Menangle virus: one of the first of the novel viruses from fruit bats
Peter D KirklandElizabeth Macarthur Agriculture Institute, Woodbridge Road, Menangle, NSW, Australia. Tel: +61 2 4640 6331, Fax: +61 2 4640 6429, Email: peter.kirkland@dpi.nsw.gov.au
Microbiology Australia 38(1) 22-24 https://doi.org/10.1071/MA17007
Published: 9 February 2017
‘Brainless pig disease swoops on Sydney.’ This was a media headline that threatened to emerge during the early stages of a disease outbreak in pigs in NSW. However, identification of the viral cause and epidemiological studies that supported a sound management program minimised the impact of this outbreak on animal and human health.
Disease outbreak and pathology
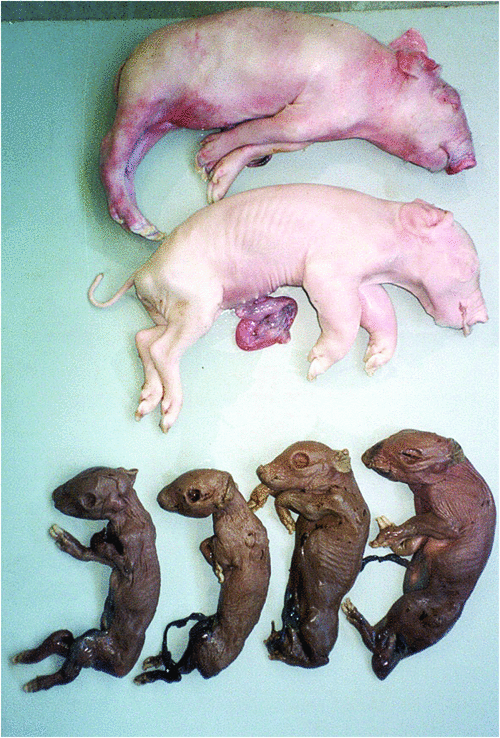
In autumn 1997, an outbreak of severe reproductive failure occurred in a 2600-sow intensive piggery near Sydney, New South Wales1. Initially there was a high incidence of stillborn piglets and the delivery of mummified foetuses. Foetal deaths, considered to probably be associated with an in-utero infection, continued for a period of five months. Towards the end of the outbreak, many stillborn piglets with a range of severe defects were delivered (Figure 1). These congenital abnormalities mainly involved the central nervous system (reduced size of cerebellum, cerebral hemispheres, brain stem and spinal cord; hydranencephaly) and the skeletal system (arthrogryposis of the limbs; craniofacial deformities; scoliosis/kyphosis)2. Histologically there was a non-suppurative multifocal meningitis, and occasionally myocarditis and hepatitis. Intranuclear and intracytoplasmic inclusion bodies were observed in neurons of the cerebrum and spinal cord. At the peak of the outbreak almost half of litters and most of the piglets in them were affected. The progression of the outbreak through different sections of the farm complex and the associated gross and microscopic pathological changes in affected piglets were consistent with a viral infection.
|
Virus discovery and characterisation
Attempts to isolate virus in a range of cell cultures was initially unsuccessful, but when tissues from piglets with severe defects were examined, a number of isolates of a novel virus were obtained3. Virus was isolated most frequently from brain, lung and heart and replicated well in BHK21 and HmLu-1 cells. Cytopathology, consisting of vacuolation of cells and large syncitia formation, was not usually observed until three passages in cell culture. A novel paramyxovirus, later named Menangle virus (MenV), was identified by electron microscopy. Subsequent molecular characterisation4 confirmed that this was a novel virus and its genome most closely aligned with those of viruses in the Rubulavirus genus, which includes human parainfluenza viruses 2 and 4 and mumps virus.
Diagnosis and epidemiology
Isolation of the causative viral agent allowed the development of a virus neutralisation test. Archival sera gave negative results, demonstrating that the virus had recently entered the population5. In contrast, positive results were obtained for serum from sows giving birth to infected piglets. Piglets born during the early stages of the outbreak, and presumptively infected late in gestation when they are able to produce an immune response, also had positive serological results, which may explain the initial failure to isolate MenV. Using a virus neutralisation test to test collections of archived sera, it was possible to monitor the spread of this virus through different sections of the farming complex and also the spread at two associated farms that had only growing pigs. Epidemiological studies demonstrated that MenV spread through the population relatively slowly and that young pigs became infected from about 12–16 weeks of age but were consistently immune by the time that they had reached breeding age, resulting in a natural cessation of the disease. The patterns and rate of spread of MenV infection among pigs suggested that transmission was more likely to be oro-nasal from faecal material or urine rather than via aerosols from the respiratory tract.
Public health and anxious weeks ahead
As a part of the epidemiological studies, serum samples were collected from all workers on the affected farms, regular visitors, including medical researchers involved in xenotransplantation studies and abattoir employees. Two seropositive male farm workers were identified6. Follow-up medical examinations revealed that both had experienced a severe febrile illness lasting for 10–14 days, with severe headaches and myalgia. Although one person lost a considerable amount of body weight, both eventually returned to normal health. During sampling of staff, a young female worker confided that she had recently become pregnant but had not advised anyone. Although seronegative, she remained on stress-related leave until after the birth of a normal child.
Origin of the virus
The close proximity of a large colony of grey-headed flying foxes (Pteropus poliocephalus) to the piggery and the recent discovery of Hendra virus in Australia raised suspicion that flying foxes could be a source of MenV. Serological evidence of MenV infection was detected in samples from four different species of flying fox at various locations in Australia, including samples taken from two P. poliocephalus colonies in the vicinity of the affected piggery3,5. More recently, MenV has been isolated from the urine of flying foxes7 while 6.5% of 5000 urine samples collected from flying boxes at locations along the coast of NSW between 2012 and 2015 gave positive qRT-PCR results (K Wernike and PD Kirkland, unpubl. data). Collectively these data suggest that Menangle virus is endemic in Australian flying fox populations while there is serological evidence to suggest that MenV is also present in Papua New Guinea8.
Eradication of the virus from pigs
Epidemiological studies5 had established that there were high levels of immunity in adult animals and the age range during which seroconversion occurred was defined. Using this information, a management program was developed that led to the successful eradication of MenV from the piggery. This involved cleaning and disinfection of pig sheds, and then re-populating with pigs of a single age, usually from the time of weaning when they still had high maternal antibody titres that were expected to provide additional protection from infection. To eliminate in utero infection, before becoming pregnant, sows known to be immune were placed in sheds that had been cleaned and disinfected. Not only did their immunity prevent new infections but their progeny also had passively acquired immunity. Collectively these measures provided additional time for any residual virus to become inactivated in the environment and also a long time interval before young pigs had lost protective antibody and become susceptible to infection. Successful eradication was confirmed by demonstrating that subsequent generations of pigs remained seronegative after losing maternally derived antibodies.
Where is Menangle virus today?
To the best of our knowledge, there are no terrestrial animals currently infected with Menangle virus, but it is still circulating in the fruit bat population. The emergence of other zoonotic viruses such as the Hendra-related Nipah virus from Pteropus spp in SE Asia, has provided additional incentive to minimise opportunities for contact of terrestrial animals with environments contaminated by excreta from flying foxes. Had there not been rapid control of spread through the implementation of farm quarantine and control measures, MenV could have spread throughout the Australian pig population and, with amplification of the virus in pigs, posed an ongoing human occupational health hazard.
References
[1] Love, R.J. et al. (2001) Reproductive disease and congenital malformations caused by Menangle virus in pigs. Aust. Vet. J. 79, 192–198.| Reproductive disease and congenital malformations caused by Menangle virus in pigs.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD3M3kvVSguw%3D%3D&md5=bc687ad21b8c73e1bf464924feebf6fbCAS |
[2] Philbey, A.W. et al. (2007) Skeletal and neurological malformations in pigs congenitally infected with Menangle virus. Aust. Vet. J. 85, 134–140.
| Skeletal and neurological malformations in pigs congenitally infected with Menangle virus.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD2s7pslSmsQ%3D%3D&md5=fdadbd9d3ba9973b9a0242baa57b8a90CAS |
[3] Philbey, A.W. et al. (1998) An apparently new virus (family Paramyxoviridae) infectious for pigs, humans, and fruit bats. Emerg. Infect. Dis. 4, 269–271.
| An apparently new virus (family Paramyxoviridae) infectious for pigs, humans, and fruit bats.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK1c3osVaitQ%3D%3D&md5=e24100b0447a9b5f3be0694147647abdCAS |
[4] Bowden, T.R. et al. (2001) Molecular characterization of Menangle virus, a novel paramyxovirus which infects pigs, fruit bats, and humans. Virology 283, 358–373.
| Molecular characterization of Menangle virus, a novel paramyxovirus which infects pigs, fruit bats, and humans.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXjt1Wgtb0%3D&md5=c3821c213aff6645381626a8a05ad231CAS |
[5] Kirkland, P.D. et al. (2001) Epidemiology and control of Menangle virus in pigs. Aust. Vet. J. 79, 199–206.
| Epidemiology and control of Menangle virus in pigs.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD3M3kvVSnsg%3D%3D&md5=cf86dc1f4bfbce3e98fe788492cb6347CAS |
[6] Chant, K. et al. (1998) Probable human infection with a newly described virus in the family Paramyxoviridae. Emerg. Infect. Dis. 4, 273–275.
| Probable human infection with a newly described virus in the family Paramyxoviridae.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK1c3osVaiug%3D%3D&md5=8e85c4ff40f4575e22346607eaa38670CAS |
[7] Barr, J.A. et al. (2012) Evidence of bat origin for Menangle virus, a zoonotic paramyxovirus first isolated from diseased pigs. J. Gen. Virol. 93, 2590–2594.
| Evidence of bat origin for Menangle virus, a zoonotic paramyxovirus first isolated from diseased pigs.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXjvVWjug%3D%3D&md5=17be8d66b47e8f8f311e624fc9cca274CAS |
[8] Breed, A.C. et al. (2010) Prevalence of Henipavirus and Rubulavirus antibodies in Pteropid bats, Papua New Guinea. Emerg. Infect. Dis. 16, 1997–1999.
| Prevalence of Henipavirus and Rubulavirus antibodies in Pteropid bats, Papua New Guinea.Crossref | GoogleScholarGoogle Scholar |
Biography
Dr Peter Kirkland is the Head of the Virology Laboratory at the state government Elizabeth Macarthur Agriculture Institute at Menangle NSW. Dr Kirkland has had a long career in diagnostic and research projects in animal health. He has been instrumental in the identification of several new viruses, including Menangle virus that was transmitted from flying foxes to pigs, a novel pestivirus that was responsible for a major disease outbreak in pigs and viruses that have caused blindness and sudden deaths in macropods. In 2007 he led the EMAI team during the diagnosis and response to the equine influenza outbreak and in 2011 the investigation of the large West Nile virus outbreak in horses in NSW. His research interests include vector borne viruses and the development and evaluation of rapid diagnostic assays for viral diseases of animals.


