Dengue introduced by travellers, Australia
Allison ImrieSchool of Biomedical Sciences
Faculty of Health and Medical Sciences
University of Western Australia
Email: allison.imrie@uwa.edu.au
Microbiology Australia 39(2) 67-71 https://doi.org/10.1071/MA18019
Published: 26 April 2018
Dengue is a mosquito-borne acute viral infection that can develop into a potentially lethal complication known as severe dengue. It is endemic in more than 100 tropical and subtropical countries where the mosquito vectors, predominantly Aedes aegypti and Aedes albopictus, are found. Non-immune travellers are at risk of infection and with the rise in international travel and the availability of cheap holiday packages to endemic countries, many of which are popular tourist destinations, there has been a significant increase in spread of dengue viruses.
In recent years dengue outbreaks have occurred in countries where the disease has never been reported or has not occurred for many decades, coinciding with increased global distribution of the primary vectors. In Australia, dengue is currently restricted to northern Queensland where epidemics occur following introduction of virus by travellers; however, dengue is regularly imported to other states and territories where the vectors have not been present for several decades. Recent detection of the vectors at international air and sea ports in Australia is of concern in light of widespread dengue activity throughout the country up until the 1960s. Genetic analysis of dengue viruses (DENV) imported by travellers provides important information on DENV circulating in the region and introduced into Australia.
Epidemiology
Dengue is a mosquito-borne viral disease that is endemic in most tropical and sub-tropical countries. Epidemics were described in Australia, Asia, the Americas, the Pacific and the Caribbean in the 18th and 19th centuries and outbreaks described in central America and the Caribbean in the 17th century are also believed to be dengue1. Increased incidence of dengue and emergence of dengue haemorrhagic fever in Southeast Asia in the second half of the 20th century was a consequence of uncontrolled urbanisation, human movement and population growth during the Second World War. The incidence has continued to increase dramatically since that time and dengue is now endemic in more than 100 tropical and subtropical countries, where the principal mosquito vectors Aedes aegypti (Figure 1) and Aedes albopictus are found. An estimated 390 million dengue virus (DENV) infections have been estimated to occur annually, of which approximately one quarter result in clinical disease2. The number and magnitude of dengue epidemics have increased consistently in Southeast Asia and the Western Pacific since 2000 and more than 70% of the global dengue disease risk is currently borne by people who live in this region3. Autochthonous dengue transmission has recently been reported in Europe4, the United States5 and Japan6, areas where local outbreaks have not been previously reported or had not occurred for many decades.

|
Clinical features
Infection with any of the four dengue viruses (DENV-1-4) causes a spectrum of illness ranging from a mild or severe acute flu-like illness known as dengue fever (DF) to severe dengue (previously known as dengue hemorrhagic fever and dengue shock syndrome)7. Symptoms become apparent 4–10 days after the bite of an infected mosquito and usually last for 2–7 days. In up to 20% of cases decrease in fever is accompanied by sudden onset of complications due to a vascular leak syndrome consisting of plasma leakage, severe bleeding, respiratory distress or organ impairment. Medical care and maintenance of blood fluid volume during the 24–48 h critical phase can decrease mortality from more than 20% to less than 1%. Warning signs that predict severe dengue are useful for deciding which patients need hospital admission and more intensive monitoring8; however, they may not be recognised and case management may not be optimal, exposing patients to unnecessary risk9.
Dengue is an acute viral infection and virus can be detected in blood by RT-PCR or NS1 protein ELISA from disease onset for up to 9 days. During the viremic phase infected people may transmit DENV to a susceptible host via the bite of the appropriate mosquito vector, after an extrinsic incubation period of 8–12 days following the blood meal during which the virus replicates within the mosquito and is amplified in the salivary glands.
Immune responses
Immunity to DENV infection is thought to be lifelong, and DENV-specific neutralising antibody responses have been detected more than 60 years after infection9,10. Infection with one serotype does not protect against infection with the other three serotypes, and indeed epidemiological observations indicate secondary infection with heterologous DENV serotypes is associated with increased probability of severe dengue11; 80–90% of severe dengue cases occur in individuals experiencing secondary infection with heterologous DENV.
DENV genomes and correlates of epidemic virulence
The dengue viruses are RNA viruses which form their own antigenic complex within the Flaviviridae family. Like many other RNA viruses DENV undergo high rates of mutation, and comparison of DENV sequence data allows analysis of evolutionary relationships and epidemiological linkages. The four serotypes, DENV-1–DENV-4, share amino acid sequence homology of approximately 70% and are further classified into genetically distinct genotypes, and lineages within the genotypes, based on phylogenetic analysis of the E gene or whole genomes. DENV-1 and DENV-2 can be divided into five and six genotypes, respectively, and DENV-3 and DENV-4 into four genotypes, including the sylvatic lineages found in non-human primates. DENV epidemic virulence has been linked to introduction and transmission of specific serotypes, genotypes and lineages. Introduction of a Southeast Asian genotype of DENV-2 into the Caribbean resulted in the first epidemic of severe dengue in the region, with over 10 000 cases of dengue haemorrhagic fever (DHF) and 158 deaths12. The Southeast Asian genotype replaced the American genotype which had circulated for some time and was not associated with severe disease. Similarly, introduction of a lineage of DENV-3 genotype III, a variant associated with DHF in India and East Africa, into Sri Lanka coincided with emergence of DHF in 198913. In contrast, an outbreak of severe dengue in the Solomon Islands in 2013 that resulted in hospitalisations and deaths was caused by a lineage of DENV-3 circulating in Madang on the northern coast of Papua New Guinea (PNG) in 2007–200814. Unlike the previous instances in Cuba and Sri Lanka where the introduced virus was associated with dengue haemorrhagic fever, severe disease was not identified among patients in Madang. Dengue virulence has been associated with viral and host factors.
Dengue in Australia
Dengue was first reported in Australia in 1873, with a report of 8 cases in Melbourne in May 1873 on board the Charles Auguste, a ship originating in Mauritius15. Local cases were prevalent in northern Queensland in Townsville in 1879 and Rockhampton in 1885, and epidemics in Queensland, northern NSW, Northern Territory and Western Australia occurred up until the 1940s16. Dengue activity in Australia since the first reports at the end of the 19th century has been linked to distribution of its major vector Ae. aegypti, a highly efficient vector that preferentially feeds on human blood and is capable of biting several people in a short period for one blood meal. It is highly adapted to urban environments, living in intimate association with humans. The species was widely distributed in the north-east coastal areas of Australia at the end of the 19th century and spread along road and rail links with the movement of people17. Disappearance of Ae. aegypti from New South Wales, Western Australia and the Northern Territory in the 1960s, and a decline in distribution in Queensland in the 1960s and 1970s followed changes in water storage practices and the introduction of scheme water supply18. Established populations of Ae. albopictus, the other major dengue vector, were first recognised in Australia when they were discovered in the Torres Strait in 200519, and may have served as a vector in a DENV-2 outbreaks in Torres Strait in 201620. Ae. Albopictus is a less efficient DENV vector than Ae. aegypti21 and under most conditions would be unlikely to be responsible for large-scale dengue outbreaks. This species is not known to be present in mainland Australia.
Introduction of DENV in travellers
Dengue reappeared in northern Queensland in 1981 after an absence of 26 years with an outbreak of DENV-122. A significant increase in the number and frequency of outbreaks was observed after the international airport was opened in Cairns in 198423, linked to DENV importation by international visitors and returning residents. Major epidemics occurred, for example introduction of DENV-2 in 1992 resulted in an outbreak that lasted for 64 weeks, with >1000 cases of DENV-224.
Travellers from countries where dengue is not transmitted and who do not have pre-existing immunity are at significant risk of DENV infection in endemic countries. The frequency of dengue diagnoses in febrile returned travellers increased from 2% in the early 1990s to 16% by 200525. Many countries in the Asia-Pacific region are popular tourist destinations and national notification data show that dengue is the most significant introduced mosquito-borne disease in Australia26 (Figure 2). Severe dengue has been identified in Australia, however a systematic analysis of incidence among imported and local cases has not been undertaken.
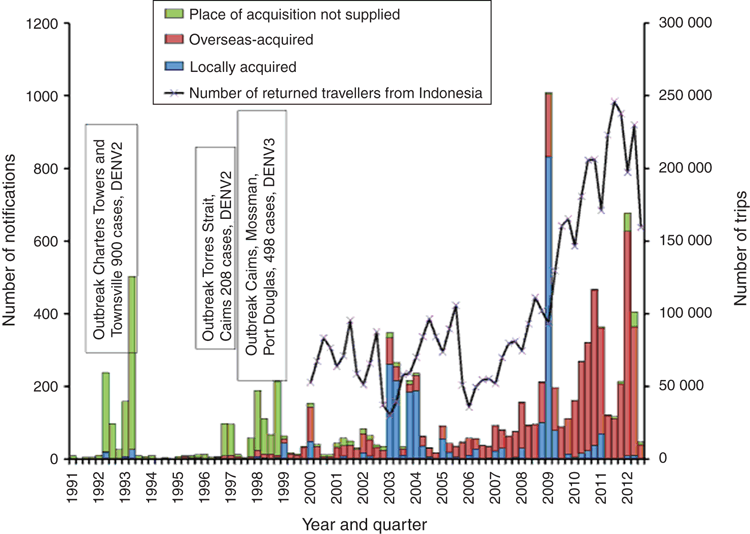
|
Between 2002–2010 DENV infections notified in Queensland were most commonly acquired in Southeast Asia – predominantly Indonesia, Thailand and the Philippines – followed by Papua New Guinea and other Pacific Island nations27. Imported DENV was identified as the source of outbreaks of DENV-2 in 2003–200428 and DENV-3 in 2008–200929. The DENV-2 outbreaks that began in late February 2003 in Cairns were likely imported by a PNG national who developed a febrile illness in late January soon after arriving from PNG. DENV E gene sequencing showed the Cairns DENV-2 was similar to a strain isolated from an outbreak that began in Townsville in May 2003, as was DENV-2, isolated from a second outbreak that began in Townsville in October 2003. The Townsville and Cairns viruses were very similar to an imported strain isolated from a traveller from PNG into Townsville in April 2003, and all three viruses represented the same lineage within the Cosmopolitan genotype of DENV-2, indicating that PNG was the source of the February, May and October outbreaks in Cairns and Townsville. PNG was also the likely source of a distinct lineage of DENV-2, Cosmopolitan genotype, that was isolated from a resident of Yam Island in the Torres Strait during an outbreak that began in early November 2003. PNG nationals had attended a funeral ceremony on the island in mid-September. The Yam Island virus showed 100% homology with DENV-2 isolated during an outbreak that began in Cairns in February 2004, suggesting that the Cairns virus belonged to a lineage that originated in PNG. A different strain of DENV-2, belonging to the Asian I genotype, was isolated from a small outbreak that began in Cairns in later October 2003; this virus was very similar to one isolated from an outbreak in Kuranda, near Cairns, in 2002 and both strains were very similar to DENV imported from Thailand in 2001. Genetic analysis thus illustrated separate introductions into northern Queensland of distinct lineages of DENV-2 that circulated in different countries in the region between 2002 and 2003. A clear linkage between DENV importation by travellers and local transmission was also shown for DENV-3: an explosive outbreak in Cairns from November 2008 to May 2009 was likely initiated by a traveller infected in Kalimantan, Indonesia. The epidemic virus was distinct from DENV-3 isolated earlier in Townsville in 2006 and in Cairns in 1998.
Although dengue has not been present in Western Australia since the 1940s, the state notifies the most dengue cases among the 8 Australian States and territories despite having only 10% of the Australian population and no sustained endogenous transmission, due to the absence of the mosquito vectors26. In recent years new budget airlines began offering affordable package holidays and travel increased 300-fold between 2006 and 2010, coinciding with a sharp increase in dengue cases notified to the Communicable Diseases Division of the WA Department of Heath30. Analysis of DENV derived from febrile travellers entering WA after visiting seven countries throughout Asia between 2010–2012 identified a diverse range of DENV genotypes and lineages within all four DENV serotypes31 (Figure 3). Most of the travellers had entered from Bali, a popular holiday destination for residents of WA. Many of the imported DENV were local regional variants with strong epidemic potential known to have circulated in the region, particularly in Indonesia and Singapore, for some time. Other DENV were more recently introduced into Bali from other countries in the region. A new lineage of DENV-2 that appeared to be associated with a major outbreak in Bali in 2012 was also identified. Importation of this lineage has continued up to 2017. The data from this study suggest that Bali is a melting pot of substantial DENV diversity and serves as a hub for DENV transmission and mixing.
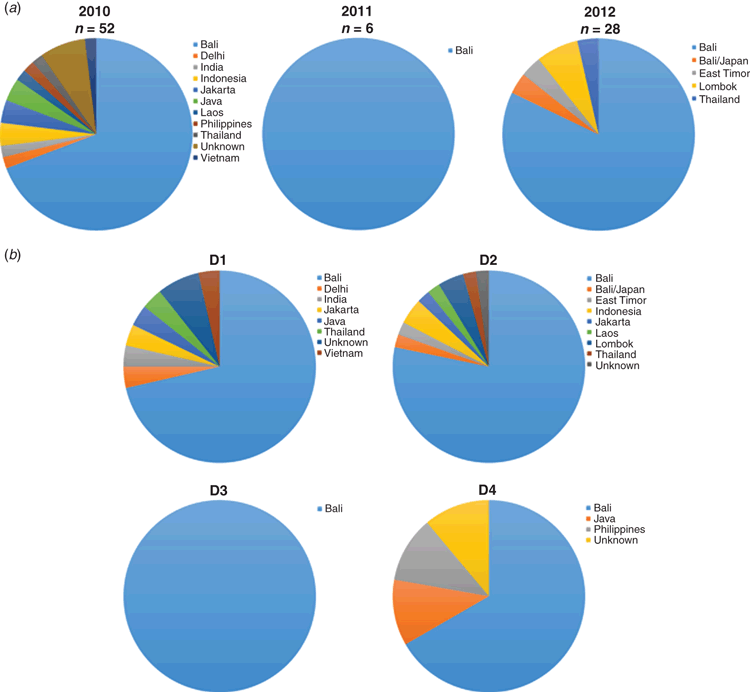
|
The dengue vectors were present in WA up until the end of the 1960s and Ae. aegypti was recorded as far south as Harvey, approximately 150 km south of Perth18. Changes in water storage and supply practices led to complete elimination of Ae. aegypti, and no native mosquito species are known to have the potential to transmit DENV. In October 2013 the first case of locally acquired dengue fever occurred in a male with no history of travel outside WA for many years and who was likely exposed in the Pilbara region in north-west WA32. The case may have been exposed to infected mosquitoes imported on international cargo vessels docked at local iron ore export ports or via direct international flights into Port Hedland or Perth airports. Passive transportation of mosquitoes by both air and sea has been demonstrated in previous studies in Southeast Asia and has been suggested as a mechanism for global spread of DENV33,34. Between February 2014 and March 2016 Ae. aegypti was frequently detected at Australian international airports including Perth, Melbourne, Adelaide, Brisbane, Sydney and Darwin35,36. The most regular detections occurred at Perth International Airport32 but there is no evidence that Ae. aegypti has become established.
Conclusion
Surveillance of DENV imported by travellers provides information on origin and movement of DENV in the region and in countries where locally generated detailed genetic data may not be available. Ongoing public health surveillance and rapid response measures are necessary to detect incursion of Ae. aegypti and Ae. Albopictus and to prevent re-introduction of dengue into states where the disease has not been present for many decades.
References
[1] Mayer, S.V. et al. (2017) The emergence of arthropod-borne viral diseases: a global perspective on dengue, chikungunya and Zika fevers. Acta Trop. 166, 155–163.| The emergence of arthropod-borne viral diseases: a global perspective on dengue, chikungunya and Zika fevers.Crossref | GoogleScholarGoogle Scholar |
[2] Bhatt, S. et al. (2013) The global distribution and burden of dengue. Nature 496, 504–507.
| The global distribution and burden of dengue.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXltlOnsLs%3D&md5=f1a956c26f805a51a250b69f319221c3CAS |
[3] World Health Organization (2018) Emerging disease surveillance and response: dengue in the Western Pacific Region. http://www.wpro.who.int/emerging_diseases/Dengue/en/ (accessed February 2018).
[4] La Ruche, G. et al. (2010) First two autochthonous dengue virus infections in metropolitan France, September 2010. Euro Surveill. 15, 19676.
| 1:STN:280:DC%2BC3cfnvFKjsA%3D%3D&md5=03dad8d4becaf91449d72686e0c74748CAS |
[5] Murray, K.O. et al. (2013) Identification of dengue fever cases in Houston, Texas, with evidence of autochthonous transmission between 2003 and 2005. Vector Borne Zoonotic Dis. 13, 835–845.
| Identification of dengue fever cases in Houston, Texas, with evidence of autochthonous transmission between 2003 and 2005.Crossref | GoogleScholarGoogle Scholar |
[6] Kutsuna, S. et al. (2015) Autochthonous dengue fever, Tokyo, Japan, 2014. Emerg. Infect. Dis. 21, 517–520.
| Autochthonous dengue fever, Tokyo, Japan, 2014.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC28XitVOisbfP&md5=489de7a19b92c06d2aa4b965c9764865CAS |
[7] World Health Organization (2015) Dengue: fact sheet no. 117. http://www.who.int/mediacentre/factsheets/fs117/en/ (accessed February 2018).
[8] World Health Organization (2009) Dengue: guidelines for diagnosis, treatment, prevention and control. New edition. Geneva: WHO. http://www.who.int/tdr/publications/documents/dengue-diagnosis.pdf?ua=1 (accessed February 2018).
[9] Tal, A.Y.C. et al. (2017) Management of dengue in Australian travellers: a retrospective multicentre analysis. Med. J. Aust. 206, 295–300.
| Management of dengue in Australian travellers: a retrospective multicentre analysis.Crossref | GoogleScholarGoogle Scholar |
[10] Imrie, A. et al. (2007) Antibody to dengue 1 detected more than 60 years after infection. Viral Immunol. 20, 672–675.
| Antibody to dengue 1 detected more than 60 years after infection.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXhsVKitb%2FI&md5=bd018ca6c49a184f0386d15470e40a47CAS |
[11] Burke, D.S. et al. (1988) A prospective study of dengue infections in Bangkok. Am. J. Trop. Med. Hyg. 38, 172–180.
| A prospective study of dengue infections in Bangkok.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaL1c7jtFClsw%3D%3D&md5=6b7b719b9c396f9ef4da51db66f2f9f8CAS |
[12] Rico-Hesse, R. et al. (1997) Origins of dengue type 2 viruses associated with increased pathogenicity in the Americas. Virology 230, 244–251.
| Origins of dengue type 2 viruses associated with increased pathogenicity in the Americas.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2sXivVGrsr4%3D&md5=96720e2137986c40b98dc6b5c44da925CAS |
[13] Messer, W.B. et al. (2003) Emergence and global spread of a dengue serotype 3, subtype III virus. Emerg. Infect. Dis. 9, 800–809.
| Emergence and global spread of a dengue serotype 3, subtype III virus.Crossref | GoogleScholarGoogle Scholar |
[14] Luang-Suarkia, D. et al. (2018) Hyperendemic dengue transmission and identification of a locally evolved DENV-3 lineage, Papua New Guinea 2007–2010. PLoS Negl. Trop. Dis. , .
| Hyperendemic dengue transmission and identification of a locally evolved DENV-3 lineage, Papua New Guinea 2007–2010.Crossref | GoogleScholarGoogle Scholar |
[15] Medical Society of Victoria (1873) Local topics. Aust. Med. J. May, 160. https://digitised-collections.unimelb.edu.au/bitstream/handle/11343/23137/267571_UDS2010779-209.pdf?sequence=5&isAllowed=y (accessed February 2018).
[16] Lumley, G.F. (1942) Dengue. Part 1. Medical. In: Lumley GF, Taylor FH (eds). Dengue. Service Publication (School of Public Health and Tropical Medicine) No.3 Sydney, University of Sydney and Commonwealth Department of Health: 9–142.
[17] Mackenzie, J.S. et al. (1996) Dengue in Australia. J. Med. Microbiol. 45, 159–161.
| Dengue in Australia.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK28vgs1ymsQ%3D%3D&md5=25b5d4a81113e72fe59d7d8971acd926CAS |
[18] Beebe, N.W. et al. (2009) Australia’s dengue risk driven by human adaptation to climate change. PLoS Negl. Trop. Dis. 3, e429.
| Australia’s dengue risk driven by human adaptation to climate change.Crossref | GoogleScholarGoogle Scholar |
[19] Ritchie, S.A. et al. (2006) Discovery of a widespread infestation of Aedes albopictus in the Torres Strait, Australia. J. Am. Mosq. Control Assoc. 22, 358–365.
| Discovery of a widespread infestation of Aedes albopictus in the Torres Strait, Australia.Crossref | GoogleScholarGoogle Scholar |
[20] Moore, P.R. et al. (2007) Infection and dissemination of dengue virus type 2 in Aedes aegypti, Aedes albopictus, and Aedes scutellaris from Torres Strait, Australia. J. Am. Mosq. Control Assoc. 23, 383–388.
| Infection and dissemination of dengue virus type 2 in Aedes aegypti, Aedes albopictus, and Aedes scutellaris from Torres Strait, Australia.Crossref | GoogleScholarGoogle Scholar |
[21] Lambrechts, L. et al. (2010) Consequences of expanding global distribution of Aedes albopictus for dengue virus transmission. PLoS Negl. Trop. Dis. 4, e646.
| Consequences of expanding global distribution of Aedes albopictus for dengue virus transmission.Crossref | GoogleScholarGoogle Scholar |
[22] Kay, B.H. et al. (1984) Dengue fever. Reappearance in northern Queensland after 26 years. Med. J. Aust. 140, 264–268.
| 1:STN:280:DyaL2c7ktFCgug%3D%3D&md5=63bbda8f6992dd1367cd6fc64a45b71bCAS |
[23] Huang, X. et al. (2013) Imported dengue cases, weather variation and autochthonous dengue incidence in Cairns, Australia. PLoS One 8, e81887.
| Imported dengue cases, weather variation and autochthonous dengue incidence in Cairns, Australia.Crossref | GoogleScholarGoogle Scholar |
[24] Hanna, J.N. and Ritchie, S.A. (2009) Outbreaks of dengue in north Queensland, 1990–2008. Commun. Dis. Intell. 33, 32–33.
[25] Wilder-Smith, A. and Schwartz, E. (2005) Dengue in travelers. N. Engl. J. Med. 353, 924–932.
| Dengue in travelers.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXpsFChur0%3D&md5=6f08f4eeb1a1f5148e83a32ec689868fCAS |
[26] Knope, K. et al. (2013) Increasing notifications of dengue in Australia related to overseas travel, 1991 to 2012. Commun. Dis. Intell. 37, E55–E59.
[27] Warrilow, D. et al. (2012) Sources of dengue viruses imported into Queensland, Australia, 2002-2010. Emerg. Infect. Dis. 18, 1850–1857.
| Sources of dengue viruses imported into Queensland, Australia, 2002-2010.Crossref | GoogleScholarGoogle Scholar |
[28] Hanna, J.N. et al. (2006) Multiple outbreaks of dengue serotype 2 in north Queensland, 2003/2004. Aust. N. Z. J. Public Health 30, 220–225.
| Multiple outbreaks of dengue serotype 2 in north Queensland, 2003/2004.Crossref | GoogleScholarGoogle Scholar |
[29] Ritchie, S.A. et al. (2013) An explosive epidemic of DENV-3 in Cairns, Australia. PLoS One 8, e68137.
| An explosive epidemic of DENV-3 in Cairns, Australia.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXht1Shu7fJ&md5=634d2fead9e8a25d1356894f7f8ed42eCAS |
[30] Department of Health Western Australia (2012) Disease watch. Bali travel behind spike in dengue fever. http://www.health.wa.gov.au/diseasewatch/vol16_issue5/bali_travel.cfm (accessed February 2018).
[31] Ernst, T. et al. (2015) Emergence of a new lineage of dengue virus type 2 identified in travellers entering Western Australia from Indonesia, 2010–2012. PLoS Negl. Trop. Dis. 9, e0003442.
| Emergence of a new lineage of dengue virus type 2 identified in travellers entering Western Australia from Indonesia, 2010–2012.Crossref | GoogleScholarGoogle Scholar |
[32] Lindsay, M.D.A. et al. (2015) Investigation of the first case of dengue virus infection acquired in Western Australia in seven decades: evidence of importation of infected mosquitoes? PLoS Negl. Trop. Dis. 9, e0004114.
| Investigation of the first case of dengue virus infection acquired in Western Australia in seven decades: evidence of importation of infected mosquitoes?Crossref | GoogleScholarGoogle Scholar |
[33] Russell, R.C. (1987) Survival of insects in the wheel bays of a Boeing 747B aircraft on flights between tropical and temperate airports. Bull. World Health Organ. 65, 659–662.
| 1:STN:280:DyaL1c7gvFyjtA%3D%3D&md5=253b9f6c0f58058de9e3478a6fe22741CAS |
[34] Fonzi, E. et al. (2015) Human-mediated marine dispersal influences the population structure of Aedes aegypti in the Philippine archipalego. PLoS Negl. Trop. Dis. 9, e0003829.
| Human-mediated marine dispersal influences the population structure of Aedes aegypti in the Philippine archipalego.Crossref | GoogleScholarGoogle Scholar |
[35] Pettit, W. et al. (2016) A series of exotic mosquito detections at Darwin International Airport, Northern Territory between February 2015 and January 2016. The Northern Territory Disease Control Bulletin Vol. 23, no. 4, December.
[36] Australian Government Department of Health August (2017) Response guide for exotic mosquito detections at Australian first ports of entry. http://www.health.gov.au/internet/main/publishing.nsf/Content/E4A2B36B23CBBF64CA257F6A001B058B/$File/Exotic-Mosquito-Detections-Australian-Borders.pdf (accessed February 2018).
Biography
Associate Professor Allison Imrie is a teaching and research academic in the School of Biomedical Sciences within the Faculty of Health and Medical Sciences at the University of Western Australia, and a Research Scientist at PathWest Laboratory Medicine WA. Her current research interests focus on arboviruses, anti-viral immunity and viral genetic epidemiology, influenza and other respiratory viruses, discovery, and laboratory-based surveillance of infectious diseases. She has previously worked with HIV and AIDS.


