The impact of novel lyssavirus discovery
Ashley C Banyard A C and Anthony R Fooks A B DA Wildlife Zoonoses and Vector-borne Diseases Research Group, Animal and Plant Health Agency (APHA), Weybridge, UK
B Institute for Infection and Immunity, St George’s Hospital Medical School, University of London, London, UK
C Tel: +44 (0) 20 8026 9463 Email: ashley.banyard@apha.gsi.gov.uk
D Tel: +44 (0) 20 8415 2238 Email: tony.fooks@apha.gsi.gov.uk
Microbiology Australia 38(1) 17-21 https://doi.org/10.1071/MA17006
Published: 9 February 2017
The global discovery of novel lyssaviruses is of continued scientific interest through its importance to both public and animal health. Lyssaviruses cause an invariably fatal encephalitis that is more commonly known as rabies. The term rabies has a long history in human society, as rabies virus (RABV) is the only pathogen that is associated with 100% fatality once the onset of clinical disease has started. Although predominantly associated across the globe with domestic and feral dog populations, the association of bats is clear. Whilst evolutionarily associated with bats, RABV is most commonly transmitted to human populations through the bite of an infected dog and dogs are considered the primary reservoir of disease. Indeed, RABV does cause more than an estimated 70 000 deaths every year globally in human populations and whilst this is largely in areas where the disease is endemic, areas that remain free of rabies must remain vigilant to the risk of re-incursion of disease. Characterisation of novel lyssaviruses is of importance on several levels. Not least to investigate the pathogenesis and potential transmission routes of different lyssavirus species but also to assess the potential effect of post-exposure treatments and vaccination should human exposure occur. Bat lyssaviruses and the problems associated with novel discoveries and the potential impact they have on both human and animal populations are discussed.
The global discovery of lyssaviruses is of continued scientific interest through its importance to both public and animal health. Lyssaviruses cause an invariably fatal encephalitis referred to as rabies. The term rabies has a long history in human society, as rabies virus (RABV) is the only pathogen that is associated with 100% fatality once the onset of clinical disease has started1. Whilst predominantly associated across the globe with domestic and feral dog populations, the association of bats with lyssaviruses is clear. Historically, rabies has a long association with hematophagous bats across Central and South America and this association, with ‘mysterious blood feeding creatures of the night’, has cemented rabies into the conscience of human populations. Alongside this the more typical association of the virus with aggressive dogs, the horrific clinical disease seen in human infection, and the invariably fatal nature of infection, has led to rabies being the most feared pathogen known to man. Such clinical manifestations have held the imagination of humanity since the earliest reports of canine and human madness. Whilst evolutionarily associated with bats, rabies virus is most commonly transmitted to human populations through the bite of an infected dog. Indeed, RABV does cause thousands of deaths every year globally in human populations and whilst this is largely in areas where the virus is endemic, areas that remain free of rabies must remain vigilant to the risk of re-incursion of disease2. Alongside the burden seen from dogs, wildlife species also play an important role in the epidemiology of disease although the paucity of data on wild animal populations, their distribution and the generally sporadic interactions between different wildlife populations and domesticated carnivore species means that the role of wildlife and the epidemiology of the virus is often unclear.
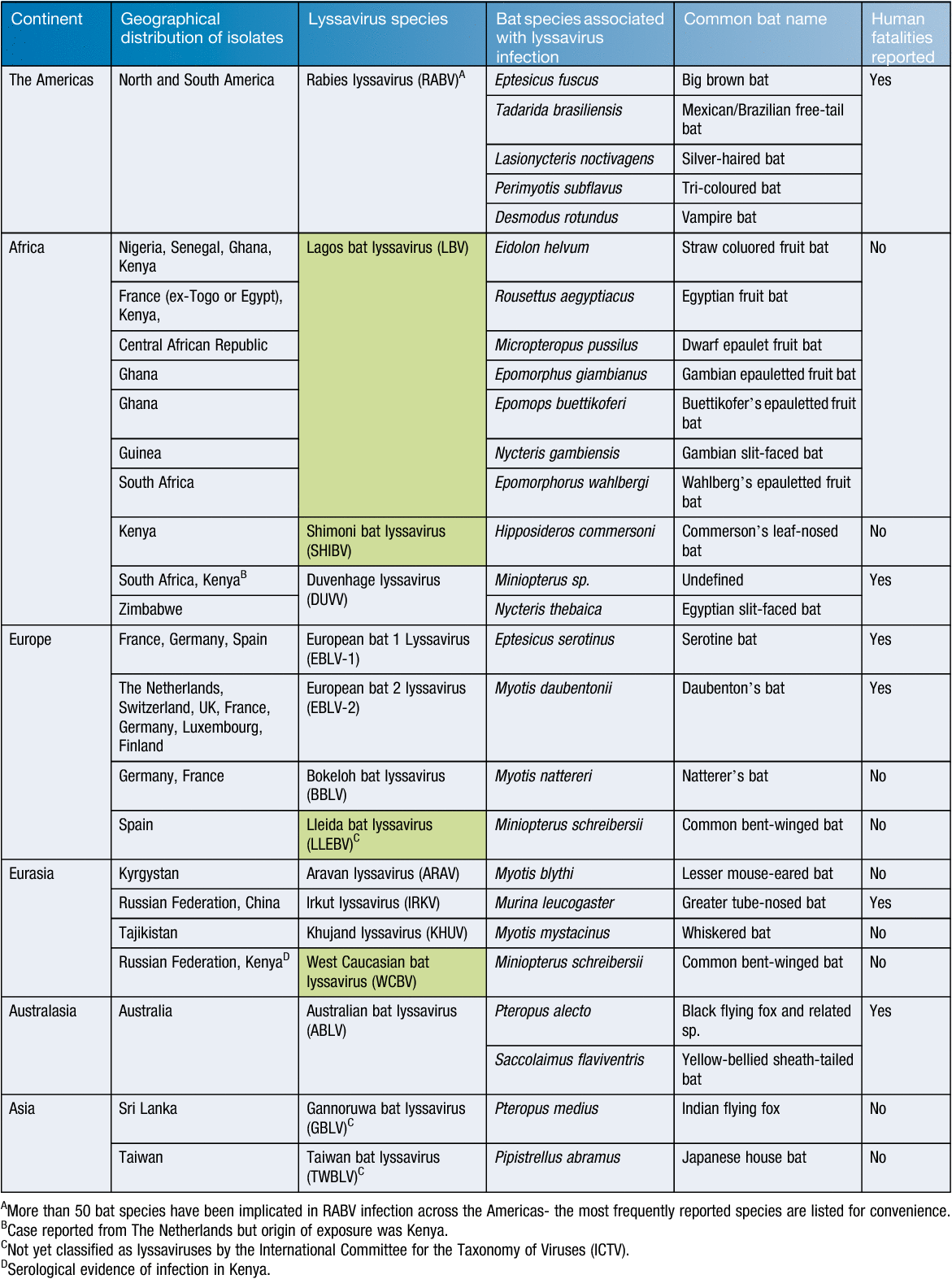
Alongside RABV strains that are predominantly associated with terrestrial carnivore species, a number of other genetically and to some extent antigenically related viruses exist within the lyssavirus genus3. Currently, 14 viruses, in addition to RABV are associated with bats and are classified within the genus4, while few are well characterised from either an epidemiological or clinical disease perspective. Three bat-associated viruses, Lleida bat lyssavirus, Gannoruwa bat lyssavirus and Taiwan bat lyssavirus, are not officially classified within the Lyssavirus genus, and remain as tentative species only. Although RABV causes a significant annual disease burden to human populations globally, the association of these other lyssaviruses with human fatalities is poorly characterised (Table 1). This may be because there are genuinely fewer cases of infection with these viruses across human and animal populations or, conversely may reflect the inability of the most commonly used diagnostic tool, the antigen detection based Fluorescent Antibody Test (FAT), to differentiate between lyssavirus species and a lack of a secondary confirmatory test in endemic regions that can genetically type virus5. This feature is important and as more divergent lyssaviruses are discovered the ability of commercial conjugates to detect them requires assessment. These factors mean that the true epidemiological situation regarding these lyssaviruses remains unclear and consequently the burden of disease to animal and human health remains undefined6.
Divergent lyssaviruses have been recognised for over 65 years. Initial discoveries of viruses causing rabies disease originated in the Old World in the 1950s where serological profiling using monoclonal antibodies characterised viruses similar to RABV7. A steady flow of virus discovery then continued until molecular methods were developed that superseded antibody based classification of new pathogens. Currently, sequencing technologies are able to rapidly type viruses genetically and as such lyssaviruses continue to be discovered8,9. Apart from those already detected and partially characterised, novel species continue to be detected that then require characterisation and classification. The importance of novel lyssaviruses remains unknown but the potential for fatal infection following spill over events dictates that some importance must be placed on their virological characterisation. This has become increasingly important with the heightened interest in bats as biological entities and as reservoirs of zoonotic pathogens that have crossed species barriers to drive large mortality events in human and animal populations. Furthermore, the potential for interactions with bat species has increased with the encroachment of human populations into areas of forestation and the popularity of human activities such as caving and potholing10.
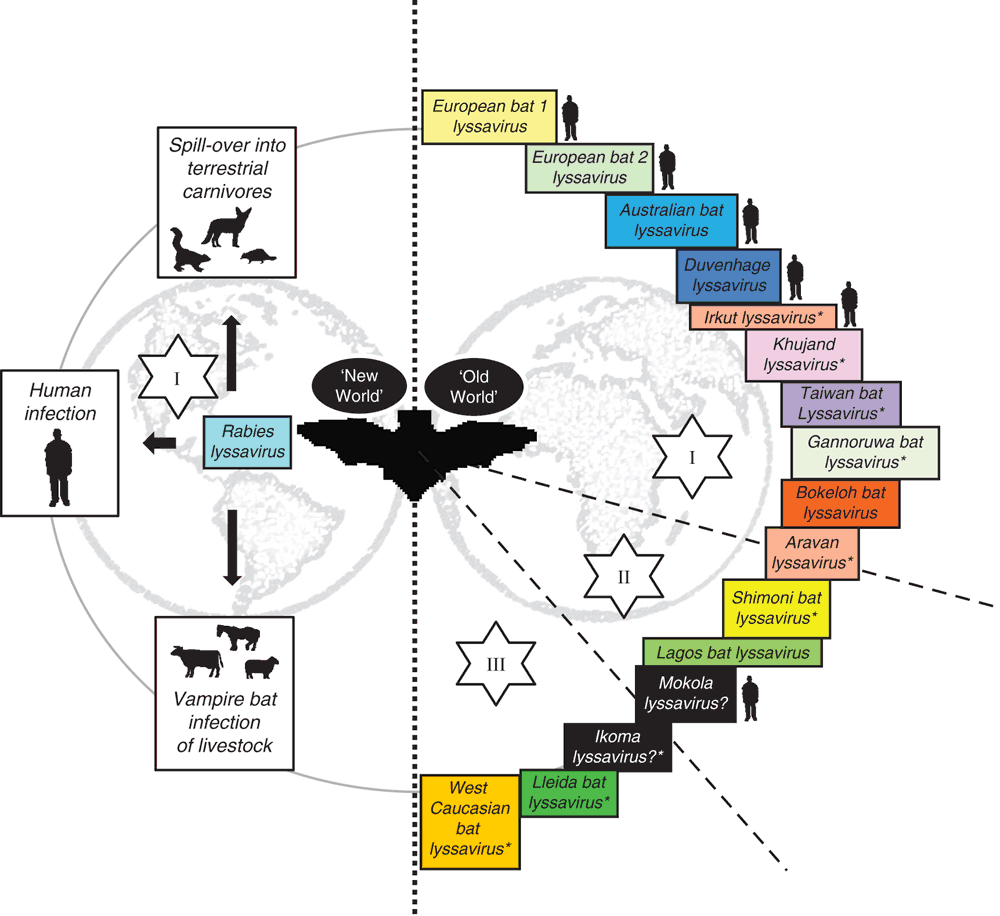
The association of lyssaviruses with bats provides several interesting conundrums regarding the epidemiology of these viruses11. RABV is present across terrestrial carnivore populations across the globe but is only associated with the infection of insectivorous, hematophagous and to a lesser extent frugivorous bats across the Americas. In the Americas, following the successful elimination of rabies from domestic carnivore species and, following extensive oral vaccination programs, reduction of disease within wildlife populations, bats represent an ever present remaining source of virus for potential infection and spill over transmission events that continue to cause fatalities in the human populations. Indeed, recent reports have highlighted the importance of seeking post-exposure prophylaxis wherever interaction with a potentially rabid bat has occurred, even in the absence of any actual contact between the animal and the human individual in question12. Interestingly however, the detection of classical RABV has never been reported across the ‘Old World’ in bats13. In contrast, the other viruses that are classified within the lyssavirus genus have, in the main, been isolated from different bat species across the Old World with a complete lack of reporting of any lyssavirus species, apart from RABV, in the Americas (Figure 1). The basis for this apparent division remains unknown. It is probable that RABV has existed in blood-sucking and insectivorous bats in the Americas for millennia. With the introduction of European dog rabies and large agricultural hosts (cattle, horses etc.) following the European colonisation of the Americas, the incidence of RABV in these new hosts likely increased exponentially. Further, increased agricultural prey species likely impacted significantly to the increase in vampire bat populations.
A key factor that defines the relationship between viruses and their hosts is the ability to be transmitted and maintained within a population. The maintenance of lyssaviruses within bat populations is poorly understood. Whilst bats can be clearly identified as reservoir hosts for lyssaviruses, where associations have been defined, they do not fulfil the classic ideal of a symbiotic relationship between microbe and host. In this sense, the ability of viruses to cause clinical disease is of interest. For numerous other pathogens for which bats are considered reservoirs, including high profile viral zoonotic pathogens such as Ebola virus and Nipah virus, infection generally occurs in the absence of clinical disease in the bat. For lyssaviruses, although our understanding may be limited, this feature of virus host interactions is not true. Indeed, lyssaviruses do cause clinical disease in the bats they infect and in the vast majority of cases it is only through the observation of clinical manifestations of disease that these viruses are discovered. This is a feature not restricted to bat infection, but one that covers lyssavirus infection of all mammals. However, recent evidence has suggested that mammals can be exposed to virus, mount an immunologically detectable response (generally a humoral response) and clear the virus. Both in bat populations where serological positivity can range significantly across a roost and human populations, evidence of exposure in the absence of clinical disease has been reported. What however constitutes exposure against an interaction that leads to productive infection and disease is ill defined, as are the mechanisms that dictate the outcome of any potential exposure/infection event. Assessment of innate signalling mechanisms in bats, and other species, following exposure needs to be completed to understand the mechanisms that drive infection.
Another key question when considering bat lyssavirus infection is the potential for cross species transmission events (CSTs)14. Whilst few CSTs have been reported where the bat variant has been maintained in terrestrial hosts15,16, the potential for such events remains. Certainly across the Old World, where non-rabies lyssaviruses appear to predominate in bat populations whilst RABV appears to circulate terrestrially, such events may occur more often but without any evidence for viral adaptation and transmission. Current diagnostic methods to genetically type rabies virus at post mortem cannot differentiate between lyssaviruses and as such CST events may be missed. Certainly, without the adoption of molecular tools to genetically type circulating virus variants such events will remain undefined.
A final element of importance when considering the detection of novel lyssaviruses, and their effect on human and animal populations, is the role of neutralising antibodies. Whilst nearly 100% fatal following the development of clinical disease, it has long been established that a neutralising antibody titre over a defined threshold will protect individuals from the development of disease17. However, the cut-off for a serological neutralising antibody titre is poorly defined for numerous viruses within the continually expanding lyssavirus genus and as such the discovery of novel viruses requires investigation as to the efficacy of existing pre- and post-exposure preparations6,18–23. Of the more divergent viruses, there appears to be no vaccine afforded protection whilst the less divergent may be neutralised by a vaccinal response5,6,24,25.
The relative impact of these viruses on human populations is low, as evidenced by the low number of human fatalities associated with these viruses in countries that are free of terrestrial rabies where diagnostic capabilities are able to thoroughly investigate cases of encephalitis. Maintenance of such rabies free areas is important and the OIE definition of what constitutes freedom from rabies disease is clear and valid. Numerous island nations, including the United Kingdom and Australia, are defined by the OIE as ‘rabies free’, because rabies virus is absent in terrestrial mammals. However, both serological and virological evidence for lyssaviruses continues to increase across bat populations. As such, an increased knowledge of the mechanisms behind how these viruses persist, and how they cross the species barrier will be critical in ensuring that risk assessments surrounding human and animal health are optimised.
Acknowledgements
ACB and ARF were jointly supported by the UK Department for Environment, Food and Rural Affairs (Defra), Scottish Government and Welsh Government under project grant SV3500.
References
[1] Fooks, A.R. et al. (2014) Current status of rabies and prospects for elimination. Lancet 384, 1389–1399.| Current status of rabies and prospects for elimination.Crossref | GoogleScholarGoogle Scholar |
[2] Banyard, A.C. et al. (2010) Reassessing the risk from rabies: a continuing threat to the UK? Virus Res. 152, 79–84.
| Reassessing the risk from rabies: a continuing threat to the UK?Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXps1ejs7c%3D&md5=db000ec02b2f9e6ade666f46fad233d6CAS |
[3] Baer, G.M. (1994) Rabies--an historical perspective. Infect. Agents Dis. 3, 168–180.
| 1:STN:280:DyaK2M7ivVGmsQ%3D%3D&md5=3cf2422d8bcee6b2243dcb355e3b4000CAS |
[4] Afonso, C.L. et al. (2016) Taxonomy of the order Mononegavirales: update 2016. Arch. Virol. 161, 2351–2360.
| Taxonomy of the order Mononegavirales: update 2016.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC28XosVSqu7Y%3D&md5=66b6fb67fe18f6caa0c79e740f8feea9CAS |
[5] Fooks, A. (2004) The challenge of new and emerging lyssaviruses. Expert Rev. Vaccines 3, 333–336.
| The challenge of new and emerging lyssaviruses.Crossref | GoogleScholarGoogle Scholar |
[6] Evans, J.S. et al. (2012) Rabies virus vaccines: is there a need for a pan-lyssavirus vaccine? Vaccine 30, 7447–7454.
| Rabies virus vaccines: is there a need for a pan-lyssavirus vaccine?Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38Xhs1Gkt7%2FF&md5=ab3eee15a922417054ac4531a200e7cdCAS |
[7] King, A.A. et al. (2004) Lyssavirus infections in European Bats. In: King, A.A., et al., eds. Historical perspective of Rabies in Europe and the Mediterranean basin. Paris, France: OIE. pp. 221–241.
[8] Aréchiga Ceballos, N. et al. (2013) Novel lyssavirus in bat, Spain. Emerg. Infect. Dis. 19, 793–795.
| Novel lyssavirus in bat, Spain.Crossref | GoogleScholarGoogle Scholar |
[9] Gunawardena, P.S. et al. (2016) Lyssavirus in Indian Flying Foxes, Sri Lanka. Emerg. Infect. Dis. 22, 1456–1459.
| Lyssavirus in Indian Flying Foxes, Sri Lanka.Crossref | GoogleScholarGoogle Scholar |
[10] Engering, A. et al. (2013) Pathogen-host-environment interplay and disease emergence. Emerg. Microbes Infect. 2, e5.
| Pathogen-host-environment interplay and disease emergence.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXit1Ghtr8%3D&md5=4c6d3a9568c9a1ea266e741c0f25a886CAS |
[11] Badrane, H. and Tordo, N. (2001) Host switching in Lyssavirus history from the Chiroptera to the Carnivora orders. J. Virol. 75, 8096–8104.
| Host switching in Lyssavirus history from the Chiroptera to the Carnivora orders.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXmtFejtbs%3D&md5=c34bec0c7844bb92025a330938d1e607CAS |
[12] Dato, V.M. et al. (2016) A systematic review of human bat rabies virus variant cases: evaluating unprotected physical contact with claws and teeth in support of accurate risk assessments. PLoS One 11, e0159443.
| A systematic review of human bat rabies virus variant cases: evaluating unprotected physical contact with claws and teeth in support of accurate risk assessments.Crossref | GoogleScholarGoogle Scholar |
[13] Rupprecht, C.E. et al. (2008) Can rabies be eradicated? Dev. Biol. (Basel) 131, 95–121.
| 1:STN:280:DC%2BD1cvlvVKiug%3D%3D&md5=3e63bc8276683c57140a73d4c2ee8a1bCAS |
[14] Streicker, D.G. et al. (2010) Host phylogeny constrains cross-species emergence and establishment of rabies virus in bats. Science 329, 676–679.
| Host phylogeny constrains cross-species emergence and establishment of rabies virus in bats.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXps1ehtLc%3D&md5=18f5698c3b4aea2d7c9af523e5d0a5f3CAS |
[15] Leslie, M.J. et al. (2006) Bat-associated rabies virus in skunks. Emerg. Infect. Dis. 12, 1274–1277.
| Bat-associated rabies virus in skunks.Crossref | GoogleScholarGoogle Scholar |
[16] Daoust, P.Y. et al. (1996) Cluster of rabies cases of probable bat origin among red foxes in Prince Edward Island, Canada. J. Wildl. Dis. 32, 403–406.
| Cluster of rabies cases of probable bat origin among red foxes in Prince Edward Island, Canada.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK283pvVKjug%3D%3D&md5=957fbc7002617d8093298d129fbe4cc9CAS |
[17] Warrell, M.J. (2012) Current rabies vaccines and prophylaxis schedules: preventing rabies before and after exposure. Travel Med. Infect. Dis. 10, 1–15.
| Current rabies vaccines and prophylaxis schedules: preventing rabies before and after exposure.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC383ntVaiuw%3D%3D&md5=0de3c248efd5a327cc79dacd5c8533c4CAS |
[18] Brookes, S.M. et al. (2006) Ability of rabies vaccine strains to elicit cross-neutralising antibodies. Dev. Biol. (Basel) 125, 185–193.
| 1:STN:280:DC%2BD28vlsFWqtQ%3D%3D&md5=4a63494eac4c7ee7a420bad33078b81fCAS |
[19] Brookes, S.M. et al. (2005) Rabies human diploid cell vaccine elicits cross-neutralising and cross-protecting immune responses against European and Australian bat lyssaviruses. Vaccine 23, 4101–4109.
| Rabies human diploid cell vaccine elicits cross-neutralising and cross-protecting immune responses against European and Australian bat lyssaviruses.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXlt1Kjtr8%3D&md5=cf94a6afd9e85f3910f2931e56727b9dCAS |
[20] Hanlon, C.A. et al. (2005) Efficacy of rabies biologics against new lyssaviruses from Eurasia. Virus Res. 111, 44–54.
| Efficacy of rabies biologics against new lyssaviruses from Eurasia.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXkt1Cku7s%3D&md5=95ce359aa15d900ae3210d006b2c5c41CAS |
[21] Horton, D.L. et al. (2010) Quantifying antigenic relationships among the Lyssaviruses. J. Virol. 84, 11841–11848.
| Quantifying antigenic relationships among the Lyssaviruses.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhsVKksLvK&md5=2144716ee1d038543f4a41f931366c30CAS |
[22] Nolden, T. et al. (2014) Comparative studies on the genetic, antigenic and pathogenic characteristics of Bokeloh bat lyssavirus. J. Gen. Virol. 95, 1647–1653.
| Comparative studies on the genetic, antigenic and pathogenic characteristics of Bokeloh bat lyssavirus.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXhsFaksr%2FO&md5=2ba25f181737c75ff8d08138d76afde4CAS |
[23] Badrane, H. et al. (2001) Evidence of two Lyssavirus phylogroups with distinct pathogenicity and immunogenicity. J. Virol. 75, 3268–3276.
| Evidence of two Lyssavirus phylogroups with distinct pathogenicity and immunogenicity.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXitVGitbc%3D&md5=5aba28cba46f1925327cb13152859393CAS |
[24] Horton, D.L. et al. (2014) Antigenic and genetic characterization of a divergent African virus, Ikoma lyssavirus. J. Gen. Virol. 95, 1025–1032.
| Antigenic and genetic characterization of a divergent African virus, Ikoma lyssavirus.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXhtVyms7jJ&md5=0f827c9308c795c334c3d5c367cd749fCAS |
[25] Kuzmin, I.V. et al. (2008) Possible emergence of West Caucasian bat virus in Africa. Emerg. Infect. Dis. 14, 1887–1889.
| Possible emergence of West Caucasian bat virus in Africa.Crossref | GoogleScholarGoogle Scholar |
Biographies
Dr Banyard is a senior researcher in the Wildlife Zoonoses and Vector-Borne Diseases Research Group at the Animal and Plant Health Agency (National Reference Laboratory for rabies). He has 20 years’ experience working with emerging veterinary pathogens and zoonoses. Dr Banyard’s research interests include molecular pathogenesis of viral infections, novel pathogen discovery and alternative approaches to vaccine development.
Dr Fooks leads the Wildlife Zoonoses and Vector-Borne Diseases Research Group at the Animal and Plant Health Agency. Since 2002, he was appointed director of a World Health Organization Communicable Disease Surveillance and Response Collaborating Centre for the characterisation of rabies and rabies-related viruses. In 2006, he was appointed as an OIE Reference Expert for Rabies. He holds Honorary Visiting Professor positions in the Department of Veterinary Pathology at the University of Liverpool, UK and in the Institute for Infection and Immunity at St Georges Medical School, University of London, UK. Dr Fooks’ research interests are focused on RNA viruses, especially viral diseases of the CNS and emerging/exotic viral zoonoses.