Rethinking the targets for antifungal development
Jessica L Chitty A and James A Fraser A BA School of Chemistry and Molecular Biology, University of Queensland, St Lucia, Qld 4072, Australia
B Email: jafraser@uq.edu.au
Microbiology Australia 36(2) 88-89 https://doi.org/10.1071/MA15028
Published: 20 March 2015
Cryptococcus neoformans is the leading cause of fungal meningoencephalitis and one of the major causes of death in immunocompromised individuals; this AIDS-defining illness has a reported fatality rate of up to 20% in high-income countries such as Australia, and as high as 65% in developing nations1,2. The current treatment regime recommended by the World Health Organization is induction therapy with flucytosine and amphotericin B, followed by maintenance and consolidation therapy of fluconazole3. Development of resistance to these drugs is an ever-present threat given the pathogen undergoes microevolution while infecting the host, with evidence that this contributes to the high rate of relapse. It is therefore essential that we develop additional classes of antifungal drugs, particularly ones that are more effective than those currently available. But due to the shared eukaryotic physiology of fungi and humans, gross differences that can be exploited as drug targets such as those targeted by current antifungals are limited.
Rather than focus on large differences between fungal and human physiology, one approach that can be taken in the pursuit of new antifungal targets is a rational drug design approach to exploit subtle differences in otherwise conserved pathways. Rational drug design was pioneered in the purine metabolic pathway4, and this is one of the pathways providing exciting new avenues for antifungal development. The purine metabolic pathway is extremely well characterised in humans, and is the target for drugs such as mercaptopurine and mycophenolic acid that compromise cells with an increased demand for nucleotides due to their rapid proliferation5. However, little investigation has been undertaken into purine metabolism as a potential antifungal target. Given its environmental niche of purine-rich pigeon guano, C. neoformans is an ideal candidate in which to study the necessity of purine biosynthesis during infection.
With the aid of a phosphoribosyltransferase, C. neoformans can readily salvage purines from pigeon guano in its environmental niche. However, upon entering the human host, purine availability plummets6 and the pathogen becomes dependent on de novo biosynthesis of ATP and GTP. Several studies have shown the dependence of C. neoformans, Aspergillus fumigatus and Candida albicans on this primary metabolic pathway during the infection of an animal host, a dependence that could be exploited. This potential is currently being investigated through study of C. neoformans IMP dehydrogenase, the first dedicated step in the synthesis of GTP from the purine intermediate inosine monophosphate. C. neoformans strains lacking IMP dehydrogenase are avirulent in a murine model of infection, and efforts to develop a fungal-specific IMP dehydrogenase inhibitor are being facilitated by the elucidation of high resolution crystal structures. While sequence alignments reveal high identity of this enzyme between the host and pathogen, comparison of the fungal and animal crystal structures has enabled the identification of a striking conformational change in the active site pocket. With the aid of in silico docking studies, this difference is informing the design of what may be a new class of antifungal drugs7.
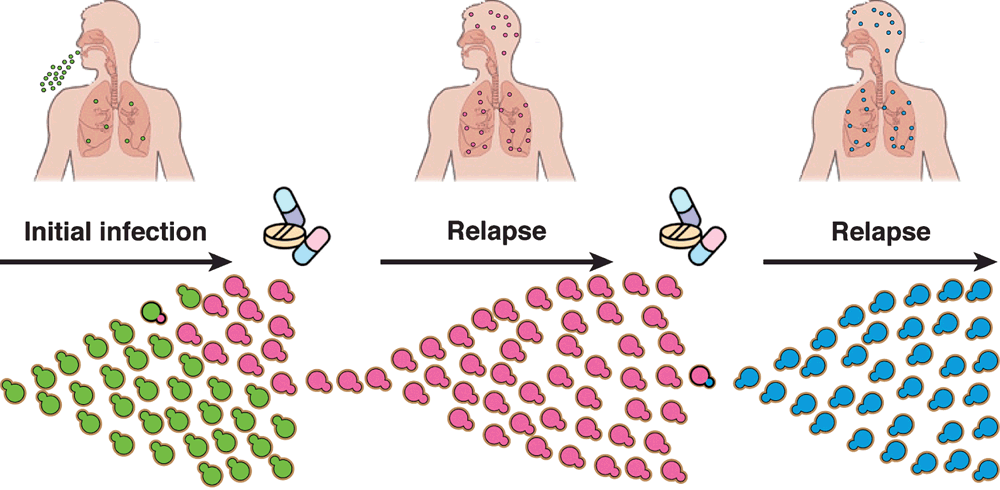
While the study of purine metabolism as an antifungal target builds upon a vast repository of information, an alternative approach is to initiate studies of completely uncharacterized fungal pathways that have been shown to be associated with the infection process. One method of identifying these genes is through microevolutionary studies. Upon infection of a mammalian host C. neoformans faces new environmental challenges as it moves from its traditional niche into a new hostile environment requiring its rapid adaptation for survival. Infection occurs via inhalation of fungal cells that lodge in the alveoli and disseminate into the bloodstream. From there, the infection is able to cross the blood brain barrier to cause meningoencephalitis. Importantly, even if this infection is cured relapse subsequently occurs in 6–23% of cases8. Studies of relapse isolates has revealed that they often exhibit phenotypes that differ from the original infection isolate, and it has been proposed that this microevolution likely facilitates relapse1,9,10 (Figure 1).
Comparison of the closely related C. neoformans var. grubii, C. neoformans var. neoformans and Cryptococcus gattii genomes have shown the genome to be remarkably stable, with no major chromosomal rearrangements in var. grubii for the last several million years. However whole genome analyses of clinical isolates from AIDS patients who have suffered from cryptococcal meningoencephalitis and subsequently relapsed have revealed surprising changes. Upon infection of a human host, a microevolutionary burst takes place, with gross chromosomal rearrangements occurring just as commonly as single nucleotide polymorphisms10. Furthermore, genome analysis from a range of isolates from several patients has shown a remarkable trend. Of the roughly 7,000 protein coding genes in the genome, several were found to be mutated in independent strains isolated from multiple patients, implying these genes are disadvantageous during the infection process11. Unlike the well-understood genes of purine metabolism, these examples of parallel evolution show little homology to characterised genes in other species, making their characterisation more challenging. But excitingly, the information provided by microevolutionary studies is revealing potential vulnerabilities of C. neoformans that may help inform the development of much-needed new classes of antimycotic therapeutics.
References
[1] Perfect, J.R. (2014) Cryptococcosis: a model for the understanding of infectious diseases. J. Clin. Invest. 124, 1893–1895.| Cryptococcosis: a model for the understanding of infectious diseases.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXotVSns7g%3D&md5=4f93097d936fa5c87807f369fe001a56CAS | 24743152PubMed |
[2] Chen, S.C. (2002) Australasian Society for Infectious Diseases Mycoses Interest G. Cryptococcosis in Australasia and the treatment of cryptococcal and other fungal infections with liposomal amphotericin B. J. Antimicrob. Chemother. 49, 57–61.
| Australasian Society for Infectious Diseases Mycoses Interest G. Cryptococcosis in Australasia and the treatment of cryptococcal and other fungal infections with liposomal amphotericin B.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XhsFChtbk%3D&md5=bbc9669321f27dc89f4ae43c0991ef7eCAS | 11801583PubMed |
[3] WHO (2011) Rapid advice: diagnosis, prevention and management of cryptococcal disease in HIV-infected adults, adolescents and children: World Health Organization; December 2011. Available from: http://www.who.int/hiv/pub/cryptococcal_disease2011/en/index.html (accessed 14 July 2014).
[4] Elion, G.B. (1989) The purine path to chemotherapy. Science 244, 41–47.
| The purine path to chemotherapy.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL1MXitVCru7s%3D&md5=a81a3d8f49c9e9cf88897c6b100737b7CAS | 2649979PubMed |
[5] Christopherson, R.I. et al. (2002) Inhibitors of de novo nucleotide biosynthesis as drugs. Acc. Chem. Res. 35, 961–971.
| Inhibitors of de novo nucleotide biosynthesis as drugs.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XmsVCnt78%3D&md5=875cd31db8df0cc111ad1a8dd2b2a7c2CAS | 12437321PubMed |
[6] Eells, J.T. and Spector, R. (1983) Purine and pyrimidine base and nucleoside concentrations in human cerebrospinal fluid and plasma. Neurochem. Res. 8, 1451–1457.
| Purine and pyrimidine base and nucleoside concentrations in human cerebrospinal fluid and plasma.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL2cXitVymsw%3D%3D&md5=39b0d6f2526815db17d4ee19474eb178CAS | 6656991PubMed |
[7] Morrow, C.A. et al. (2012) De novo GTP biosynthesis is critical for virulence of the fungal pathogen Cryptococcus neoformans. PLoS Pathog. 8, e1002957.
| De novo GTP biosynthesis is critical for virulence of the fungal pathogen Cryptococcus neoformans.Crossref | GoogleScholarGoogle Scholar | 23071437PubMed |
[8] Musubire, A.K. et al. (2013) Diagnosis and management of cryptococcal relapse. J. AIDS Clin. Res. Suppl 3, S3-003.
[9] Fries, B.C. et al. (1996) Karyotype instability in Cryptococcus neoformans infection. J. Clin. Microbiol. 34, 1531–1534.
| 1:STN:280:DyaK28zislyqsQ%3D%3D&md5=ef7af5cef8db05c57876c83b57db6f3fCAS | 8735111PubMed |
[10] Ormerod, K.L. et al. (2013) Comparative genomics of serial isolates of Cryptococcus neoformans reveals gene associated with carbon utilization and virulence. G3 (Bethesdaz) , g3.113.005660v1.
| Comparative genomics of serial isolates of Cryptococcus neoformans reveals gene associated with carbon utilization and virulence.Crossref | GoogleScholarGoogle Scholar |
[11] Ormerod, K.L. and Fraser, J.A. (2013) Balancing stability and flexibility within the genome of the pathogen Cryptococcus neoformans. PLoS Pathog. 9, e1003764.
| Balancing stability and flexibility within the genome of the pathogen Cryptococcus neoformans.Crossref | GoogleScholarGoogle Scholar | 24348244PubMed |
Biographies
Jessica Chitty is a PhD student at the University of Queensland. Previously she completed her undergraduate degree at the University of Sussex followed by an internship at Procter and Gamble.
James Fraser is an Associate Professor in the School of Chemistry and Molecular Biosciences at the University of Queensland. His research team is investigating microevolution of human fungal pathogens, and using that information to inform antifungal development.