Sporotrichosis: an Australian perspective of a global infection
Ian Arthur A C , Michael Leung A and Elin Westergaard BA Department of Microbiology, PathWest Laboratory Medicine, QEII Medical Centre, Nedlands, WA, Australia
B School of Pathology and Laboratory Medicine, The University of Western Australia, Crawley, WA, Australia
C Corresponding author. Email: Ian.Arthur@health.wa.gov.au
Microbiology Australia 36(2) 90-92 https://doi.org/10.1071/MA15029
Published: 19 March 2015
Sporotrichosis is a fungal infection caused by Sporothrix schenckii sensu lato usually acquired after a penetrating injury with contaminated material1,2. The infection may establish at the site of the injury, potentially disseminate along the lymphatics, or rarely cause systemic infections including occasional primary pulmonary sporotrichosis3. New knowledge of the organism reveals a diverse infection with regard to its epidemiology, geographical distribution, and species characteristics.
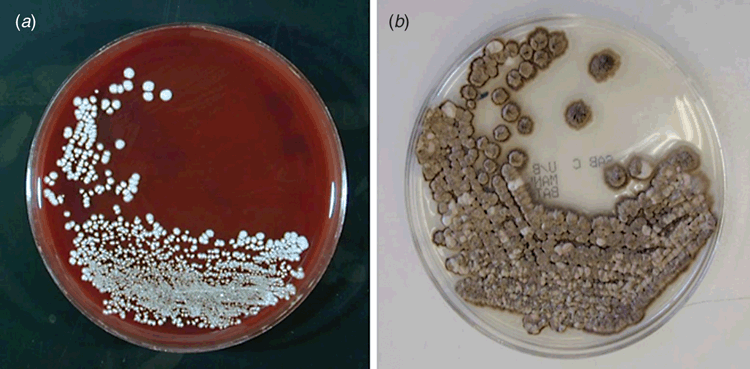
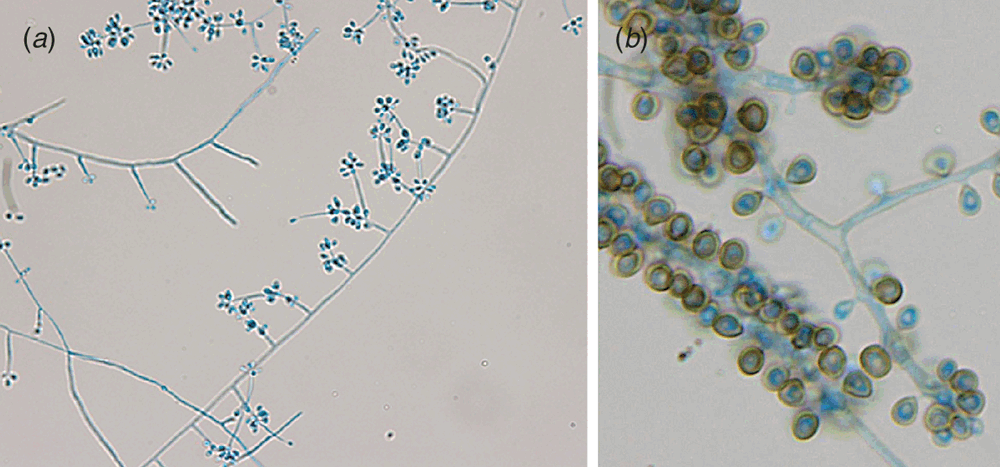
S. schenckii sensu lato is a thermally dimorphic fungus characterised by its ability to grow as a yeast-like organism at 35°C (i.e. at body temperatures, Figure 1a). However at temperatures <30°C it grows as a mould (Figure 1b) demonstrating both clavate/subglobose conidia on denticles arranged in clusters on a short conidiophore (Figure 2a), and larger pigmented sessile conidia which may proliferate with age to appear like sleeves along the hyphae (Figure 2b).
|
|
Despite its dimorphic characteristics, 18S rDNA sequencing indicates S. schenckii sensu lato is related to the environmental saprophyte Ophiostma stenoceras rather than other clinically relevant dimorphic fungi1. In the environment S. schenckii sensu lato is mostly associated with a variety of dead organic substrates, with growth accelerated by warmth and humidity. The environmental mycelia produce abundant conidia that may establish areas of endemicity, but when implanted into the body may cause infection in a variety of warm blooded animals. Zoonotic transmission has been recorded amongst armadillo hunters and from other animals, but cat-to-cat and cat-human transmission is notable2.
Patients with sporotrichosis outside of endemic areas may go undiagnosed having undergone unsuccessful treatment with antibacterial agents2. Despite the organism growing on a wide range of laboratory media, diagnosis may be hampered by the lack of extended incubation of microbiological cultures or a lack of recognition of the organism particularly if the yeast phase is disregarded as a non-albicans Candida.
At least as early as 1979, differences in the virulence and morphology of clinical isolates were noted4. Now supported by epidemiological, morphological and molecular data, the species are considered members of a species complex with the main clinically relevant species being S. schenckii sensu stricto, S. brasiliensis, S. globosa and S. luriei.
Outbreaks have been reported in geographical localities on all continents other than Europe usually associated with specific organic substrates (e.g. hay/Australia, wood/ South Africa, sphagnum moss/USA, etc.)1. Any age/gender bias is predominantly driven by the source of transmission (e.g. male miners in South Africa acquiring the infection from contaminated timbers).
The exception to this global pattern occurs in Brazil where both S. schenckii sensu stricto and S. brasiliensis have been recorded. S. schenckii sensu stricto infection follows the classical sporotrichosis epidemiology across different regions of the country5. However, since the early 1990s S. brasiliensis has been the agent of an expanding outbreak radiating out from Rio de Janeiro and affecting many thousands of people. Remarkably this outbreak is primarily an urban zoonosis perpetuated by feline sporotrichosis. Cats not only carry the organism but they are also susceptible to infection which is transmitted via scratches from infected animals or from the environment. Given the low level of available medical and veterinary care in these poor socio-economic areas, it seems inevitable the infection will continue to spread across an increasing geographical area affecting many more individuals.
Consistent with epidemiological pattern of these species, it has been demonstrated in a separate report that S. brasiliensis is the most virulent of the species in a murine model followed by S. schenckii sensu stricto and then S. globosa, while S. mexicana and S. albicans show little or no virulence in this model of infection6. Thus expanding knowledge of the different species within the species complex gives understanding of the different epidemiology in different geographical areas, but may also indicate somewhat different susceptibility profiles of the organism5,7.
Sporotrichosis in Australia
The infection has variously been reported in areas of eastern Australia. In a review of sporotrichosis from NSW, Sivagnanam et al. described 31 cases between 2000–2010 around the Port Macquarie area8. Sporotrichosis has also been reported from Queensland including a case cluster in 1998 of 16 patients reported in the Darling Downs district of south-east Queensland9. In an earlier review 37 cases were described from the Royal Brisbane Hospital between 1965–197710.
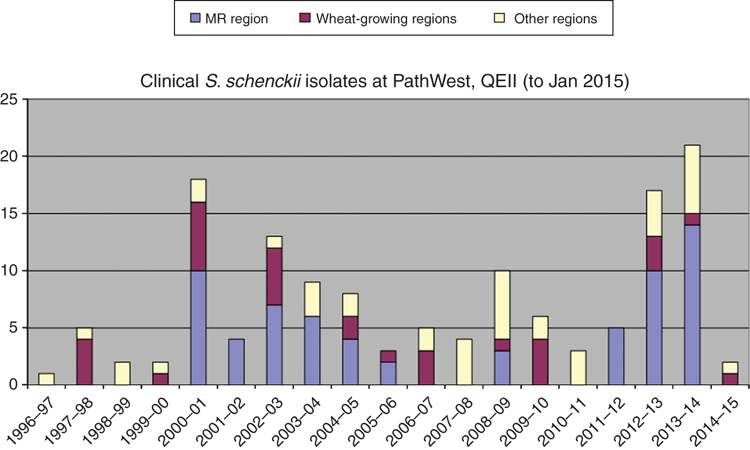
In Western Australia (WA), sporadic cases have occurred at least since 197511, mostly in certain districts of the ‘wheat belt’ in the south-west. However between 2000-2006 a cluster of 33 laboratory confirmed cases was reported from the Margaret River region where sporotrichosis had not previously been reported12. A further cluster of 29 cases were identified from 2011–2014 in the same region. Following investigations of the 2000 outbreak, a local media information campaign was instigated by local health authorities and a ‘clean up’ of an implicated hay supplier resulted in a reduction in cases. A similar community education programme instituted in 2013 seems to also have resulted in a reduction in the incidence of infection (Figure 3).
|
Then in 2014 the first cases of sporotrichosis were reported in the Northern Territory13. Again, hay was implicated as the infection source, following a remarkably similar pattern to the Margaret River infections.
The species distribution in Australia has only been studied to a limited degree. In a poster presented at the 17th ISHAM Congress in Tokyo from a small sample of isolates, all WA isolates were identified as S. schenckii sensu stricto, in comparison to isolates from eastern Australia identified as S. schenckii sensu stricto (but a separate clade to those in WA) or S. globosa14.
Conclusion
Sporotrichosis in Australia occurs as sporadic cases in certain geographical regions, with the occasional case cluster often associated with contact with hay. Distribution of organic substrates may spread the organism to new geographical areas. However, the species distribution and susceptibility pattern of the organisms have yet to be systematically studied in Australia.
References
[1] Chakrabarti, A. et al. (2015) Global epidemiology of sporotrichosis. Med. Mycol. 53, 3–14.| Global epidemiology of sporotrichosis.Crossref | GoogleScholarGoogle Scholar | 25526781PubMed |
[2] de Lima Barros, M. et al. (2011) Sporothrix schenckii and Sporotrichosis. Clin. Microbiol. Rev. 24, 633–654.
| Sporothrix schenckii and Sporotrichosis.Crossref | GoogleScholarGoogle Scholar |
[3] Aung, A. et al. (2013) Pulmonary sporotrichosis: case series and systematic analysis of literature on clinicoradiological patterns and management outcomes. Med. Mycol. 51, 534–544.
| Pulmonary sporotrichosis: case series and systematic analysis of literature on clinicoradiological patterns and management outcomes.Crossref | GoogleScholarGoogle Scholar | 23286352PubMed |
[4] Kwon-Chung, K.J. (1979) Comparison of isolates of Sporothrix schenckii obtained from fixed cutaneous lesions with isolates from other types of lesions. J. Infect. Dis. 139, 424–431.
| Comparison of isolates of Sporothrix schenckii obtained from fixed cutaneous lesions with isolates from other types of lesions.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaE1M7nsVWgtg%3D%3D&md5=64057c32efd4f06561a3dc888e5ca2edCAS | 438543PubMed |
[5] Rodrigues, A.M. et al. (2014) Genetic diversity and antifungal susceptibility profiles in causative agents of sporotrichosis. BMC Infect. Dis. 14, 219.
| Genetic diversity and antifungal susceptibility profiles in causative agents of sporotrichosis.Crossref | GoogleScholarGoogle Scholar | 24755107PubMed |
[6] Arrillaga-Moncrieff, I. et al. (2009) Different virulence levels of the species of Sporothrix in a murine model. Clin. Microbiol. Infect. 15, 651–655.
| Different virulence levels of the species of Sporothrix in a murine model.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD1MrltVOitg%3D%3D&md5=ff51df013ccf236cbccb71b43e3ee931CAS | 19624508PubMed |
[7] Stopiglia, C. et al. (2014) Antifungal susceptibilities and identification of species of the Sporothrix schenckii complex isolated in Brazil. Med. Mycol. 52, 56–64.
[8] Sivagnanam, A. et al. (2012) Sporotrichosis (Sporothrix schenckii infection) in the New South Wales mid-north coast. 2000–2010. Med. J. Aust. 196, 588–590.
| Sporotrichosis (Sporothrix schenckii infection) in the New South Wales mid-north coast. 2000–2010.Crossref | GoogleScholarGoogle Scholar |
[9] Conias, S. and Wilson, P. (1998) Epidemic cutaneous sporotrichosis: report of 16 cases in Queensland due to mouldy hay. Australas. J. Dermatol. 39, 34–37.
| Epidemic cutaneous sporotrichosis: report of 16 cases in Queensland due to mouldy hay.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK1c7ptVGqsA%3D%3D&md5=50b01cc3928aea41ed23aef2f5a6b35cCAS | 9529687PubMed |
[10] Auld, J.C. and Beardmore, G.L. (1979) Sporotrichosis in Queensland: a review of 37 cases at the Royal Brisbane Hospital. Australas. J. Dermatol. 20, 14–22.
| Sporotrichosis in Queensland: a review of 37 cases at the Royal Brisbane Hospital.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaE1M3lsFemsA%3D%3D&md5=8b3b457412c8ffc20088b00497290585CAS | 475693PubMed |
[11] Black, R.B. and McAleer, R. (1975) A case of sporotrichosis in Western Australia. Australas. J. Dermatol. 16, 32–38.
| A case of sporotrichosis in Western Australia.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaE287htFKrsQ%3D%3D&md5=0969da9587362b470f5e735996e1ca12CAS | 1212122PubMed |
[12] Feeney, K.T. et al. (2007) Outbreak of sporotrichosis, Western Australia. Emerg. Infect. Dis. 13, 1228–1231.
| Outbreak of sporotrichosis, Western Australia.Crossref | GoogleScholarGoogle Scholar | 17953099PubMed |
[13] Subedi, S. et al. (2014) Case report: sporotrichosis from the Northern Territory of Australia. Am. J. Trop. Med. Hyg. 91, 1263–1268.
| Case report: sporotrichosis from the Northern Territory of Australia.Crossref | GoogleScholarGoogle Scholar | 25200259PubMed |
[14] Madrid, H. et al. (2009) A putative new species in the Sporothrix schenckii complex and new records of Sporothrix species from Australia. Paper presented at: The 17th Congress of The International Society for Human and Animal Mycology; Tokyo, Japan. PP-04-14.
Biographies
Ian Arthur is the Senior Scientist of the Mycology Laboratory at PathWest, QEII Medical Centre where he has worked since 1992 with experience in all aspects of clinical diagnostic Mycology. He oversees the operations of the diagnostic laboratory also presenting several university lectures at UWA. The laboratory has had a long interest in superficial mycology, which now uses classical and molecular identification techniques to identify the full range of fungal pathogens. Ian is a former WA ASM branch committee member and treasurer.
Michael Leung is a clinical microbiologist and the regional microbiologist at PathWest Laboratory Medicine, QEII Medical Centre, and also a Clinical Senior Lecturer at the School of Pathology & Laboratory Medicine, University of Western Australia. He was previously Head of Department, Microbiology, at Western Diagnostic Pathology. He obtained his MBBS from the University of Melbourne (1987) and FRCPA (microbiology) in 1997. He has had a longstanding interest in clinical diagnostic mycology.
Inger Elin Westergaard completed her BSc at the University of Bergen, Norway, in 2011, and graduated from Master of Infectious Diseases at UWA in 2014. In her master thesis she investigated the possible source of the sporotrichosis case cluster that occurred in the Busselton-Margaret River region in 2011–2014. She is currently studying for her PhD at UWA, in a co-operation with the Norwegian Public Health Institute. Her research interests include rapid detection of antimicrobial resistance and improving public health in developing countries.


