Experience of HPV primary screening: a cross-sectional survey of ‘Let’s test for HPV’ study participants in Aotearoa New Zealand
Sally B. Rose 1 * , Lynn McBain 1 , Rebecca Bell 2 , Carrie Innes 2 , Sarah Te Whaiti 2 , Alexandria Tino 2 , Peter Sykes 2
1 * , Lynn McBain 1 , Rebecca Bell 2 , Carrie Innes 2 , Sarah Te Whaiti 2 , Alexandria Tino 2 , Peter Sykes 2
1
2
Abstract
In 2022–2023, a multi-region implementation study (‘Let’s test for HPV’) was undertaken in New Zealand primary care to inform the National Cervical Screening Programme shift to human papillomavirus (HPV) primary screening in September 2023.
This study aimed to describe ‘Let’s test for HPV study participants’ experiences with HPV primary screening.
Implementation study participants were invited to complete an anonymous online survey in September 2023. Survey data were summarised using descriptive statistics. Free text comments were analysed using inductive thematic analysis.
Forty-two percent of those invited began a survey (969/2302) and 921 were included in analyses. Respondents were aged 24–71, represented each of the three regions of New Zealand and different ethnic groups and included never and under-screened participants. Most people chose to self-test for comfort, convenience and privacy. Gaps were identified in participant understanding about HPV, how HPV testing differs from cervical cytology and the implications of HPV test results. Key topics requiring further explanation were identified by participants. Around 8% did not find self-testing easy or comfortable. Intent to screen again was high (92.4%), with greater preference for self-testing at home (48.2%) over the GP practice (33.5%).
HPV primary screening incorporating the option to self-test was highly acceptable to primary care-based study participants. Despite having participated in the new pathway, knowledge gaps were evident. Clear communication from screen-takers will be key to support participant knowledge, understanding and confidence in the efficacy of HPV primary screening. Ongoing programme evaluation, including patients’ perspectives, will be essential in the pursuit of equity and progress towards cervical cancer elimination.
Keywords: cervical cancer, cervical screening, human papillomavirus (HPV), self-sampling, self-test, survey, patient perspectives.
| WHAT GAP THIS FILLS |
| What is already known: HPV is the major cause of cervical cancer and can be detected from a self-collected vaginal or clinician-collected cervical sample with comparable sensitivity and specificity. HPV self-testing has been shown to be an effective tool to improve participation in cervical screening among never and under-screened people. |
| What this study adds: HPV primary screening incorporating self-testing was widely accepted among screening-eligible primary care participants, but key messages about this new approach were not well understood. Education, information provision and clear communication at all stages of the screening pathway will be critical to support patient understanding of and confidence in HPV primary screening. |
Introduction
Cervical cancer is preventable and treatable through vaccination, regular screening and effective management, yet globally it remains the fourth most common cancer for women and people with a cervix.1 In 2020 the World Health Organization released a strategy to accelerate worldwide elimination of cervical cancer, striving for a target of fewer than four cases per 100,000 women in every country.1 Persistent infection with high-risk types of human papillomavirus (HPV) is the leading cause of cervical cancer.2 HPV primary screening identifies individuals at greater risk for development of precancerous abnormalities, as it detects the presence of high-risk types of HPV that can cause abnormal cell changes that may progress to cancer.3 When HPV is detected, clinical follow-up with cytology and/or colposcopy identifies people requiring treatment or monitoring to prevent cancer developing.4 When HPV is absent there is a very low risk of developing precancerous abnormalities, and the screening interval can be safely extended to 5 years.5
A key advantage of an HPV test is that it can be performed on a self-collected vaginal or clinician-collected cervical sample with comparable sensitivity and specificity.6 Numerous studies show that self-testing is highly acceptable and preferred by many cervical screening participants,7 including those who have never screened or screened more than 5 years ago (under-screened).8–11 Historically, Māori (the indigenous peoples of New Zealand, NZ), Pacific peoples and people living in areas with higher socioeconomic deprivation have been under-served by the National Cervical Screening Programme (NCSP), with lower rates of screening coverage than European/other ethnic groups and people living in less deprived areas.12 Improving screening coverage in these under-served populations has significant potential to reduce disparities in the incidence and morbidity from cervical cancer. An NZ study found that never- and under-screened participants were significantly more likely to accept a self-test at home than a standard speculum-assisted cervical cytology test at a clinic.11 This preference for home self-testing over cervical cytology was almost 10 times higher among Māori participants, six times higher among Pacific participants and five times higher among Asian participants.11 Self-testing is more acceptable to many people than cytology because it is easier, less invasive, more affirming of bodily autonomy, comfortable and private.7,13–15
In September 2023, the NZ NCSP moved to HPV primary screening with the universal option to self-test.16 Notably, NZ was the first high-income country with an organised screening programme to move directly to HPV primary screening with self-testing available to everyone. This change followed years of delays, campaigning, lobbying and advocacy by both individuals and organisations.17 It is backed by international clinical data6,18 and strong local evidence demonstrating consumer preferences for self-testing, particularly among under-screened Māori and Pacific people.8–11,13,19,20 This shift is intended to progress NZ towards elimination of cervical cancer, improve equity in screening participation and cancer outcomes.16 To inform the implementation of HPV primary screening in primary care, Health NZ Te Whatu Ora funded a multi-region trial ‘Let’s test for HPV’ that was undertaken between August 2022 and April 2023. Participating screen-takers interviewed towards the end of the study were positive about the new HPV testing pathway, citing benefits for patients and providers.21 The present study was carried out in September 2023, utilising an online survey to understand participant perspectives on HPV primary screening.
Methods
Let’s test for HPV study participants
The Let’s test for HPV implementation trial (‘main study’) involved 17 general practices from three regions: Canterbury (5 practices), Wellington (7) and Whanganui (5). The study enrolled 3308 registered patients aged 21–81 years between August 2022 and February 2023. An estimated 78% of all eligible patients were screened during the study. Patients who were due or overdue for cervical screening were offered the choice of an HPV self-test (clinic or home) or a clinician-taken cervical test (using a speculum) that enabled reflexive cytology if HPV was detected. A cervical test was clinically indicated for some participants. Let’s test for HPV study methods are described in full at https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=383849. Consent was received from 2394 of the 3308 participants to be re-contacted about future research (current study).
‘Let’s test for HPV’ was approved in 2022 (2022 Southern Health and Disability Ethics Committee [HDEC] FULL 12546). This survey was approved as an amendment (Southern HDEC 2023 AM 12546, 11 September 2023).
Survey recruitment
Supplementary Fig. S1 presents a flowchart of survey recruitment and response rates. Of the 2394 main study participants who consented for follow-up, 98.6% provided an email address or mobile number (2361/2394). The first survey invitation was sent on 14 September 2023, and three reminders were sent at 2-week intervals. Paper surveys and completion via (free)phone call with the research nurse were offered. The secure Qualtrics software survey platform was used to collect anonymous responses to encourage frank feedback. Responses could not be matched with data collected in the main study. When clicking on the link, potential respondents were presented with the information sheet and asked to click a ‘consent to participate’ checkbox to proceed.
Data collection
A customised questionnaire was developed to capture information specific to our study context. Articles reporting relevant local10,13,19,20 and international22–27 research informed response options for some questions. Feedback was obtained from members of the Māori Steering Group and Pacific Advisory Group (convened to provide support to the main study) and culturally appropriate modifications incorporated into the final draft. We collected demographic information, cervical screening history, choice of screening method (and why), understanding of HPV testing, appropriateness of information resources, ease of participation (decision-making, sample collection/return/results/clinical follow-up), issues encountered, suggested improvements and future screening preferences. The format included multiple choice, Likert scale (agree–disagree) and open-ended questions that asked about (i) gaps in information provision, (ii) experience with self-testing, (iii) what they liked about HPV primary screening, (iv) what they did not like, and (v) suggestions for improvement.
The current paper reports on quantitative data and qualitatively analysed responses regarding (i) gaps in information provision. To enable full reporting of all survey data, additional papers report on the subset of participants with an HPV-detected result,28 qualitatively analysed responses to open-ended questions (ii–v)29 and the experience of Māori participants.
Analysis
Responses were reviewed to check legitimacy of response patterning, there was no evidence of fraudulent responses. Some cleaning was undertaken before exporting the file to Excel for collation and analysis. Age was re-categorised into 10-year age bands and self-identified ethnicity data were categorised following established protocols,30 with prioritised output used for reporting. Response frequencies were tabulated for quantitative survey items, and number, percentages and 95% confidence intervals (CIs) calculated. An inductive thematic approach31 was used to analyse comments shared in response to open-ended questions. SR read all comments in the context of the full dataset several times then extracted text alongside participant descriptors (age band, ethnicity, screening method, result and study region) and stored them in an Excel file for analysis. Content was coded by SR using an iterative and emergent process (reviewing and refining topic headings). Key topics were finalised and grouped into themes for review by co-authors. Quotes were selected to illustrate the range of questions and concerns shared by participants for each theme, inclusive of common and unique reflections. Quotes were intentionally selected to highlight the diverse demographic characteristics of participants who shared comments (in terms of age, ethnicity and region of residence). Ethnicity and 10-year age bands are shown alongside quotes to reflect this, with region omitted to preserve participant anonymity.
Results
Survey invitations were delivered to 2302 of 2361 participants, of whom 969 ticked ‘consent to participate’ and began the survey (42.1% partial completion rate, 969/2302). Most reached the end of the survey (89.6%, 868/969). We reviewed 101 incomplete responses and included an additional 23 respondents who completed at least 50% of questions, giving a total of 921 respondents (40%, 921/2302).
Participant characteristics
The demographic characteristics of survey and main study respondents are presented in Table 1. Respondents were aged 24–71 years, 92% chose a self-test and 10% returned an HPV detected result. Comparing demographics of survey participants with the main study cohort, the proportion of European participants who opted to participate in the survey was higher than the proportions of Māori, Pacific and Asian participants. Survey participants were older on average (median age 49) than the main study group (median age 45), and a higher proportion of Wellington region participants completed a survey than from other regions.
| Characteristics A | Total participants (n = 921) | Main study participants (n = 3308) B | |||||
|---|---|---|---|---|---|---|---|
| n | % | 95% CI | n | % | 95% CI | ||
| Age band (years) | |||||||
| <30 | 82 | 8.9 | (7.1–10.9) | 424 | 12.8 | (11.7–14.0) | |
| 30–39 | 183 | 19.9 | (17.3–22.6) | 818 | 24.7 | (23.3–26.2) | |
| 40–49 | 204 | 22.1 | (19.5–25.0) | 743 | 22.5 | (21.1–23.9) | |
| 50–59 | 251 | 27.3 | (24.4–30.3) | 742 | 22.4 | (21.0–23.9) | |
| 60 and over | 193 | 21.0 | (18.4–23.7) | 581 | 17.6 | (16.3–18.9) | |
| Region of residence | |||||||
| Whanganui | 276 | 30.0 | (27.0–33.0) | 1061 | 32.1 | (30.5–33.7) | |
| Wellington | 383 | 41.6 | (38.4–44.8) | 1174 | 35.5 | (33.9–37.1) | |
| Canterbury | 260 | 28.2 | (25.3–31.3) | 1073 | 32.4 | (30.1–34.1) | |
| Ethnicity (prioritised) | |||||||
| Māori | 176 | 19.1 | (16.6–21.8) | 796 | 24.1 | (22.6–25.6) | |
| Pacific | 20 | 2.2 | (1.3–3.3) | 192 | 5.8 | (5.1–6.7) | |
| NZ and other European | 632 | 68.6 | (65.5–71.6) | 1733 | 52.4 | (50.7–54.1) | |
| Asian | 62 | 6.7 | (5.2–8.5) | 484 | 14.6 | (13.5–15.9) | |
| Middle eastern, Latin American, African | 11 | 1.2 | (0.6–2.1) | 85 | 2.6 | (2.1–3.2) | |
| Other | 16 | 1.7 | (1.0–2.8) | 18 | 0.5 | (0.3–0.8) | |
| Gender C | |||||||
| Female | 913 | 99.1 | (98.3–99.6) | – | – | – | |
| Male, non–binary, Takatāpui, gender diverse | 7 | 0.8 | (0.3–1.6) | – | – | – | |
| Language(s) spoken D | |||||||
| English | 914 | 99.2 | (98.4–99.7) | – | – | – | |
| Māori | 40 | 4.3 | (3.1–5.9) | – | – | – | |
| NZ Sign | 6 | 0.7 | (0.2–1.4) | – | – | – | |
| Samoan | 3 | 0.3 | (0.1–0.9) | – | – | – | |
| Other languages | 86 | 9.3 | (7.5–11.4) | – | – | – | |
| Cervical screening history | |||||||
| Screened within 5 years | 730 | 79.3 | (76.5–81.8) | 2308 | 70.1 | (68.5–71.6) | |
| Screened more than 5 years ago ‘under-screened’ | 103 | 11.2 | (9.2–13.4) | 671 | 20.4 | (19.0–21.8) | |
| Screened but can’t recall when | 54 | 5.9 | (4.4–7.6) | – | – | – | |
| Never screened ‘un-screened’ E | 30 | 3.3 | (2.2–4.6) | 315 | 9.6 | (8.6–10.6) | |
| Unsure | 2 | 0.2 | (0–0.8) | – | – | – | |
| If screened, ever had abnormal cytology F | 298 | 32.4 | (29.3–35.5) | – | – | – | |
| Screening method in ‘Let’s test for HPV’ | |||||||
| HPV self-test (clinic) | 585 | 63.5 | (60.3–66.6) | 2340 | 70.7 | (69.2–72.3) | |
| HPV self-test (home) | 266 | 28.9 | (26.0–31.9) | 744 | 22.5 | (21.1–23.9) | |
| HPV self-test (location not reported) | 0 | 0 | 0 | 48 | 1.5 | (1.1–1.9) | |
| Clinician taken cervical test | 70 | 7.6 | (6.0–9.5) | 176 | 5.3 | (4.6–6.1) | |
Adequacy of information provision
Information deemed most helpful to understand what HPV testing was about included ‘talking with the doctor or nurse at my practice’ (86.2%, 794/921) and ‘reading the information sheet I was given myself’ (69.8%, 643/921). An online video created for Whanganui participants was regarded as helpful by 6.5% (18/276) of this subset, although it is unknown how many accessed the video. A small group found having someone read the information to/with me helpful (5.2%, 48/921). ‘Other’ helpful sources described in comments included: own research including information from online sources (n = 20), own knowledge as a health professional or talking with family members who are health professionals (n = 8), talking with a nurse on the phone or as part of ‘support to screen’32 outreach work (n = 6), talking to someone who had done an HPV test (n = 4) and hearing about it on the news/radio (n = 2).
When asked about other information preferences to support decision-making about which test to have, most responded that they ‘had enough information’ (81.7%, 752/921). Around one in 10 wanted to access information via online videos (10.4%, 96/921) and information with more pictures and photos (8.6%,79/921). Others wanted information presented in a way that considers their culture and worldview (2.6%, 24/921), via a freephone number (1.7%, 16/921), in another language (1.4%, 13/921 of all participants, 7% or 10/141 participants who spoke a language other than English), via Māori television (1.4%, 13/921 of all participants and 6.3% or 11/176 Māori participants) or Pasifika radio (0.5%, 5/921 overall and 5% or 1/20 Pacific participants). Thirty people suggested other helpful information sources including email, web-links to ‘independent, reliable websites’, brochures/pamphlets and social media.
Around one in five participants (23.5%, 216/921) commented on questions or concerns they had about HPV primary screening. Comments are summarised in Table 2 (with selected illustrative quotes that reflect the main themes), with content relating to five topic areas: (1) facts about HPV, (2) old vs new test, (3) self-testing, (4) HPV test results, and (5) the extended screening interval.
| Themes | Description and selected participant quotes A | |
|---|---|---|
| 1. Facts about HPV and cervical cancer | Participants wanted to know the ‘basic facts’ about HPV including how common it is, how it is passed on, the duration of infection and the fact that the immune system is able to clear an HPV infection: | |
| How common HPV is, there definitely needs to be greater education about it throughout the life course – I think that now we are switching to HPV testing this is an opportunity for it. (Pacific, <30) | ||
| How you may have contracted the HPV virus and how long ago. (European, 50–59) | ||
| That HPV can be cleared by the immune system. (European, <30) | ||
| They also had questions about other risk and protective factors for cervical cancer: | ||
| Are there other risk factors for cervical cancer, not caused by HPV? (European, 40–49) | ||
| If a vaccine is available if the test is negative. (European, 40–49) | ||
| 2. Old vs new test (‘smear’ vs HPV test) | Uncertainty about how HPV testing differs to cervical cytology was common, with related concerns about missed detection of any abnormal cells or cancer: | |
| Wasn't sure if it would pick up as many potential issues as a normal smear. (Māori, <30) | ||
| I would have liked more detailed information about the difference between a smear and the HPV test. The nurse was very helpful but I wanted to know more, to fully understand why the HPV test was ‘better’ and the difference between the different variants of the virus they might detect. The information provided was at quite a basic level. (European, 40–49) | ||
| What was it looking for? How often should I test. More information to help prevent any concerns moving forward would’ve been helpful. (Pacific, 30–39) | ||
| I still feel nervous that only HPV 16 and 18 are the only things being checked to determine if I might have cervical cancer. I felt more reassured by the lab examination of cervical cells. (European, 40–49) | ||
| 3. Self-sample collection | The instructions were not clear for everyone, with questions raised about the correct use of the swab, including where to swab, how far to insert the swab, opening the packet, unscrewing the tube. | |
| What the apparatus I was to use looked like, it wasn't until I opened it, it was all a bit vague. (European, 60+) | ||
| Where to put the swab. (European, 40–49) | ||
| How far into swab yourself when doing self-swab. (Māori, 40–49) | ||
| A range of questions were raised about eligibility for self-sampling (including consideration of screening history, being in same sex relationship and ability to self-sample during menstruation). | ||
| I probably shouldn’t have been able to self-test due to previous abnormal results. (Māori,40–49) | ||
| Do women in same sex couples have to do HPV testing? (Asian, 30–39) | ||
| Doubts were expressed about correct technique and how this might impact on accuracy: | ||
| The consequences of not doing the self-test correctly. (Māori, 40–49) | ||
| I was concerned that I may not have done the test correctly. I have had irregular test results before and have had colposcopies before so I want to be sure that I get it right. (European, 60+) | ||
| I was very nervous thinking I hope I swab myself correctly. I feel like I want to go and get a swab from the nurse just to make sure I did it right. (European, 30–39) | ||
| Some felt ill-informed about the possibility of experiencing pain or discomfort with self-sampling. | ||
| It wasn't explained at the appointment with the nurse that it would potentially hurt!! Nor that I would bleed afterwards, or have potential pain when urinating days after! (Māori, 40–49) | ||
| How uncomfortable I would feel immediately afterwards. (European, 50–59) | ||
| 4. HPV test results | Several people were unsure about expected wait time for results and how they would be delivered: | |
| Delivery of results | How long it would take to have the result; how would the result be delivered; will there be a follow-up call discussing the results. (Asian, 30–39) | |
| Many participants commented that getting results via text or a patient portal notification meant they were unable to ask questions, find out next steps or seek reassurance about results: | ||
| I got my results through a text so felt I couldn't ask any questions and they took a while to come back. (European, 30–39) | ||
| Understanding results | Results and next steps were inadequately explained in some cases: | |
| Lab result is not always easy to read due to formatting and the medical language used. (Asian,30–39) | ||
| The results were not explained well. (Māori, 30–39,) | ||
| What type of HPV would require more tests. (Māori, <30) | ||
| The actual results from the nurse at my practice wasn’t educated enough to give the results therefore I didn’t actually understand them until I spoke with the Dr at the hospital. (European, <30) | ||
| Do I need to be worried? (MELAA, 30–39) | ||
| A common misconception was that an HPV detected result followed by normal cytology meant the HPV test was inaccurate or indicative of a ‘false positive’: | ||
| I had confusion on the HPV test being positive and the smear being negative. (European, 50–59) | ||
| [Re. the information provided] It wasn't enough. I really didn't understand about the high rates of false positives. (European, 60+) | ||
| Questions were raised seeking clarity about implications of an HPV detected result on future health and relationships. | ||
| I have only had one sexual partner in the past 6 years. Before him no sex in many years. What does this mean? My partner has cheated on me? (European, 50–59) | ||
| I was terrified as I had no idea how and where I got the disease, I had to inform my partner that I possibly have it and I could have transmitted to him. (Asian, 40–49) | ||
| Comments from some people with an HPV not detected result were suggestive of low trust in the result, likely exacerbated by a lack of opportunity to discuss it with their screen-taker: | ||
| I am concerned just because HPV doesn’t show up in swab it doesn’t mean you don’t have concerning changes to your cervix that could indicate cancer? (European, 40–49) | ||
| If you are negative for HPV but still have cancer cells present is this picked up? (European, 60+) | ||
| 5. Screening interval | Concerns were expressed about the safety implications of extending the screening interval from 3 to 5 years, reflecting low awareness of the rationale for the extension: | |
| I don't feel secure with screening only done every 5 years. (European, 40–49) | ||
| I would like to see these tests done yearly as it was easy to do. (European, 40–49) | ||
| It worries me that it will now be every 5 years instead of 3 years. That’s too long between tests. (European, 50–59) |
At the end of the survey, five ‘true/false’ statements were presented to determine participant understanding of key messages about HPV testing (see Table 3). Only one-quarter of respondents answered three or more questions correctly (25.3%, 228/901). Two-thirds of participants (64.3%, 571/888) were correct that the statement ‘HPV is very rare’ is false, and almost three-quarters were correct that ‘the HPV test is used to look for a virus’ (72.4%, 642/887) is true. The majority (78.4%, 697/889) incorrectly thought ‘the HPV test is used to identify abnormal cell changes in the cervix.’ Few people knew ‘HPV infections often clear up on their own’ (19.1%, 170/889) or that ‘the risk of HPV causing serious health problems is very low’ (12.5%, 111/890).
| Statement (Correct answer) | Response (n = 875) A | Interpretation | ||||||
|---|---|---|---|---|---|---|---|---|
| True | False | Unsure | ||||||
| n | % | n | % | n | % | |||
| HPV is very rare (F) | 65 | 7.3 | 571 | 64.3 | 242 | 27.3 | Around 1/3 unaware that HPV is very common. | |
| HPV infections often clear up on their own (T) | 170 | 19.1 | 494 | 55.6 | 219 | 24.6 | More than 3/4 unaware our bodies usually clear HPV. | |
| The HPV test is used to look for a virus (T) | 642 | 72.4 | 99 | 11.2 | 141 | 15.9 | Nearly 1/3 unaware that the HPV test detects the presence of a virus. | |
| An HPV test is used to identify abnormal cell changes in the cervix (F) | 697 | 78.4 | 143 | 16.1 | 45 | 5.1 | Over 3/4 appear to have confused the HPV test with cervical cytology. | |
| The risk of HPV causing serious health problems is very low (T) | 111 | 12.5 | 563 | 63.3 | 210 | 23.6 | Most appear to think HPV means cancer. | |
Choice of screening method
Over 90% of participants agreed with statements asking about choice of screening method (see Fig. 1), and most disagreed with the statement ‘I didn’t have a choice about which test to have’. The majority of participants self-tested (92.4%, 851/921) for reasons including comfort (57.9%, 493/851), convenience (53.7%, 457/851) and ability to test in private (48.4%, 412/851). Around a third of self-testers also cited not having to show anyone private parts (39.1%, 333/851), GP/nurse recommendation (30.4%, 259/851), avoiding whakamā or embarrassment (29.7%, 253/851), mana motuhake – I am in charge and have control over my body (29.3%, 249/851) and less time consuming (29.5%, 251/851) as reasons supporting their choice. Comfort, convenience and privacy were the most common reasons for opting to self-test at home. See Supplementary Table S1 for full data describing reasons for choice of screening method. Commonly selected reasons for choosing a cervical test were: GP/nurse recommendation (31.4%, 22/70), worry about doing the self-test wrong (25.7%, 18/70), convenience (21.4%, 15/70), comfort (14.3%, 10/70) and symptoms/wanting something else checked (14.3%, 10/70).
Percentage agreement with statements about choice of screening method, self-testing and receipt of results.
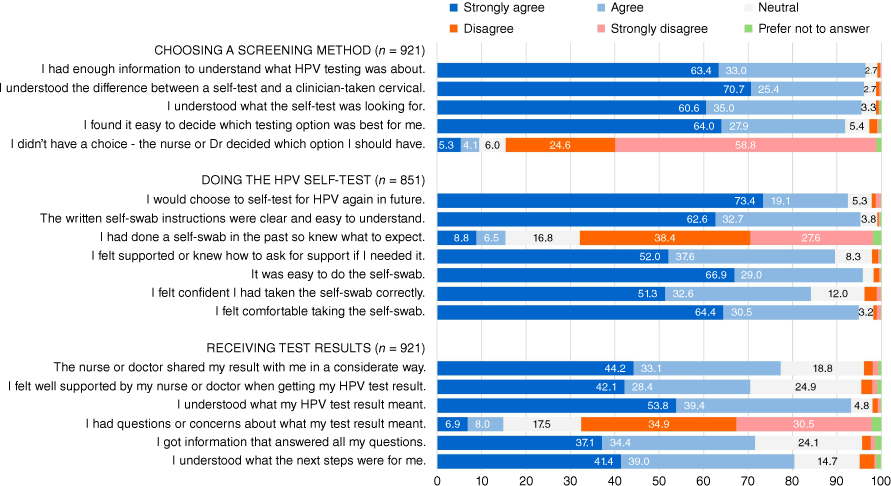
Of those who self-tested, most reported a positive experience, agreeing that they found it easy, comfortable, confident and well supported, despite only a minority having done a self-swab in the past. Participant responses to statements about self-testing are summarised in Fig. 1. A demographically diverse group of 81 people (9.5%) aged 24–68 years, of all ethnicities, disagreed or were neutral about clarity of instructions (n = 35), sampling ease (n = 32), feeling comfortable (n = 40) and/or supported (n = 81). Fifty-four people did not find it easy and/or comfortable (6.3%, 54/851). Comments shared by this subset described the process as awkward or physically difficult to do by themselves, or they experienced pain, cramps, stinging, discomfort or bleeding.
Receipt of results
‘HPV not detected’ results were typically conveyed electronically with patients’ permission (via text or a notification of a new lab result in a patient portal) and an ‘HPV detected’ result shared via phone call or in person. Most people reported having received their cervical screening result (96.2%, 886/921), 29 people said they had not, and six preferred not to answer. Note that all results were followed up as part of the main study protocol. Of those reporting having received their result, 87.2% (766/878) received an HPV not detected result, 10.8% (95/878) had an HPV detected result, eight had an invalid result, nine were unsure what their result was and eight did not answer this question.
Fig. 1 summarises participant responses to statements about receipt of results. Most participants (70% or more) agreed results were shared in a considerate way, understood what results meant, felt well supported and understood next steps. A small percentage (3.7%) disagreed that they felt well supported on receipt of HPV test results.
When asked to select one or more emotions from a list, most people with an HPV not detected result said they felt happy (84.9%, 650/766). Smaller proportions felt relieved (34.2%, 262/766), neutral (9.2%, 71/766), worried (0.9%, 7/766) or were unsure how they felt (0.78%, 6/766). Some people shared concerns that they hadn’t swabbed correctly so didn’t trust their result. Those receiving an ‘HPV detected’ result reported feeling one or more negative emotions including worry, shock, fear, distress or upset (65.3%, 62/95). One-quarter were surprised (25.3%, 24/95) and around 15% or fewer felt confused (14.7%, 14/95), neutral (15.8%, 15/95) or unsure (9.5%, 9/95).
Future screening preferences
Table 4 describes future screening intent and preferences, with a high proportion of participants intending to screen again (92.4%). Most survey respondents expressed a preference for screening via self-testing (81.8%), and almost half wanted to self-test at home (48.2%). When considering participant’s overall responses to Likert-scale questions asked to understand acceptability of screening (ie the statements shown in Fig. 1), it was apparent that those who felt well informed, comfortable, confident and supported at each stage were more likely to intend to screen again (94%, 654/696, 95% CI 91.9–95.6) than those who did not (87.1%, 183/213, 95% CI 81.8–91.4, P = 0.005).
| Future screening intent and preferences | Total (n = 906) | |||
|---|---|---|---|---|
| n | % | (95% CI) | ||
| Intent to screen again | ||||
| Yes | 837 | 92.4 | (90.5–94) | |
| No | 17 | 1.9 | (1.1–3.0) | |
| Not sure | 46 | 5.1 | (3.7–6.7) | |
| Prefer not to answer | 6 | 0.7 | (0.2–1.4) | |
| Preferred screening method A | ||||
| Self-test (total) | 722 | 81.8 | (79.1–84.3) | |
| Self-test at home | 426 | 48.2 | (44.9–51.6) | |
| Self-test at the GP practice | 296 | 33.5 | (30.4–36.7) | |
| Nurse/doctor taken vaginal sample | 16 | 1.8 | (1.0–2.9) | |
| Depends on nurse/doctor recommendation | 57 | 6.5 | (4.9–8.3) | |
| Nurse/doctor taken test from cervix | 39 | 4.4 | (3.2–6.0) | |
| No preference | 37 | 4.2 | (3.0–5.7) | |
| Not sure | 10 | 1.1 | (0.5–2.1) | |
| Prefer not to answer | 2 | 0.2 | (0–0.8) | |
| Preferred invitation source | ||||
| Usual healthcare provider | 279 | 31.6 | (28.6–34.8) | |
| National Cervical Screening Programme | 94 | 10.7 | (8.7–12.9) | |
| No preference | 509 | 57.7 | (54.4–61) | |
| Preferred invitation method | ||||
| 516 | 58.6 | (55.3–61.9) | ||
| Text message | 452 | 51.4 | (48.0–54.7) | |
| Letter | 201 | 22.8 | (20.1–25.8) | |
| No preference | 193 | 21.9 | (19.2–24.8) | |
| Phone call | 99 | 11.3 | (9.2–13.5) | |
| Face to face (when at clinic) | 97 | 11.0 | (9.0–13.3) | |
Discussion
This study highlights a preference for, and widespread acceptability of, HPV self-testing among a screening-eligible primary care population. Across all demographic groups, small but not insignificant numbers of people identified one or more aspects of the screening pathway that did not meet their needs or expectations. While most felt well informed during their screening experience, around 20% identified something they were unsure about or thought wasn’t well explained. Areas of uncertainty included general facts about HPV, understanding how HPV testing and cervical cytology differ, accuracy of self-testing and HPV test results, self-sample collection technique and understanding test results.
Talking with the screen-taker and reading written information were deemed the most helpful ways to get information about HPV primary screening. However, many had questions and concerns about the new pathway, and general knowledge questions about HPV and HPV testing were often answered incorrectly. This suggests information provided about HPV may not have been sufficient to appropriately inform the screening population. This is not unexpected as past research also describes low awareness and poor knowledge of HPV and its role in cervical screening among cervical screening participants24,25 and screening-eligible people in countries that have already shifted to HPV primary screening.33,34 Knowledge gaps about HPV were also identified among screen-takers themselves when surveyed at the start of the Let’s test for HPV trial.35 Our survey findings support suggestions made by Let’s test for HPV screen-takers that ongoing health promotion and educational activities are needed to accompany the shift to the HPV primary screening pathway.21
Consistent with past research, most participants found self-testing easy, convenient and comfortable, and they were confident they had done it correctly.20,23,27 Around one in 12 participants either found instructions unclear, the process uncomfortable, physically difficult and/or experienced pain or discomfort during or after self-testing – a finding that is also consistent with past work.19,20 The annual volume of screening participants exceeds 50,000 in NZ (53,313 in 2023),12 which could equate to around 4000 people each year for whom self-testing may not be straightforward. It is important that any avoidable issues are mitigated for future screening participants. Providing very clear verbal and illustrated instructions about how and where to swab (ie the walls of the vagina, not the urethra or cervix) is important, with the offer of assistance to anyone who may find the process physically difficult. Checking in to ask how an individual got on with their sample collection could help pick up any issues (pain, bleeding, discomfort) that need to be checked. Further research may be needed to identify strategies to alleviate pain and discomfort experienced by a small percentage when self-testing.
One in four participants expressed uncertainty or confusion about their HPV test results. Fear and worry were common on receipt of an HPV detected result, and people wanted more information about next steps regardless of the test outcome. For some people with an HPV not detected result, lack of an opportunity to discuss it with a health provider left them feeling concerned about accuracy of results. Explaining the implications of potential results – where HPV is detected or not, or where cytology detects cervical cell abnormality or not, both at the time of screening and again when results are shared would help pre-empt concerns and alleviate doubts. Low knowledge of key messages about HPV also points to the need for reassurance, eg to emphasise that the risk of HPV causing serious problems is low.
Future intent to participate in cervical screening was high, with a strong preference for self-testing. Of note, people who encountered one or more issues during their screening experience were less likely to want to screen again. Given that past research also shows that a negative cervical screening experience impacts on future screening participation,36,37 this finding underscores the importance of ensuring that every part of the screening pathway is carried out in a way that addresses the varied needs found within primary care populations. Around half of survey participants wanted to self-test at home, and a third at their GP practice. Past research involving under-screened participants has reported a greater preference for home over clinic-based testing.20 Although not routinely offered in the NSCP, self-testing at home was an attractive option utilised by approximately 20% in Let’s test for HPV. Kits were typically given out by practices opportunistically during the main study. Some screen-takers interviewed afterwards saw a role for home testing, particularly for rural dwellers or people whose work commitments make it hard to attend in-person consultations.21 On balance, however, they viewed in-clinic testing more favourably, due to the time needed to follow-up with participants to confirm completion of self-tests at home.21 Given that many screening participants prefer home self-tests, it is important to identify ways to minimise the challenges of completing and following-up on these tests. Facilitating participants’ preference for home testing could better engage under-screened populations, contributing to equitable outcomes for those who have historically been under-served by the NSCP.
Strengths and limitations
This study describes the experience of a primary care population offered HPV testing as their primary cervical screen. Although offered in the context of implementation research, the screening process and clinical pathways closely resembled those now in place for NSCP participants. The recruitment rate into the main trial was excellent but was limited to enrolled patients at participating practices. Our survey response rate reached 40%, with good participation by Māori who made up 19% of survey participants (but 24% of the main study group). European were over-represented whereas Pacific and Asian participants were under-represented among respondents. Although we offered options for paper and phone survey completion, invitations were only sent by email and mobile phone, so favoured people with connectivity and available time. Furthermore, as we were unable to offer translations of this survey, participation would have been easier for native English speakers and those with high English proficiency. A higher proportion of responses came from the Wellington region (where fewer participants live in higher socioeconomic deprivation), therefore people who face fewer barriers to health care and potentially had a more positive screening experience are probably over-represented among respondents. The survey was undertaken 4 months after final recruitment closed so recall bias or difficulty recalling aspects of the experience may have impacted some responses.
Implications
HPV primary screening with the choice of self-testing was highly acceptable to primary care trial participants screened before the NCSP change in September 2023. Our findings emphasised the need for education for the screening-eligible population to achieve widespread understanding of HPV primary screening, and to raise awareness of the self-test option. Participant feedback also stressed the importance of good communication and information provision by the screen-taker at all stages of the screening pathway to support participation, understanding and trust in HPV primary screening. Ongoing evaluation of programme delivery, including patients’ perspectives, will be vital to identify and resolve any ongoing process or equity issues, and to support progress towards the elimination of cervical cancer.
Data availability
All survey data are being reported in full and are not being made publicly available. We did not seek participant permission to share data or receive ethics approval to do so. Any queries about the content of the dataset can be directed to sally.rose@otago.ac.nz.
Conflicts of interest
S. R. has no conflicts of interest to declare. L. M., R. B., C. I. and P. S. received funding from Te Whatu Ora Health New Zealand to conduct the main study. S. T. W. received funding from the main study fund as Chair of the Māori Steering Committee and A. T. received funding from the main study fund as Chair of the Pacific Advisory group. Te Whatu Ora Health New Zealand did not have any input into the study design, collection, analysis or interpretation of data, nor in the writing of the manuscript or decision to submit the article for publication.
Declaration of funding
This research was supported by a 2023 GRACI General Research Grant (Reference No: COOC2301_07), funds awarded in 2022 by Te Whatu Ora Health New Zealand (Contract No. 374890/00) for the Let’s test for HPV Implementation trial and University of Otago funding.
Acknowledgements
We thank all those participants who took the time to share their screening experience by completing a survey. Thanks also to Tania Batley from the Māori Steering Committee for input into the survey draft, Debra Smith for inviting feedback from participating practice nurses, Sue Garrett for assisting with survey set-up in Qualtrics and Janine Nip who helped prepare study documents for ethics committee review and locality assessment. We also acknowledge the work of Let’s test for HPV study investigators and team members (John McMenamin, Ben Hudson, Beverley Lawton, Melanie Gibson) as well as staff at the 17 participating practices.
References
1 World Health Organization. Eliminating cervical cancer. 2020. Available at https://www.who.int/publications/i/item/9789240014107 [accessed 11 September 2024].
2 Burd EM. Human papillomavirus and cervical cancer. Clin Microbiol Rev 2003; 16(1): 1-17.
| Crossref | Google Scholar | PubMed |
3 Koliopoulos G, Nyaga VN, Santesso N, et al. Cytology versus HPV testing for cervical cancer screening in the general population. Cochrane Database Syst Rev 2017; 8: CD008587.
| Crossref | Google Scholar | PubMed |
4 Best Practice Advocacy Centre (BPAC) New Zealand. Cervical cancer - early detection and referral. 2022. Available at https://bpac.org.nz/2022/docs/cervical-cancer.pdf [accessed 12 September 2024].
5 Ronco G, Dillner J, Elfström KM, et al. Efficacy of HPV-based screening for prevention of invasive cervical cancer: follow-up of four European randomised controlled trials. Lancet 2014; 383(9916): 524-32.
| Crossref | Google Scholar | PubMed |
6 Arbyn M, Smith SB, Temin S, et al. Detecting cervical precancer and reaching underscreened women by using HPV testing on self samples: updated meta-analyses. BMJ 2018; 363: k4823.
| Crossref | Google Scholar | PubMed |
7 Nelson EJ, Maynard BR, Loux T, et al. The acceptability of self-sampled screening for HPV DNA: a systematic review and meta-analysis. Sex Transm Infect 2017; 93(1): 56.
| Crossref | Google Scholar | PubMed |
8 Brewer N, Foliaki S, Bromhead C, et al. Acceptability of human papillomavirus self-sampling for cervical-cancer screening in under-screened Māori and Pasifika women: a pilot study. N Z Med J 2019; 132(1497): 21-31.
| Google Scholar | PubMed |
9 Adcock A, Cram F, Lawton B, et al. Acceptability of self-taken vaginal HPV sample for cervical screening among an under-screened Indigenous population. Aust N Z J Obstet Gynaecol 2019; 59(2): 301-07.
| Crossref | Google Scholar | PubMed |
10 MacDonald EJ, Geller S, Sibanda N, et al. Reaching under-screened/never-screened indigenous peoples with human papilloma virus self-testing: a community-based cluster randomised controlled trial. Aust N Z J Obstet Gynaecol 2021; 61(1): 135-41.
| Crossref | Google Scholar | PubMed |
11 Brewer N, Bartholomew K, Grant J, et al. Acceptability of human papillomavirus (HPV) self-sampling among never- and under-screened Indigenous and other minority women: a randomised three-arm community trial in Aotearoa New Zealand. Lancet Reg Health West Pac 2021; 16: 100265.
| Crossref | Google Scholar | PubMed |
12 Health New Zealand Te Whatu Ora. National Cervical Screening Programme Coverage Report; Wellington: Health New Zealand. 2024. Available at https://tewhatuora.shinyapps.io/nsu-ncsp-coverage/ [accessed 11 November 2024].
13 Adcock A, Stevenson K, Cram F, et al. He Tapu Te Whare Tangata (sacred house of humanity): under-screened Māori women talk about HPV self-testing cervical screening clinical pathways. Int J Gynaecol Obstet 2021; 155(2): 275-81.
| Crossref | Google Scholar | PubMed |
14 Camara H, Zhang Y, Lafferty L, et al. Self-collection for HPV-based cervical screening: a qualitative evidence meta-synthesis. BMC Public Health 2021; 21(1): 1503.
| Crossref | Google Scholar | PubMed |
15 Brewer N, Foliaki S, Gray M, et al. Pasifika women’s knowledge and perceptions of cervical-cancer screening and the implementation of self-testing in Aotearoa New Zealand: a qualitative study. Lancet Reg Health West Pac 2022; 28: 100551.
| Crossref | Google Scholar | PubMed |
16 Health New Zealand Te Whatu Ora. About HPV screening. 2024. Available at https://www.tewhatuora.govt.nz/health-services-and-programmes/ncsp-hpv-screening/about-hpv-screening [accessed 30 October 2024].
18 Ronco G, Giorgi-Rossi P, Carozzi F, et al. Efficacy of human papillomavirus testing for the detection of invasive cervical cancers and cervical intraepithelial neoplasia: a randomised controlled trial. Lancet Oncol 2010; 11(3): 249-57.
| Crossref | Google Scholar | PubMed |
19 Bromhead C, Wihongi H, Sherman SM, et al. Human Papillomavirus (HPV) self-sampling among never-and under-screened Indigenous Māori, Pacific and Asian women in Aotearoa New Zealand: a feasibility study. Int J Environ Res Public Health 2021; 18(19): 10050.
| Crossref | Google Scholar | PubMed |
20 Sherman SM, Brewer N, Bartholomew K, et al. Human papillomavirus self-testing among unscreened and under-screened Māori, Pasifika and Asian women in Aotearoa New Zealand: a preference survey among responders and interviews with clinical-trial nonresponders. Health Expect 2022; 25(6): 2914-23.
| Crossref | Google Scholar | PubMed |
21 Borchowsky K, Rush M, Mullally T, et al. Primary care experiences in the ‘Let’s test for HPV’ study: a qualitative analysis. J Prim Health Care 2023; 15(2): 147-54.
| Crossref | Google Scholar | PubMed |
22 O'connor M, Costello L, Murphy J, et al. ‘I don’t care whether it’s HPV or ABC, I just want to know if I have cancer.’ Factors influencing women’s emotional responses to undergoing human papillomavirus testing in routine management in cervical screening: a qualitative study. Bjog 2014; 121(11): 1421-9.
| Crossref | Google Scholar | PubMed |
23 Sultana F, Mullins R, English DR, et al. Women’s experience with home-based self-sampling for human papillomavirus testing. BMC Cancer 2015; 15: 849.
| Crossref | Google Scholar | PubMed |
24 Tiro JA, Betts AC, Kimbel K, et al. Understanding Patients’ Perspectives and Information Needs Following a Positive Home Human Papillomavirus Self-Sampling Kit Result. J Womens Health (Larchmt) 2019; 28(3): 384-92.
| Crossref | Google Scholar | PubMed |
25 Dodd RH, Mac OA, McCaffery KJ. Women’s experiences of the renewed National Cervical Screening Program in Australia 12 months following implementation: a qualitative study. BMJ Open 2020; 10(7): e039041.
| Crossref | Google Scholar | PubMed |
26 McBride E, Tatar O, Rosberger Z, et al. Emotional response to testing positive for human papillomavirus at cervical cancer screening: a mixed method systematic review with meta-analysis. Health Psychol Rev 2021; 15(3): 395-429.
| Crossref | Google Scholar | PubMed |
27 Creagh NS, Zammit C, Brotherton JM, et al. The experience of under-screened and never-screened participants using clinician-supported self-collection cervical screening within the Australian National Cervical Screening Program. Womens Health 2022; 18: 17455065221075905.
| Crossref | Google Scholar | PubMed |
28 Rose SB, McBain L, Bell R, et al. “Kind of scared but happy something was detected.” Cross-sectional survey of Let’s test for HPV participants to understand perspectives on an HPV detected result. Aus NZ J Obstet Gyn 2024; In production.
| Crossref | Google Scholar |
29 Rose SB, McBain L, Garrett SM, et al. “I felt so empowered, respected and shame free.” Let’s test for HPV participants’ experience of HPV primary screening. J Prim Health Care 2024;
| Crossref | Google Scholar |
30 Ministry of Health. Ethnicity data protocols. HISO 10001:2017. Wellington, Ministry of Health. 2017. Available at https://www.tewhatuora.govt.nz/assets/Our-health-system/Digital-health/Health-information-standards/HISO-10001-2017-Ethnicity-Data-Protocols.pdf [accessed 1 November 2024].
31 Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol 2006; 3(2): 77-101.
| Crossref | Google Scholar |
32 Health New Zealand Te Whatu Ora. Screening Support Services in Aotearoa New Zealand. 2024; Available at https://info.health.nz/keeping-healthy/cancer-screening/screening-support-services-in-aotearoa-new-zealand [accessed 13 November 2024].
33 Waller J, Waite F, Marlow L. Awareness and knowledge about HPV and primary HPV screening among women in Great Britain: an online population-based survey. J Med Screen 2024; 31: 91-98.
| Crossref | Google Scholar | PubMed |
34 Kola-Palmer S, Dhingra K. Awareness and knowledge of human papilloma virus in UK women aged 25 years and over: results from a cross-sectional internet-based survey. Eur J Cancer Care (Engl) 2020; 29(1): e13181.
| Crossref | Google Scholar | PubMed |
35 Ingamells S, Bell R, Nip J, et al. Perceived barriers to self-collected HPV testing for cervical cancer screening, and knowledge of HPV: a survey of primary healthcare smear-takers across Aotearoa New Zealand. NZ Med J 2024; 137(1590): 57-76.
| Crossref | Google Scholar | PubMed |
36 Chorley AJ, Marlow LA, Forster AS, et al. Experiences of cervical screening and barriers to participation in the context of an organised programme: a systematic review and thematic synthesis. Psychooncology 2017; 26(2): 161-72.
| Crossref | Google Scholar | PubMed |
37 Wearn A, Shepherd L. Determinants of routine cervical screening participation in underserved women: a qualitative systematic review. Psychol Health 2024; 39(2): 145-70.
| Crossref | Google Scholar | PubMed |


