On the surface of it: the role of materials science in developing antifungal therapies and diagnostics
Bryan R CoadMycology/Surface Interfaces Group
Mawson Institute
University of South Australia
Mawson Lakes, SA 5059, Australia
Tel: +61 8 8302 3152
Email: bryan.coad@unisa.edu.au
Microbiology Australia 36(2) 71-73 https://doi.org/10.1071/MA15024
Published: 16 March 2015
Surfaces are often considered to play a passive role in clinical mycology; that is, the outward face of a medical device to which fungal cells attach and form biofilms. However, materials chemistry and nanotechnology are now transforming passive surfaces into active interfaces and driving innovation into antifungal agents, their surface delivery and mechanisms, and diagnostic devices. Beyond technological improvements, there is great opportunity to drive basic research into fungal-surface interactions; however, this can only be accomplished with combined and concerted efforts of materials scientists, polymer chemists and mycologists.
Fungal biofilms on medical devices
Implanted devices are the source of 81% of nosocomial infections1, costing the US $5–10 billion per year2. Fungal pathogens are the third most common cause of infections from catheters, leading to candidaemia3, which has a mortality rate greater than 50% in Australia4. Particularly troublesome for treatment are fungal biofilms because they are pervasively established on surfaces through a process of rapid colonisation and spreading, followed by secretion of a protective extracellular matrix. Because of this, systemically administered antifungal drugs are nearly always ineffective at penetrating and eradicating a mature biofilm, necessitating the removal and replacement of infected devices. Clearly, new strategies are required for understanding the fungal-surface interface in order to reduce the unacceptably high rate of morbidity and mortality associated with infected urinary, central and venous catheters, endotracheal tubes, and other implanted biomedical devices.
Delivery from the surface: antifungal material coatings
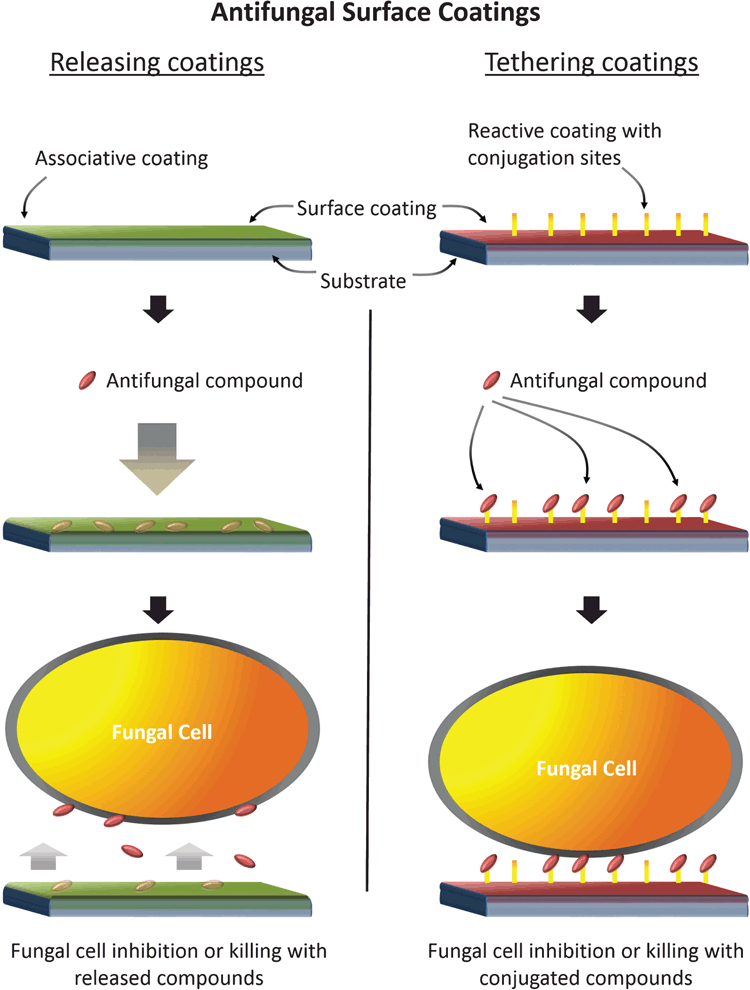
Incorporation of antifungal agents onto the surface of medical devices in the form of surface coatings offers a novel way to prevent biofilm formation from the bottom up. This strategy allows concentration of antifungal agents at the material interface, killing potential colonisers or preventing them from attaching. Through the application of surface coatings, an active interface is furnished without altering the bulk properties of the substrate. Associated antifungal agents could take the form of currently licensed antifungal drugs or experimental compounds. There are broadly two types of coatings: (1) those that release a payload of antifungal agents, and (2) those where the active compound is irreversibly bound at the surface interface (Figure 1). The former comprise thin sponge-like coatings that are initially loaded with antifungal agents and become released into local tissues and fluids once implanted5,6. Releasing coatings with defined elution profiles would be suitable for short-dwelling implants in specific applications. The second strategy is to irreversibly bind or tether antifungal agents to the surface coating, providing a surface-contact effect without systemic release. Tethered antifungal agents would ideally utilise compounds with a known effect in the fungal cell wall (e.g. the echinocandins) that could act through a contact-killing mechanism. New research has shown this strategy to be remarkably effective against Candida spp. In our lab, we have prepared surface coatings with covalently attached caspofungin and demonstrated reduced surface attachment of 98% of C. albicans cells compared with untreated surfaces (unpublished data).
Moving from 2D to 3D: through the envelope
Tethering agents to the surface is a particularly attractive idea because it may be possible to formulate surface coatings using antifungal agents too toxic to be administered systemically. For some of the polyene class of drugs, which associate with ergosterol in the fungal cell membrane, many effective compounds have been developed but cannot be used because of their toxicity profile. One challenge that must be overcome using the tethering strategy is delivery of the agent to the cell membrane, which is protected by the thick cell wall. Using polymer surface grafting techniques it is possible to design nanoscale control of the linker with desired length, density and rigidity or fluidity. Such 3D approaches have successfully been used to study fibroblast adhesion by locating cell binding peptides onto and within so-called polymer brushes7. Beyond the delivery of antifungal agents, this technology could be harnessed to probe structures within the cell envelope to investigate changes in cell wall components that are important in morphogenesis and virulence8. This could be studied using live-cell imaging techniques allowing real-time monitoring of morphological changes to the hyphal filaments9. Thus the combination of 3D grafting approaches with live-cell imaging provides a means to correlate the physical and chemical properties of the coating with observable changes to the structure and function of invasive filaments. This has potential to be a powerful method in pathogenesis studies. Furthermore, 3D grafting techniques that probe specific targets within the cell envelope will allow a more complete knowledge of cell wall structure and function, leading to a new understanding of drug mechanisms, organism pathology, and discovery of new diagnostic biomarkers.
Understanding mycology at the surface interface: new research, new opportunities
Breakthroughs in the biology of surface interfaces can only be accomplished by teams possessing an array of specialised skills: materials science, surface analysis, polymer chemistry, microscopy, biochemistry, and cellular biology. Compared to anti-bacterial surfaces, progress has been slow in antifungal surfaces, evidenced by a 10:1 ratio of publications in these fields to date10. Clearly there is a disconnect between the importance of this topic to human health and the research outputs, despite calls by key opinion leaders for new strategies and therapies to combat invasive fungal infections3,11 and labelling fungi as ‘the unknown superbugs’12. The Mycology/Surface Interfaces Group is beginning to address these research gaps through basic research understanding of fungal-surface interactions. Part of this will feed into a greater understanding of clinically relevant poly-microbial infections13. Broader research will seek to apply discoveries to other areas including food and water safety, environmental moulds, and new diagnostic devices. Our long term goal is to develop partnerships with industries and translate research outcomes into innovative new products and therapies.
Acknowledgements
I acknowledge the project leadership of Professor Hans J Griesser, Professor Harm-Anton Klok, Professor Nick D Read, A/Professor Anton Y Peleg and Dr Ana Traven on ARC DP150101674 and NHMRC APP1066647 and for their contributions to the Mycology/Surface Interfaces group.
References
[1] Hidron, A.I. et al. (2008) NHSN annual update: antimicrobial-resistant pathogens associated with healthcare-associated infections: annual summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2006–2007. Infect. Control Hosp. Epidemiol. 29, 996–1011.| NHSN annual update: antimicrobial-resistant pathogens associated with healthcare-associated infections: annual summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2006–2007.Crossref | GoogleScholarGoogle Scholar | 18947320PubMed |
[2] Stone, P.W. et al. (2005) The economic impact of infection control: making the business case for increased infection control resources. Am. J. Infect. Control 33, 542–547.
| The economic impact of infection control: making the business case for increased infection control resources.Crossref | GoogleScholarGoogle Scholar | 16260329PubMed |
[3] Crump, J.A. and Collignon, P.J. (2000) Intravascular catheter-associated infections. Eur. J. Clin. Microbiol. Infect. Dis. 19, 1–8.
| Intravascular catheter-associated infections.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD3c7mvVKruw%3D%3D&md5=24edf4f32ebea329b411cf2faa4494b4CAS | 10706172PubMed |
[4] Marriott, D. et al. (2006) Candidaemia in the Australian intensive care unit: epidemiology, clinical features and outcome from a 3 year nationwide study. Int. J. Infect. Dis. 10, S77–S78.
| Candidaemia in the Australian intensive care unit: epidemiology, clinical features and outcome from a 3 year nationwide study.Crossref | GoogleScholarGoogle Scholar |
[5] Hachem, R. et al. (2009) Novel antiseptic urinary catheters for prevention of urinary tract infections: correlation of in vivo and in vitro test results. Antimicrob. Agents Chemother. 53, 5145–5149.
| Novel antiseptic urinary catheters for prevention of urinary tract infections: correlation of in vivo and in vitro test results.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhsF2gs7bO&md5=75c8d722fe8312d5cf5a5f0e1d831ea1CAS | 19805562PubMed |
[6] Zumbuehl, A. et al. (2007) Antifungal hydrogels. Proc. Natl. Acad. Sci. USA 104, 12994–12998.
| Antifungal hydrogels.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXpt1KrtLY%3D&md5=c3f1fba1ff4b921d5571f600ae848544CAS | 17664427PubMed |
[7] Desseaux, S. and Klok, H.-A. (2015) Fibroblast adhesion on ECM-derived peptide modified poly(2-hydroxyethyl methacrylate) brushes: ligand co-presentation and 3D-localization. Biomaterials 44, 24–35.
| Fibroblast adhesion on ECM-derived peptide modified poly(2-hydroxyethyl methacrylate) brushes: ligand co-presentation and 3D-localization.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2MXhs1KisQ%3D%3D&md5=0efe46809c0ffefc95873e2e6d15fe6eCAS | 25617123PubMed |
[8] Gow, N.A. et al. (2012) Candida albicans morphogenesis and host defence: discriminating invasion from colonization. Nat. Rev. Microbiol. 10, 112–122.
| 1:CAS:528:DC%2BC3MXhs1aitr%2FK&md5=588e7420bcd309172a4857162b3f2783CAS |
[9] Hickey, P.C. et al. (2004) Live-cell imaging of filamentous fungi using vital fluorescent dyes and confocal microscopy. Method. Microbiol. 34, 63–87.
| Live-cell imaging of filamentous fungi using vital fluorescent dyes and confocal microscopy.Crossref | GoogleScholarGoogle Scholar |
[10] Coad, B.R. et al. (2014) Biomaterials surfaces capable of resisting fungal attachment and biofilm formation. Biotechnol. Adv. 32, 296–307.
| Biomaterials surfaces capable of resisting fungal attachment and biofilm formation.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhvVagt77M&md5=5176e1a71e22e7843e55d3475ab18048CAS | 24211473PubMed |
[11] Brown, G. D. et al. (2012) Hidden killers: human fungal infections. Sci. Transl. Med. 4, 165rv13.
| Hidden killers: human fungal infections.Crossref | GoogleScholarGoogle Scholar | 23253612PubMed |
[12] Gow, N.A.R. et al. (2012) Waging war on fungi – the unknown superbugs. Microbiol. Today 39, 208–211.
[13] Peleg, A.Y. et al. (2010) Medically important bacterial-fungal interactions. Nat. Rev. Microbiol. 8, 340–349.
| Medically important bacterial-fungal interactions.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXjvFars7g%3D&md5=a51a08457e9d01185bafbcabf719a2cdCAS | 20348933PubMed |
Biography
Dr Bryan Coad is a Senior Research Fellow at the Mawson Institute, University of South Australia. His background is in physical chemistry and for the past 14 years has been active in the area of biomaterials design and surface analysis. He currently leads the Mycology/Surfaces Interfaces group. Recently, he was jointly awarded an Australian Research Council Discovery Project on combating fungal biofilm growth on surfaces.