Changing epidemiology of invasive meningococcal disease in Australia 1994–2016
Helen V Smith A B and Amy V Jennison A CA Public Health Microbiology, Forensic and Scientific Services
Queensland Department of Health
Coopers Plains, Brisbane
Qld 4108, Australia
Tel: +61 7 3096 2825
B Tel: +61 7 3096 2826
Email: Helen.Smith4@health.qld.gov.au
C Email: Amy.Jennison@health.qld.gov.au
Microbiology Australia 38(4) 184-186 https://doi.org/10.1071/MA17064
Published: 9 November 2017
Invasive meningococcal disease (IMD) has a relatively low incidence in Australia, however remains a serious public health issue, with a case fatality rate of approximately 10% despite antimicrobial treatment. IMD is particularly seen in young children, but can affect all age groups. The disease has non-specific early symptoms, rapid clinical progression mainly manifesting as septicaemia and/or meningitis, and has the potential for long term sequelae in the survivors, including skin scarring, amputation, deafness and seizures. There are 13 serogroups, although most invasive infections worldwide are caused by serogroups A, B, C, W, and Y, with some recent outbreaks in Africa caused by serogroup X. The prevalent circulating serogroups can undergo dynamic shifts, generating dramatic changes in IMD epidemiology. Such serogroup shifts have important ramifications for vaccination programs and constant surveillance is crucial.
The National Neisseria Network (NNN) established the Australian Meningococcal Surveillance Program (AMSP) in 1994, after a number of serogroup C (MenC) outbreaks in the early 1990s highlighted the need for ongoing coordinated surveillance. The NNN consists of a network of reference laboratories in each state and territory that collaborate to provide a national laboratory-based program for the examination of N. meningitidis from cases of invasive meningococcal disease (IMD)1.
Laboratory definitive evidence of IMD, according to the Communicable Diseases Network Australia criteria, is defined as isolation of N. meningitidis, or a positive nucleic acid amplification testing (NAAT) from a normally sterile site2. Since 1997, the incidence of molecular only diagnosis have been increasing and from 2000–2016 represented 35.2% (range 15.7–42.3%) of all diagnoses, although this varies by jurisdiction.
The NNN provides data derived from a range of phenotypic and genotypic techniques, including antibiotic resistance, serogrouping and antigen sequence based fine typing. Recently, laboratories have also utilised whole genome sequencing (WGS) for both in silico generation of fine-typing and phylogenetic cluster analysis. WGS-based analysis has already proved valuable in localised outbreaks and in understanding the changing epidemiology of IMD in Australia over the recent years3.
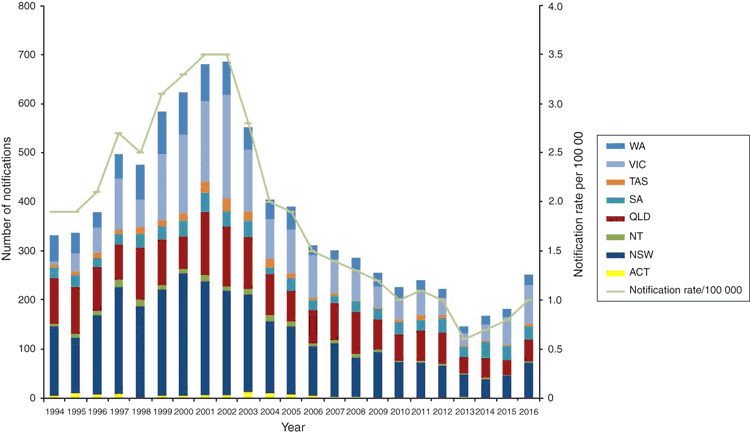
The NNN data supplements the clinical notification data from the National Notifiable Diseases Surveillance System (NNDSS), which includes cases of probable IMD (based on clinical or epidemiological grounds) as well as laboratory confirmed IMD by state4 (see Figure 1). The NNN also publishes annual IMD Laboratory Surveillance reports in Communicable Diseases Intelligence.
Australia has experienced a number of serogroup shifts since the NNN began national surveillance in 1994. This is of particular importance as vaccines for N. meningitidis are serogroup specific. The AMSP national results in 1995–96 showed that serogroup B (MenB) was the predominant serogroup causing sporadic meningococcal disease in all jurisdictions6. In 1997 New South Wales saw an increase in Men C:2a:P1.5 disease with 66% of the Australian serogroup C strains that year isolated in New South Wales. Victoria, in 1999, noted a number of infections with a novel phenotype, C:2a:P1.47, which contributed 53% of Victorian invasive cases in 2000, in contrast to 1998 when only 17.5% were MenC. In 2002 there was the highest number of notifications of IMD (688 cases: rate of 3.5 per 100 000) seen since the NNDSS began in 1991, with 79% of all MenC IMD cases seen in New South Wales and Victoria8. The national introduction of the conjugate Men C vaccine in 2003 resulted in the significant and sustained reduction in serogroup C disease (225 notifications in 2002 to 3 notifications in 2016)9.
From 2002 to 2015, the predominant serogroup seen in Australia was serogroup B, although notifications of serogroup B IMD decreased over this time period, alleviating fears of serogroup replacement with the introduction of the serogroup C vaccine. The lowest notification rate for IMD (0.6 cases per 100 000 population) was seen in Australia in 2013 potentially due to the overall decrease in MenB cases10. A recombinant multi-component serogroup B vaccine (Bexsero®) has been licenced for use in Australia since 2014. It is not part of the National Immunisation Program but is available for private purchase. The availability of the vaccine is unlikely to explain the decrease in MenB disease over the extended time frame.
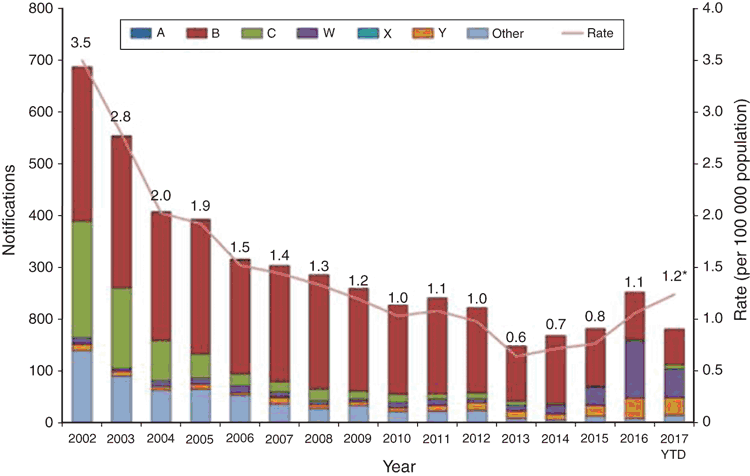
There has been a steady increase in the rate of IMD since the record low of 0.6 cases per 100 000 in 2013 to 1.0 cases per 100 000 seen in 2016, which can be attributed to a significant increase in MenW and MenY cases. Initially, the increase in MenW cases was seen in Victoria, with MenW representing in 2013 only 4% of cases to 30% of their cases in 2015. The majority of these strains when molecularly typed were determined to be the fine type MenW:P1.5,2:F1-1: ST11, a hypervirulent lineage shown by WGS to be related to the MenW ST11 strains from the United Kingdom and South America and genetically distant from the MenW strains involved in the Hajj outbreak11. In 2016 MenW became the predominant serogroup in Australia with 109 cases (43.2% of the IMD notifications to the NNDSS) (see Figure 2). Of further concern, is the rise of penicillin resistance (0.5–1 mg/L ) seen in these MenW:P1.5,2:F1-1: ST11 isolates. Work done in Western Australia demonstrated that the penA_253 allele has a major role in the increasing penicillin resistance and this has also been seen in Europe13. Currently penicillin is the standard recommended empiric treatment in IMD14 even though in 2015 before the large increase in MenW, 86% IMD isolates showed a decreased susceptibility to penicillin so continued surveillance and clinical evaluation is required15. These empiric treatment guidelines are currently being re-evaluated.
|
Traditionally in Australia, MenB occurrence has exhibited a bimodal age distribution, with most infections in the 0–4 and 15–24 year-old age groups. While MenW cases are also seen in these age groups, there have been a higher proportion of cases seen in those aged 45 and greater. Of note in the MenW infections, is the number of atypical clinical presentations (around 20%) seen. These include septic arthritis, pneumonia, epiglottitis and gastrointestinal symptoms16. Higher case fatality rates have also been reported.
There has also been an increase in serogroup Y (MenY) cases around Australia, the majority of which are genotype Y:P1.5-1,10-1:F4-1:ST-23. These have occurred across a range of age groups whereas in the past MenY IMD was more usually confined to persons over 50 years17.
Australia is geographically large and differences in epidemiology of meningococcal serogroups are seen across the jurisdictions. South Australia has had a predominance of Men B:P1.7-2,4:F1-5, a genotype responsible for the New Zealand 20 year+ epidemic (late 1990–2015), while the eastern states and Western Australia have experienced the increase in MenW incidence. Unlike the MenC vaccine implementation in 2003, which was coordinated nationally, the rise of MenW in particular jurisdictions has resulted in five states (New South Wales, Queensland, Tasmania, Victoria and Western Australia) announcing individual state based meningococcal ACWY vaccination programs commencing during 2017. The campaigns will primarily target 15–19 year olds (NSW 17–18) with different implementation timeframes among the states18.
The Commonwealth Department of Health, in collaboration with the states and territories, is closely monitoring the incidence of MenW in Australia and has established a Communicable Diseases Network of Australia working group. The Department of Health has also funded the WGS of all Australian invasive meningococcal isolates by a number of state reference laboratories (all members of the NNN), and in collaboration with NNN, are continuing to monitor the change in meningococcal epidemiology and any public health implications.
It is clear that due to the ever-changing epidemiology seen in Australia and worldwide, it is essential to continue the enhanced surveillance of IMD in a collaboration of the NNN, epidemiologists and state and Commonwealth Departments of Health. This will encompass molecular typing – now aided by genomics, epidemiological surveillance of the NNDSS data, along with monitoring the effects of the implementations of the four valent vaccination program, well into the foreseeable future to ensure the continued health of Australians.
References
[1] National Neisseria Network (1995) Meningococcal isolate surveillance Australia, 1994. Commun. Dis. Intell. 19, 286–289.[2] Public Health Laboratory Network. (2006) Meningococcal laboratory case definition. http://www.health.gov.au/internet/main/publishing.nsf/Content/cda-phlncd-mening.htm (accessed 2015).
[3] Martin, N.V. et al. (2016) Rise in invasive serogroup W meningococcal disease in Australia 2013–2015. Commun. Dis. Intell. Q. Rep. 40, E454–E459.
[4] http://www.health.gov.au/internet/main/publishing.nsf/content/cda-surveil-nndss-casedefs-cd_mening.htm
[5] National Notifiable Diseases Surveillance System. (2017) http://www9.health.gov.au/cda/source/cda-index.cfm (accessed 10 September 2017).
[6] Tapsall, J. and members of the National Neisseria Network of Australia (1996) Meningococcal isolate surveillance, Australia 1995. Commun. Dis. Intell. 20, 422–424.
[7] Tapsall, J. and members of the National Neisseria Network of Australia (2000) Annual Report of the Australian Meningococcal Surveillance Programme, 1999. Commun. Dis. Intell. 24, 181–189.
[8] Tribe, D.E. et al. (2002) Increase in meningococcal disease associated with the emergence of a novel ST-11 variant of serogroup C Neisseria meningitidis in Victoria, Australia, 1999–2000. Epidemiol. Infect. 128, 7–14.
| Increase in meningococcal disease associated with the emergence of a novel ST-11 variant of serogroup C Neisseria meningitidis in Victoria, Australia, 1999–2000.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD387mtlyhsA%3D%3D&md5=ff3b72445485549b9bfe6712537c7998CAS |
[9] Australian Government Department of Health. (2017) Invasive Meningococcal Disease National Surveillance Report. 14 August 2017. http://www.health.gov.au/internet/main/publishing.nsf/Content/5FEABC4B495BDEC1CA25807D001327FA/$File/14-Aug-2017-IMD-Surveillance-report.pdf (accessed 22 August 2017).
[10] Lahra, M.M. and Enriquez, R.P. (2014) Australian Meningococcal Surveillance Programme Annual Report 2013 Commun. Dis. Intell. 38, E301–E308.
[11] Carville, K.S. et al. (2016) Increase in meningococcal serogroup W disease, Victoria, Australia 2013–2015 Emerg. Infect. Dis. 22, 1785–1787.
| Increase in meningococcal serogroup W disease, Victoria, Australia 2013–2015Crossref | GoogleScholarGoogle Scholar |
[12] Australian Government Department of Health. (2017) Meningococcal W disease. http://www.health.gov.au/internet/main/publishing.nsf/Content/ohp-meningococcal-W.htm (accessed 12 September 2017).
[13] Mowlaboccus, S. et al. (2017) Clonal expansion of new penicillin-resistant clade of Neisseria meningitidis serogroup W clonal complex, Australia Emerg. Infect. Dis. 23, 1364–1367.
| Clonal expansion of new penicillin-resistant clade of Neisseria meningitidis serogroup W clonal complex, AustraliaCrossref | GoogleScholarGoogle Scholar |
[14] Guidelines: severe sepsis. https://tgldcdp.tg.org.au/viewTopic?topicfile=severe-sepsis#toc_d1e1486
[15] Lahra, M.M. and Enriquez, R.P. (2016) Australian Meningococcal Surveillance Programme Annual Report 2015 Commun. Dis. Intell. 40, E503–E511.
[16] Campbell, H. et al. (2016) Presentation with gastrointestinal symptoms and high case fatality associated with group W meningococcal disease (MenW) in teenagers, England July 2015 to January 2016 Euro Surveill. 21, .
| Presentation with gastrointestinal symptoms and high case fatality associated with group W meningococcal disease (MenW) in teenagers, England July 2015 to January 2016Crossref | GoogleScholarGoogle Scholar |
[17] Lahra, M.M. and Enriquez, R.P. (2014) Australian Meningococcal Surveillance Programme Annual Report 2013 Commun. Dis. Intell. 38, E301–E308.
[18] Australian Government Department of Health. (2017) Meningococcal W disease. http://www.health.gov.au/internet/main/publishing.nsf/Content/ohp-meningococcal-W.htm (accessed 22 August 2017).
Biographies
Helen Smith is a public health microbiologist and molecular epidemiologist with 31 years working for Queensland Health. She is the Supervising Scientist of the Reference Microbiology, Public Health Microbiology Forensic and Scientific Services, which is the Queensland state reference laboratory for all bacterial notifiable pathogens (except TB). Helen’s particular interests include vaccine-preventable diseases, enteric diseases, outbreak investigations, emerging bacterial diseases and the use of molecular techniques to investigate outbreaks.
The biography for Amy Jennison is on page 171.