The changing epidemiology and challenges of paediatric Clostridium difficile infections
Sicilia Perumalsamy A and Thomas V Riley A B C D EA The University of Western Australia, School of Biomedical Sciences, Queen Elizabeth II Medical Centre, Nedlands, WA 6009, Australia
B School of Medical and Health Sciences, Edith Cowan University, Joondalup, WA 6027, Australia
C PathWest Laboratory Medicine, Department of Microbiology, Queen Elizabeth II Medical Centre, Nedlands, WA 6009, Australia
D School of Veterinary and Life Sciences, Murdoch University Murdoch, WA 6150, Australia
E Tel: +61 8 6457 3690, Email: thomas.riley@uwa.edu.au
Microbiology Australia 40(4) 157-160 https://doi.org/10.1071/MA19047
Published: 14 November 2019
Clostridium difficile (recently also named Clostridioides difficile)1 is an anaerobic spore-forming Gram-positive rod, the leading cause of hospital-acquired diarrhoea in high-income countries and a significant cause of community-acquired diarrhoea2. Since the first isolation of C. difficile as a commensal organism in healthy infants in 19353, this ubiquitous pathogen has led a chequered life as a pathogen of children. Despite high rates of C. difficile carriage in infants, they rarely develop clinical symptoms of disease. The reasons for this are not fully understood but may be due to a lack of toxin receptors in the gut. Nonetheless, increasing rates of C. difficile infection (CDI) in children have been observed in many parts of the world, especially in children without traditional risk factors, as well as a rising incidence of community-acquired CDI (CA-CDI)4 – this is of concern. This complicated situation is compounded by diagnostic testing issues. Molecular detection methods have been widely implemented in Australia and North America and are more sensitive than toxin enzyme immunoassays (EIAs) or culture5. They are often used routinely in paediatric populations with a high prevalence of C. difficile such as children with malignancies5. Differentiating children who require treatment from those colonised with toxigenic strains remains a challenge for health care providers.
Antimicrobials with activity against bowel flora including clindamycin, penicillins, cephalosporins and fluoroquinolones are most often associated with CDI2. C. difficile spores are most frequently acquired through faecal-oral transmission resulting in either asymptomatic colonisation or a spectrum of clinical manifestations (ranging from mild diarrhoea to pseudomembranous colitis, toxic megacolon and death)2. Most of the recent changes in the epidemiology of CDI outside Australia have been attributed to the appearance of an epidemic strain of C. difficile, RT 027 (which produces toxins A, B and binary toxin), in the early 2000s5. National surveillance for CDI in Australia was first introduced in 2010 and, since then, the rates of CDI have increased significantly4.
Rising rates of Clostridium difficile infection in children
As with adults, recent antimicrobial exposure is the most significant risk factor for acquiring CDI in children along with the presence of comorbid conditions that alter the intestinal microbiota, placement of feeding tubes and frequent exposure to health care institutions6. Children were not considered to be a population at high risk of CDI, however, increasing hospitalisations for CDI and more severe disease has been observed in this cohort7, including in the USA in children with haematological malignancies (HM) and inflammatory bowel diseases (IBD)7. The rate of CDI varies greatly based on the type of malignancy, and CDI is reported nearly 2.5 times more in patients with HM than those with solid organ tumours8. There are many gaps in knowledge of CDI epidemiology among children with HM and haematopoietic stem cell transplantation (HSCT), even though there are numerous studies in adults9. The work that has been done in children confirms CDI as an important disease in this particular group of patients10. Moreover, outbreaks of CDI in paediatric HM wards have been documented11. In Western Australia more specifically, a case of severe CDI with binary toxin-positive C. difficile in a paediatric patient with newly diagnosed IBD was detected several years ago12. Recurrences of CDI have been observed in approximately 10–25% of paediatric patients13, similar to adults. A 5-year retrospective study from Italy reported a substantial increase from 0.75/1000 hospitalisations to 9.8/1000 hospitalisations in the incidence of CA-CDI among children presenting with diarrhoea14. A case control study conducted in US reported an increased risk of CA-CDI in children within the first 30 days of antimicrobial exposure (cephalosporins specifically)15, while a more recent US case control study identified an association of recent exposure to a household member with diarrhoeal illness with paediatric CA-CDI, hinting at the possibility of household contamination playing a role in transmitting C. difficile spores16. High gastric pH levels have also been hypothesised to influence CDI risk by allowing bacterial colonisation of the upper gastrointestinal tract6. Worldwide, there is limited information about the antimicrobial susceptibility patterns of C. difficile in children; however, a single-centre study found that C. difficile strains from children are less resistant compared to strains from adults, with most paediatric isolates being susceptible to vancomycin, metronidazole and rifaximin17. This study also reported a lack of difference in susceptibility between the first and subsequent isolates in paediatric CDI17.
Colonisation of C. difficile in the neonatal and paediatric gut
The role of C. difficile in the paediatric gut remains controversial, and it is still unclear whether it is a primary pathogen capable of causing disease or a harmless bystander, often in diseases caused by other pathogens such as rotavirus18. Asymptomatic gut colonisation with C. difficile can occur at higher rates (up to 90%) in infants aged less than 1 year old, compared to adults19. Neonates and infants who harbour high numbers of toxigenic strains often do not display any signs or symptoms of CDI18. The reason for this is not fully understood although it has been implied that this could be due to the lack (or minimal expression) of mature toxin receptors18,19. Additionally, the gestational age at birth, infant feeding mode, maternal diet, family lifestyle, geographical location and host genetics shape the initial microbiota20,21. There is still uncertainty regarding the source of infant gut colonisation and there are many theories21. Some studies show the possibility of transmission from the mother’s urogenital tract (via vaginal delivery); however, this idea has been challenged22. Other plausible theories suggest the colonisation of C. difficile in the sterile neonatal gut immediately after birth comes from various environmental sources23. This high colonisation rate drops rapidly between ages 1–3 years to approximately 5–15% as the gut microflora starts to resemble that of an adult19. Postnatal factors such as breastfeeding practices further configure the microbiota in early life as breastfed infants tend to have significantly lower rates of C. difficile colonisation compared to formula fed20. Again, conflicting findings of higher colonisation rates (of toxigenic C. difficile) in children (non-infant age group) in developed countries and lower rates in low-income countries make it more difficult to diagnose CDI20. It is likely that wide variations of enteric pathogens exist between low- and high-income countries, possibly explaining the reasons for these differing findings. Interestingly, a recent study by Kociolek and colleagues supports the hypothesis that colonisation of toxigenic C. difficile is linked to humoral immune response against toxins A and B in infants, as opposed to the trans-placental transfer of maternal antibodies24.
Challenges in diagnosing CDI in children
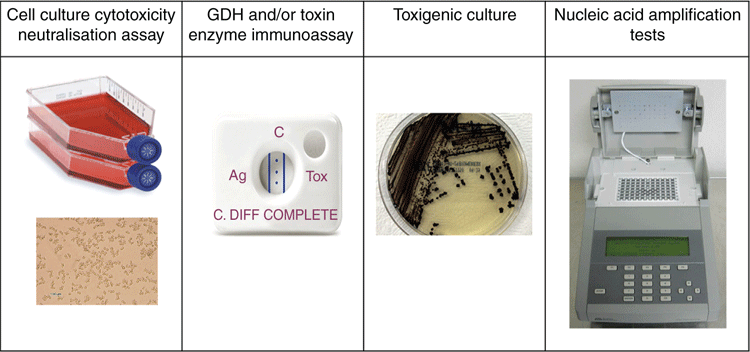
Many controversies still surround the testing methodologies for paediatric CDI5,25,26. The classification of paediatric age groups differs across countries, leading to variations in surveillance of C. difficile5. Surveillance guidelines for CDI in Australia (developed by the Australian Commission on Safety and Quality in Healthcare) exclude testing children under 2 years of age due to the high carriage rates and, as a result, most laboratories in Australia do not test this group of children26. This creates a gap in knowledge about understanding the extent of C. difficile colonisation in infants and the impact this can have in CDI. Distinguishing colonisation from symptomatic infection is also a challenge and the available laboratory tests (including molecular detection methods) are not helpful in making that distinction5,19. Identifying clinical predictors of severe CDI in children is difficult mainly because diarrheal illnesses are frequently encountered in children commonly associated with viral agents5. This places significant pressure on the importance of obtaining a full clinical history, and searching for other possible causes of diarrhoea. Also, there still exists a lack of validated clinical criteria across different countries, leading to discrepancies in defining clinically significant diarrhoea in infants and incontinent children. As a result, deciding which children presenting with diarrhoea in an ambulatory setting should be tested for C. difficile is also difficult. The IDSA (Infectious Diseases Society of America)/SHEA (Society for Healthcare Epidemiology of America) guidelines recommends specific tests such as EIAs and NAATs (Figure 1), either as a multi-step or single test (depending on whether the institutions have pre-set criteria for testing samples)25. In an Australian study, molecular methods were recommended over other testing methodologies such as a GDH algorithm or culture26.
Management of CDI in children
The IDSA and SHEA recommend either metronidazole or vancomycin for non-severe initial or first recurrence of CDI in children25,27. For moderate and severe CDI, oral vancomycin is given25. For a second or more episode of recurrent CDI, the best treatment option is yet to be determined. The current recommendations, of tapered regimens of either only vancomycin or vancomycin followed by rifaximin, stem from limited data25,28. Fidaxomicin has not been approved by the FDA for paediatric CDI, however, there was a good clinical response and tolerability in phase 2 trials17. Faecal microbiota transplantation (FMT) is regarded as the best treatment for recurrent CDI in adult patients29 due to its ability to restore the microbiota back to its initial healthy form. The constantly evolving microbiome in children and its effect on the immune system has an impact on the success of FMT. A recent review on current data on FMT in paediatric CDI reported ~90% improvement of symptoms with a 4% recurrence rate and no severe complications30. Based on adult studies and the limited data on children, FMT might be considered for treatment in children with recurrent or severe CDI episodes25,29,30. Administration of probiotics achieved 60% reduction of CDI risk in children and adults, according to a recent systematic review, and was also safe31.
Vaccines are considered the only type of intervention capable of providing long-term protection against CDI and, given the extensive testing of vaccines in clinical trials currently, a licensed C. difficile vaccine may become available within the next decade32. However, whether this vaccine (designed for adults) will be suitable for children is not known. Even if a paediatric C. difficile vaccine is developed, its use will depend on surveillance (which is not done for paediatric CDI in most parts of the world). Also, it is difficult to recognise which groups of children would benefit from vaccination and, in the context of CA-CDI, it is unclear whether it is worth vaccinating those in close contact with neonates/infants. Therefore, it is evident that there is limited data on the various treatment options for paediatric CDI, compared to the adult CDI. More important, complications and mortality in paediatric CDI are rare, making it difficult to compare data for the different treatments in children, but this may relate to a lack of surveillance.
How children play a role in the transmission of C. difficile in the context of CA-CDI is not fully understood, however, exposure to infants is a risk for getting CDI33. There are higher rates of CA-CDI in females than males possibly because women are the primary care givers for neonates and infants. There is high potential for asymptomatic carriers to shed C. difficile into the environment. Thus, young children are good examples of human reservoirs who could pose a threat to the elderly and immunocompromised in the community. Even though there are management plans in place to treat and prevent paediatric CDI in hospitals, controlling the shedding and transmission of C. difficile from paediatric carriers to other individuals still remains a challenge for health care providers.
Conclusion
The amount of research conducted on C. difficile among paediatric patients is limited and an understanding of the ability of healthy neonates and infants to tolerate high rates of toxigenic C. difficile is required. Studying the epidemiology of CDI among children and determining the main source of infection and risk factors will guide better public health responses and eventually aid in reducing the burden of CDI on the local healthcare system. Future studies investigating the duration and extent of natural C. difficile immunisation, and the age at which this happens, will aid in understanding the possibility of vaccinating children and the public health value it could have. One stumbling block to learning more about diagnosing CDI in children is the lack of a standardised scoring system for paediatric infections, thus making it harder to study disease burden that inevitably leads to confusion in deciding who to treat. More work is required to address this problem.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgements
This research did not receive any specific funding.
References
[1] Oren, A. and Rupnik, M. (2018) Clostridium difficile and Clostridioides difficile: two validly published and correct names. Anaerobe 52, 125–126.| Clostridium difficile and Clostridioides difficile: two validly published and correct names.Crossref | GoogleScholarGoogle Scholar | 30031828PubMed |
[2] Freeman, J. et al. (2010) The changing epidemiology of Clostridium difficile infections. Clin. Microbiol. Rev. 23, 529–549.
| The changing epidemiology of Clostridium difficile infections.Crossref | GoogleScholarGoogle Scholar | 20610822PubMed |
[3] Hall, I.C. and O’Toole, E. (1935) Intestinal flora in new-born infants: with a description of a new pathogenic anaerobe, Bacillus difficilis. Am. J. Dis. Child. 49, 390–402.
| Intestinal flora in new-born infants: with a description of a new pathogenic anaerobe, Bacillus difficilis.Crossref | GoogleScholarGoogle Scholar |
[4] Slimings, C. et al. (2014) Increasing incidence of Clostridium difficile infection, Australia, 2011–2012. Med. J. Aust. 200, 272–276.
| Increasing incidence of Clostridium difficile infection, Australia, 2011–2012.Crossref | GoogleScholarGoogle Scholar | 24641152PubMed |
[5] Antonara, S. and Leber, A.L. (2016) Diagnosis of Clostridium difficile infections in children. J. Clin. Microbiol. 54, 1425–1433.
| Diagnosis of Clostridium difficile infections in children.Crossref | GoogleScholarGoogle Scholar | 26912759PubMed |
[6] Allen, U.D. et al. (2014) Clostridium difficile in paediatric populations. Paediatr. Child Health 19, 43–48.
| Clostridium difficile in paediatric populations.Crossref | GoogleScholarGoogle Scholar | 24627655PubMed |
[7] Sandora, T.J. et al. (2011) Epidemiology and risk factors for Clostridium difficile infection in children. Pediatr. Infect. Dis. J. 30, 580–584.
| Epidemiology and risk factors for Clostridium difficile infection in children.Crossref | GoogleScholarGoogle Scholar | 21233782PubMed |
[8] Ochfeld, E. et al. (2019) Risk factors for Clostridioides (Clostridium) difficile infection following solid organ transplantation in children. Transpl. Infect. Dis. 21, e13149.
| Risk factors for Clostridioides (Clostridium) difficile infection following solid organ transplantation in children.Crossref | GoogleScholarGoogle Scholar | 31332916PubMed |
[9] Selvey, L.A. et al. (2016) Clostridium difficile infections amongst patients with haematological malignancies: a data linkage study. PLoS One 11, e0157839.
| Clostridium difficile infections amongst patients with haematological malignancies: a data linkage study.Crossref | GoogleScholarGoogle Scholar | 27314498PubMed |
[10] Nycz, B.T. et al. (2018) Evaluation of bloodstream infections, Clostridium difficile infections, and gut microbiota in pediatric oncology patients PLoS One 13, e0191232.
| Evaluation of bloodstream infections, Clostridium difficile infections, and gut microbiota in pediatric oncology patientsCrossref | GoogleScholarGoogle Scholar | 29746574PubMed |
[11] Nicholson, M.R. et al. (2015) Clostridium difficile infection in the pediatric transplant patient. Pediatr. Transplant. 19, 792–798.
| Clostridium difficile infection in the pediatric transplant patient.Crossref | GoogleScholarGoogle Scholar | 26403484PubMed |
[12] Androga, G.O. et al. (2015) Infection with toxin A-negative, toxin B-negative, binary toxin-positive Clostridium difficile in a young patient with ulcerative colitis. J. Clin. Microbiol. 53, 3702–3704.
| Infection with toxin A-negative, toxin B-negative, binary toxin-positive Clostridium difficile in a young patient with ulcerative colitis.Crossref | GoogleScholarGoogle Scholar | 26354812PubMed |
[13] Nicholson, M.R. et al. (2015) Novel risk factors for recurrent Clostridium difficile infection in children. J. Pediatr. Gastroenterol. Nutr. 60, 18–22.
| Novel risk factors for recurrent Clostridium difficile infection in children.Crossref | GoogleScholarGoogle Scholar | 25199038PubMed |
[14] Borali, E. et al. (2015) Community-acquired Clostridium difficile infection in children: a retrospective study. Dig. Liver Dis. 47, 842–846.
| Community-acquired Clostridium difficile infection in children: a retrospective study.Crossref | GoogleScholarGoogle Scholar | 26141927PubMed |
[15] Crews, J.D. et al. (2015) Risk factors for community-associated Clostridium difficile-associated diarrhea in children. Pediatr. Infect. Dis. J. 34, 919–923.
| Risk factors for community-associated Clostridium difficile-associated diarrhea in children.Crossref | GoogleScholarGoogle Scholar | 26164847PubMed |
[16] Weng, M.K. et al. (2019) Risk factors for community-associated Clostridioides difficile infection in young children. Epidemiol. Infect. 147, e172.
| Risk factors for community-associated Clostridioides difficile infection in young children.Crossref | GoogleScholarGoogle Scholar | 31063097PubMed |
[17] O’Gorman, M.A. et al. (2018) Safety and pharmacokinetic study of fidaxomicin in children with Clostridium difficile-associated diarrhea: a phase 2a multicenter clinical trial. J. Pediatric Infect. Dis. Soc. 7, 210–218.
| Safety and pharmacokinetic study of fidaxomicin in children with Clostridium difficile-associated diarrhea: a phase 2a multicenter clinical trial.Crossref | GoogleScholarGoogle Scholar | 28575523PubMed |
[18] Lees, E.A. et al. (2016) The role of Clostridium difficile in the paediatric and neonatal gut — a narrative review. Eur. J. Clin. Microbiol. Infect. Dis. 35, 1047–1057.
| The role of Clostridium difficile in the paediatric and neonatal gut — a narrative review.Crossref | GoogleScholarGoogle Scholar | 27107991PubMed |
[19] Enoch, D.A. et al. (2011) Clostridium difficile in children: colonisation and disease. J. Infect. 63, 105–113.
| Clostridium difficile in children: colonisation and disease.Crossref | GoogleScholarGoogle Scholar | 21664931PubMed |
[20] Penders, J. et al. (2006) Factors influencing the composition of the intestinal microbiota in early infancy. Pediatrics 118, 511–521.
| Factors influencing the composition of the intestinal microbiota in early infancy.Crossref | GoogleScholarGoogle Scholar | 16882802PubMed |
[21] Cooperstock, M. et al. (1983) Influence of age, sex, and diet on asymptomatic colonization of infants with Clostridium difficile. J. Clin. Microbiol. 17, 830–833.
| 6863502PubMed |
[22] Milani, C. et al. (2017) The first microbial colonizers of the human gut: composition, activities, and health implications of the infant gut microbiota. Microbiol. Mol. Biol. Rev. 81, e00036-17.
| The first microbial colonizers of the human gut: composition, activities, and health implications of the infant gut microbiota.Crossref | GoogleScholarGoogle Scholar | 29118049PubMed |
[23] Faden, H.S. and Dryja, D. (2015) Importance of asymptomatic shedding of Clostridium difficile in environmental contamination of a neonatal intensive care unit. Am. J. Infect. Control 43, 887–888.
| Importance of asymptomatic shedding of Clostridium difficile in environmental contamination of a neonatal intensive care unit.Crossref | GoogleScholarGoogle Scholar | 26022659PubMed |
[24] Kociolek, L.K. et al. (2019) Natural Clostridioides difficile toxin immunization in colonized infants. Clin. Infect. Dis. , ciz582.
| Natural Clostridioides difficile toxin immunization in colonized infants.Crossref | GoogleScholarGoogle Scholar | 31253983PubMed |
[25] McDonald, L.C. et al. (2018) Clinical practice guidelines for Clostridium difficile infection in adults and children: 2017 update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin. Infect. Dis. 66, e1–e48.
| Clinical practice guidelines for Clostridium difficile infection in adults and children: 2017 update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA).Crossref | GoogleScholarGoogle Scholar | 29462280PubMed |
[26] Hart, J. et al. (2014) Clostridium difficile infection diagnosis in a paediatric population: comparison of methodologies. Eur. J. Clin. Microbiol. Infect. Dis. 33, 1555–1564.
| Clostridium difficile infection diagnosis in a paediatric population: comparison of methodologies.Crossref | GoogleScholarGoogle Scholar | 24781004PubMed |
[27] Sammons, J.S. et al. (2014) Diagnosis and management of Clostridium difficile infection by pediatric infectious diseases physicians. J. Pediatric Infect. Dis. Soc. 3, 43–48.
| Diagnosis and management of Clostridium difficile infection by pediatric infectious diseases physicians.Crossref | GoogleScholarGoogle Scholar | 26624906PubMed |
[28] Mattila, E. et al. (2013) Rifaximin in the treatment of recurrent Clostridium difficile infection. Aliment. Pharmacol. Ther. 37, 122–128.
| Rifaximin in the treatment of recurrent Clostridium difficile infection.Crossref | GoogleScholarGoogle Scholar | 23095030PubMed |
[29] Cammarota, G. et al. (2019) Fecal microbiota transplant for C. difficile infection: just say yes. Anaerobe. , .
| Fecal microbiota transplant for C. difficile infection: just say yes.Crossref | GoogleScholarGoogle Scholar | 31644957PubMed |
[30] Cotter, J.M. et al.. (2019) An infectious diseases perspective on fecal microbiota transplantation for Clostridioides difficile infection in children. J. Pediatr. Infect. Dis. Soc. , piz062.
| An infectious diseases perspective on fecal microbiota transplantation for Clostridioides difficile infection in children.Crossref | GoogleScholarGoogle Scholar |
[31] Goldenberg, J.Z. et al. (2017) Probiotics for the prevention of Clostridium difficile‐associated diarrhea in adults and children. Cochrane Database Syst. Rev. , CD006095.
| Probiotics for the prevention of Clostridium difficile‐associated diarrhea in adults and children.Crossref | GoogleScholarGoogle Scholar | 29257353PubMed |
[32] Riley, T.V. et al. (2019) Status of vaccine research and development for Clostridium difficile. Vaccine. , .
| Status of vaccine research and development for Clostridium difficile.Crossref | GoogleScholarGoogle Scholar | 30902484PubMed |
[33] Wilcox, M.H. et al. (2008) A case–control study of community-associated Clostridium difficile infection. J. Antimicrob. Chemother. 62, 388–396.
| A case–control study of community-associated Clostridium difficile infection.Crossref | GoogleScholarGoogle Scholar | 18434341PubMed |
Biographies
Sicilia Perumalsamy is a first-year PhD candidate at The University of Western Australia. Her research focuses on the epidemiology of Clostridium difficile infections in the Western Australian paediatric population.
Thomas Riley is employed by various universities in Perth, as well as in Pathwest Laboratory Medicine. He has had a long-standing interest in healthcare-related infections, particularly the diagnosis, pathogenesis and epidemiology of Clostridium difficile infection, in human beings, animals and the environment.