences across geographic locales will help inform the measures that can be taken toward gonococcal control, tailored to a particular setting.
References
[1] World Health Organization (2017) Global Gonococcal Antimicrobial Surveillance Programme (GASP). http://www.who.int/reproductivehealth/topics/rtis/gonococcal_resistance/en/ (accessed 24 July 2017).[2] Lahra, M.M. et al. (2014) A new multidrug-resistant strain of Neisseria gonorrhoeae in Australia. N. Engl. J. Med. 371, 1850–1851.
| A new multidrug-resistant strain of Neisseria gonorrhoeae in Australia.Crossref | GoogleScholarGoogle Scholar |
[3] Australian Commission on Safety and Quality in Health Care (ACSQHC) (2016) AURA 2016: first Australian report on antimicrobial use and resistance in human health. Sydney: ACSQHC.
[4] World Health Organization (2016) Guidelines for the treatment of Neisseria gonorrhoeae. http://www.who.int/reproductivehealth/publications/rtis/gonorrhoea-treatment-guidelines/en/ (accessed 24 July 2017).
[5] Centres for Disease Control and Prevention (2015) Sexually transmitted diseases treatment guidelines. https://www.cdc.gov/std/tg2015/gonorrhea.htm (accessed 24 July 2017).
[6] Lahra, M.M., Enriquez, R. and National Neisseria Network (2017) Australian Gonococcal Surveillance Programme annual report, 2015. Commun. Dis. Intell. Q. Rep. 41, E60–E67.
[7] Government of Western Australia Public Health (2017) Guidelines for managing sexually transmitted infections—WA. http://silverbook.health.wa.gov.au/ (accessed 24 July 2017).
[8] Pearson J. (2017) The Western Australian Gonococcal Surveillance Programme 2016 Report. June.
[9] Goire, N. et al. (2011) Enhancing gonococcal antimicrobial resistance surveillance: a real-time PCR assay for detection of penicillinase-producing Neisseria gonorrhoeae by use of noncultured clinical samples. J. Clin. Microbiol. 49, 513–518.
| Enhancing gonococcal antimicrobial resistance surveillance: a real-time PCR assay for detection of penicillinase-producing Neisseria gonorrhoeae by use of noncultured clinical samples.Crossref | GoogleScholarGoogle Scholar |
[10] Speers, D.J. et al. (2014) Non-culture Neisseria gonorrhoeae molecular penicillinase production surveillance demonstrates the long-term success of empirical dual therapy and informs gonorrhoea management guidelines in a highly endemic setting. J. Antimicrob. Chemother. 69, 1243–1247.
| Non-culture Neisseria gonorrhoeae molecular penicillinase production surveillance demonstrates the long-term success of empirical dual therapy and informs gonorrhoea management guidelines in a highly endemic setting.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXls1OgtLs%3D&md5=eefab7fdc5fe0271cec2ace7e41aa137CAS |
[11] Natoli, L., Guy, R. and TTANGO Investigators (2014) Point-of-care testing for chlamydia and gonorrhoea: implications for clinical practice. PLoS One 9, e100518.
| Point-of-care testing for chlamydia and gonorrhoea: implications for clinical practice.Crossref | GoogleScholarGoogle Scholar |
[12] Causer, L. et al. (2016) Operational performance of a new molecular–based point-of-care test for diagnosis of Chlaymdia trachomatis and Neisseria gonorrhoeae infection: concordance with conventional laboratory testing. World STI and HIV Congress, 13–16 September 2016, Brisbane.
[13] Speers, D. et al. (2017) Detection of Neisseria gonorrhoeae and Chlamydia trachomatis from pooled rectal, pharyngeal and urine specimens in men who have sex with men. Sex. Transm. Infect. , .
[14] O’Reilly, L.C. et al. (2015) Molecular epidemiology of Neisseria gonorrhoeae using multi-antigen sequence typing and pulse-field gel electrophoresis in highly endemic Western Australian populations. BMC Infect. Dis. 15, 272.
| Molecular epidemiology of Neisseria gonorrhoeae using multi-antigen sequence typing and pulse-field gel electrophoresis in highly endemic Western Australian populations.Crossref | GoogleScholarGoogle Scholar |
[15] Suwayyid, B. et al. (2017) Genomic epidemiology and population structure of Neisseria gonorrhoeae from remote highly endemic heterosexual Western Australian populations. (submitted for publication). , .
Biography
Dr David Speers is an Infectious Diseases Physician at Sir Charles Gairdner Hospital, Head of Microbiology, QEII Network at PathWest Laboratory Medicine WA and a Clinical Associate Professor for the Department of Medicine and Pharmacology, University of WA. He is a fellow of the Royal Australasian College of Physicians, the Royal College of Pathologists of Australasia, and the Australasian College of Tropical Medicine.


