Anaerobic spirochaetes and animals
David J Hampson A B , Nyree D Phillips A C and Tom La A DA School of Veterinary and Life Sciences
Murdoch University
Murdoch, WA 6150, Australia
Tel: +61 8 9360 2287
Fax: +61 8 9360 3130
B Email: d.hampson@murdoch.edu.au
C Email: n.phillips@murdoch.edu.au
D Email: t.la@murdoch.edu.au
Microbiology Australia 36(3) 122-124 https://doi.org/10.1071/MA15043
Published: 6 August 2015
Anaerobic spirochaetes colonise the large intestine of many avian and mammalian host species. The most well known pathogenic species is the strongly haemolytic Brachyspira hyodysenteriae that was first isolated from pigs with swine dysentery (SD) in the early 1970s. Classical SD is a severe mucohaemorrhagic colitis that occurs in growing pigs and is endemic in most pig-rearing areas of the world. The spirochaete acts in concert with other components of the colonic microbiota to disrupt the integrity of the colonic epithelium and induce inflammation. In recent years two new strongly haemolytic species, the proposed ‘Brachyspira suanatina’ and ‘Brachyspira hampsonii’, both with reservoirs in migratory water birds, have been described as new and emerging agents of SD in the northern hemisphere. Weakly haemolytic species also have been described, some of which have pathogenic potential. In particular Brachyspira pilosicoli causes a mild colitis and diarrhoea in many species, including human beings, whilst Brachyspira intermedia is a common pathogen in adult poultry. Infection with B. intermedia and/or B. pilosicoli can cause wet litter, faecal staining of eggshells and delays in reaching peak egg production. Options for control of these widespread and economically significant anaerobic infections are limited.
Isolation and characterisation
Brachyspira species take at least 3–5 days to appear as a thin film of surface growth on selective blood agar that is incubated at 37–41°C in an anaerobic environment. Lack of colony formation can cause a number of technical difficulties when manipulating cultures. The various species are classified as being either strongly or weakly haemolytic (Figure 1a). Under a phase contrast microscope individual spirochaetes are seen as slender rods, usually with two or three undulating curves (Figure 1b). The cells stain weakly Gram negative, and are easier to see using silver stains. There are relatively few defining and consistent phenotypic differences between the seven officially named and other proposed Brachyspira species. Identification to the species level is important as some species are pathogenic and some are not. Identity is usually achieved using species-specific PCRs directed at pathogenic species, or by sequencing genes such as the NADH oxidase gene and constructing phylogenetic trees1,2. PCRs can be used on primary growth off the isolation plates or in DNA extracted from faeces. Multilocus sequence typing has been developed to assist with species identification as well as for sub-specific differentiation of isolates and recognition of clonal groups3.
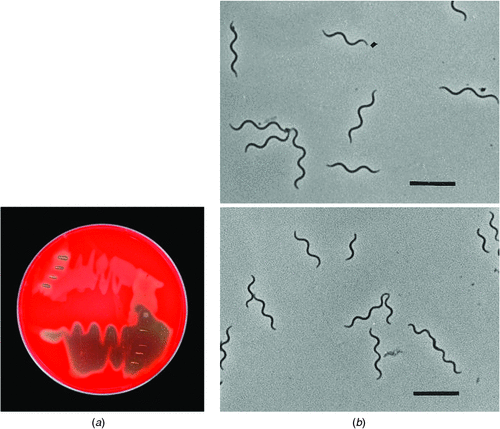
|
Swine dysentery
Swine dysentery (SD) manifests as a severe mucohaemorrhagic colitis of grower and finisher pigs (Figure 2). The disease is endemic in many countries around the world, including Australia. Although clinical manifestations can be controlled by the use of a small number of antimicrobial agents that are licensed for use in pigs, resistance to these drugs by strains of the causative agents as observed both in vitro and in vivo is a growing problem worldwide. Detecting the condition and limiting the spread of infection between farms remains a major fundamental for control. Subclinical carriage of the pathogen can present challenges for controlling transmission. The classical agent of SD is the strongly haemolytic Brachyspira hyodysenteriae, but in the last decade two newly described strongly haemolytic species, proposed as ‘Brachyspira suanatina’4 and ‘Brachyspira hampsonii’5, have been identified as emerging causes of SD. Both species appear to have reservoirs in migratory water birds6. To date description of ‘B. suanatina’ has been limited to Scandinavia, whilst ‘B. hampsonii’ has been described as an emerging clinical problem in pigs in North America and Europe.
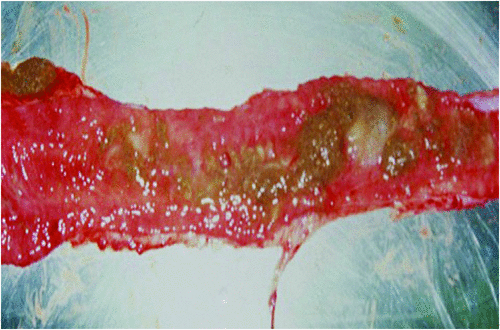
|
Intestinal spirochaetosis
A defining but inconsistent feature of intestinal spirochaetosis (IS) is the presence of spirochaetes attached by one cell end to the surface of colonic enterocytes (Figure 3). Brachyspira aalborgi can cause this manifestation in humans, but in birds and mammals (including human beings) the agent of IS is the weakly haemolytic Brachyspira pilosicoli7. This species is widely distributed, and is a particular clinical problem where individuals live in close proximity, such as with intensively reared pigs, caged adult chickens, or villagers in developing communities where basic hygiene is compromised. Problems also arise where production animals or birds that are raised outside are exposed to water or soil that has been contaminated by faeces (for example from wild water birds, which frequently carry B. pilosicoli). In pigs the infection can cause colitis and diarrhoea in groups of recently weaned or growing animals, resulting in poor and uneven growth rates that reduce the profitability of farming enterprises. Infections in dogs and horses also have been described.
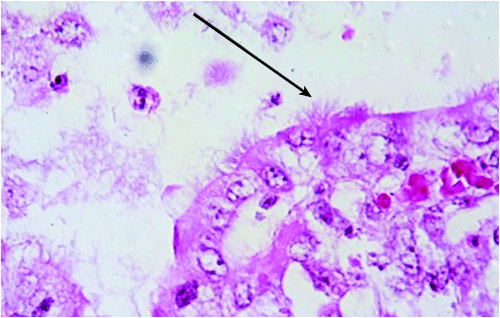
|
Avian intestinal spirochaetosis
The outcome of infection with Brachyspira species in adult chickens (laying and breeding hens) and other poultry species has been called avian intestinal spirochaetosis (AIS)8. The condition does not seem to occur naturally in young meat producing chickens (broilers), perhaps because there is insufficient opportunity and time for colonisation to occur. AIS has been associated with reductions and delays in egg production, foamy brown faeces, wet litter, and faecal staining of eggshells resulting in downgrading of valuable table eggs for use in manufacturing (Figure 4). The two main (weakly haemolytic) species that have been implicated in AIS are Brachyspira intermedia and B. pilosioli, although B. alvinipulli occasionally has been reported to be involved in the condition in the USA and Europe. AIS appears to be widespread throughout the world, including in Australia, but the condition is only infrequently diagnosed. This is probably because of the relatively non-specific nature of the signs, and the difficulty in isolating and identifying the causative anaerobic Brachyspira species without the availability of specialised diagnostic media, facilities and expertise.

|
Control
Without effective disease control the health and welfare of affected animals can be compromised. Infections with the anaerobic Brachyspira species can be suppressed by antimicrobial agents such as metronidazole, but these drugs are no longer available for use in production animals. Indeed, relatively few drugs are registered for production animal species, and resistance to the remaining drugs by strains of the various Brachyspira species is an increasing problem. Currently there are no effective commercially vaccines available, although new recombinant vaccines are being developed for control of SD9. Modifications to the diet fed to both pigs and chickens have been shown to change the colonic microenvironment and influence and potentially reduce colonisation by Brachyspira species10–12, but these diets are expensive and too specialised to be commercially viable for routine use in animals.
Control of infection within a geographical area (by State authorities or private/company veterinarians) still largely rests on provision of accessible, reliable diagnostic services and routine surveillance. When coupled with implementation of strong biosecurity measures the transmission of infection between farms can be reduced or prevented.
References
[1] Atyeo, R.F. et al. (1999) Differentiation of Serpulina species by NADH oxidase (nox) gene comparisons and nox-based polymerase chain reaction tests. Vet. Microbiol. 67, 47–60.| Differentiation of Serpulina species by NADH oxidase (nox) gene comparisons and nox-based polymerase chain reaction tests.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1MXjtFajs78%3D&md5=9a23ed918dd8b43cc69c95348e498298CAS | 10392777PubMed |
[2] Song, Y. and Hampson, D.J. (2009) Development of a multiplex qPCR for detection and quantitation of pathogenic intestinal spirochaetes in pigs and chickens. Vet. Microbiol. 137, 129–136.
| Development of a multiplex qPCR for detection and quantitation of pathogenic intestinal spirochaetes in pigs and chickens.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXltlChtb4%3D&md5=f307693e908a260b929f507e9c52402cCAS | 19171443PubMed |
[3] La, T. et al. (2009) Multilocus sequence typing as a tool for studying the molecular epidemiology and population structure of Brachyspira hyodysenteriae. Vet. Microbiol. 138, 330–338.
| Multilocus sequence typing as a tool for studying the molecular epidemiology and population structure of Brachyspira hyodysenteriae.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhtV2ksLjP&md5=96a55c0f458f755dfe151603a8f8053aCAS | 19369014PubMed |
[4] Råsbäck, T. et al. (2007) A novel enteropathogenic, strongly haemolytic spirochaete isolated from pig and mallard, provisionally designated ‘Brachyspira suanatina’ sp. nov. Environ. Microbiol. 9, 983–991.
| A novel enteropathogenic, strongly haemolytic spirochaete isolated from pig and mallard, provisionally designated ‘Brachyspira suanatina’ sp. nov.Crossref | GoogleScholarGoogle Scholar | 17359270PubMed |
[5] Chander, Y. et al. (2012) Phenotypic and molecular characterization of a novel strongly hemolytic Brachyspira species, provisionally designated ‘Brachyspira hampsonii’. J. Vet. Diagn. Invest. 4, 903–910.
[6] Rubin, J.E. et al. (2013) Isolation and characterization of Brachyspira spp. including ‘Brachyspira hampsonii’ from lesser snow geese (Chen caerulescens caerulescens) in the Canadian Arctic. Microb. Ecol. 66, 813–822.
| Isolation and characterization of Brachyspira spp. including ‘Brachyspira hampsonii’ from lesser snow geese (Chen caerulescens caerulescens) in the Canadian Arctic.Crossref | GoogleScholarGoogle Scholar | 23933825PubMed |
[7] Trott, D.J. et al. (1996) Serpulina pilosicoli sp. nov., the agent of porcine intestinal spirochetosis. Int. J. Syst. Bacteriol. 46, 206–215.
| Serpulina pilosicoli sp. nov., the agent of porcine intestinal spirochetosis.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK287jvV2rsg%3D%3D&md5=8ea55ad63cfa28f8346904a3f74ec4bdCAS | 8573497PubMed |
[8] McLaren, A.J. et al. (1997) Genetic and phenotypic characterization of intestinal spirochetes colonizing chickens, and allocation of known pathogenic isolates to three distinct genetic groups. J. Clin. Microbiol. 35, 412–417.
| 1:STN:280:DyaK2s7lslehsA%3D%3D&md5=c4935f5540eace8d3b23e68b72ce3404CAS | 9003607PubMed |
[9] Song, Y. et al. (2009) A reverse vaccinology approach to swine dysentery vaccine development. Vet. Microbiol. 137, 111–119.
| A reverse vaccinology approach to swine dysentery vaccine development.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXltlChtLY%3D&md5=8002a3abc4cecfbfdb3d9d52135929b6CAS | 19179021PubMed |
[10] Pluske, J.R. et al. (1998) Confirmation of the role of non-starch polysaccharides and resistant starch in the expression of swine dysentery in pigs following experimental infection. J. Nutr. 128, 1737–1744.
| 1:CAS:528:DyaK1cXmtlKrtLk%3D&md5=5bd17384a9fa89d74f2a009547a1f33dCAS | 9772144PubMed |
[11] Hampson, D.J. et al. (2000) Influences of diet and vaccination on colonization of pigs with the intestinal spirochaete Brachyspira (Serpulina) pilosicoli. Vet. Microbiol. 73, 75–84.
| Influences of diet and vaccination on colonization of pigs with the intestinal spirochaete Brachyspira (Serpulina) pilosicoli.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD3c7pvVGjsg%3D%3D&md5=13b46ecec209d75d20ff698c3c213a10CAS | 10731619PubMed |
[12] Phillips, N.D. et al. (2004) A wheat-based diet enhances colonisation with the intestinal spirochaete Brachyspira intermedia in experimentally-infected laying hens. Avian Pathol. 33, 451–457.
| A wheat-based diet enhances colonisation with the intestinal spirochaete Brachyspira intermedia in experimentally-infected laying hens.Crossref | GoogleScholarGoogle Scholar | 15370044PubMed |
Biographies
Dr David Hampson is a veterinarian who is Professor of Veterinary Microbiology and Dean of the School of Veterinary and Life Sciences at Murdoch University. He has maintained a special interest in Brachyspira species for most of his academic career.
Dr Nyree Phillips completed her PhD on avian intestinal spirochaetosis at Murdoch University in 2006. Nyree is a Postdoctoral Fellow at Murdoch University and works mainly on the diagnosis and control of Brachyspira infections in pigs and poultry.
Dr Tom La completed his PhD on swine dysentery vaccine development at Murdoch University in 2006. Tom is a Postdoctoral Fellow at Murdoch University and works on diagnostics and vaccine development for Brachyspira species.


