Tackling malnutrition with a new compact oral nutrient supplement among residents in aged care: a pilot study
Wendy J. O’Brien 1 , Jessica Jellicoe 1 , Hajar Mazahery 1 , Carol Wham 1 *
1 *
1 College of Health, Massey University, Level 3, SNWE Building, Fernhill Road, Auckland Campus, Auckland 0632, New Zealand.
Journal of Primary Health Care 14(4) 363-367 https://doi.org/10.1071/HC22104
Published: 14 November 2022
© 2022 The Author(s) (or their employer(s)). Published by CSIRO Publishing on behalf of The Royal New Zealand College of General Practitioners. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
Introduction: There is a high prevalence of malnutrition among older adults entering residential aged care (RAC).
Aim: To determine whether 60 mL of a compact oral nutrition supplement (ONS; daily total: 576 kcal, 35 g protein) consumed four times daily with medication rounds improves malnutrition status, body weight, and body composition measures among older adults in RAC.
Methods: Residents (n = 20; mean age: 86.7 ± 6.8 years; 50% female) screened for malnutrition (20% malnourished, 80% at risk of malnutrition) using the Mini Nutritional Assessment-short form were recruited during April–June 2021. Participants received 60 mL of an ONS four times daily using the Medication Pass Nutrition Supplement Programme (Med Pass). The ONS intake and participant compliance were recorded. Body mass index, fat, and muscle mass (bioelectrical impedance), malnutrition risk, depressive symptoms, and quality of life were assessed at baseline and following the 18-week intervention.
Results: Median overall compliance was 98.6%. An ONS intake did not significantly increase mean ± s.d. any body composition measures or improve health and wellbeing outcomes; however, it resulted in increased body weight and body mass index (BMI; 13/20 (65%) participants), body fat mass and percentage (10/16 (63%) participants) and muscle mass (9/16 (56%) participants). Malnutrition risk scores improved in 65% (13/20) of participants, resulting in 10% being assessed as malnourished, 65% at risk of malnutrition, and 25% with normal nutrition status.
Discussion: Delivery of a compact oral nutrition supplement with the medication round was accepted by residents. Its efficacy in improving malnutrition risk and body composition among residents warrants further investigation.
Keywords: aged care, BMI, malnutrition, Med Pass, MNA‐SF, muscle mass, New Zealand, oral nutrition supplement.
| WHAT GAP THIS FILLS |
| What is already known: Malnutrition at entry into RAC is high (47%) and is associated with increased risk of sarcopenia and other adverse health outcomes. The Medication Pass Nutrition Supplement Programme (Med Pass) protocol is associated with high compliance and has led to increased appetite, meal consumption, protein intake, body weight, and decreased length of stay in RAC.9 |
| What this study adds: Compliance to intake of an ONS may be enhanced using the Med Pass protocol. The use of an ONS in addition to usual dietary intake may assist residents to meet their energy and protein requirements and maintain or regain body weight. |
Introduction
Sarcopenia, including muscle wasting and low BMI, is associated with an increased risk of falls and fractures,1 chronic respiratory disease,2 and all-cause mortality.3 Sarcopenia is also exacerbated by inadequate nutrient, especially protein, intake.4,5 Previously, 47% of older adults entering residential aged care (RAC) were reportedly malnourished and 43% were at risk of malnutrition,6 highlighting the need for effective dietary interventions to improve nutrition status. Although a ‘food first approach’ is the preferred first step, the use of an oral nutrition supplement (ONS) is recommended when the food first approach does not meet nutritional needs.7 The Medication Pass Nutrition Supplement Programme (Med Pass) protocol is associated with high levels of compliance and involves dispensing small doses (~60 mL) of energy-dense ONS (≥2 kcal mL−1) three or four times daily between meals during the medication round.8 In Australia, a randomised controlled trial using the Med Pass protocol among older hospitalised adults found a significant improvement in appetite, increased meal consumption, protein intake, body weight, and decreased length of stay.9 The addition of an ONS to the usual medication round was no more time-consuming for staff and resulted in reduced ONS wastage. This pilot study aimed to determine whether use of a compact ONS using the Med Pass protocol could reduce malnutrition risk and improve body composition and health and wellbeing measures among RAC residents in New Zealand.
Methods
A single arm intervention pilot study was conducted among residents at an Auckland RAC facility.10 Although the original design was a two-armed study, restrictions due to coronavirus disease 2019 (COVID-19) lockdown prevented the researchers from taking follow-up measures in the control group. Inclusion criteria were: Mini Nutritional Assessment-Short Form (MNA-SF) score 0–11 (malnourished or at risk of malnutrition), aged ≥65 years, BMI <30 kg m−2, and deemed eligible by the RAC clinical manager. Participants not eligible to participate were those with an acute decline in cognition or function, or those in need of acute palliative care. Residents were recruited between April and June 2021, and they or their enduring power of attorney were guided through a Participant Information Sheet and provided written informed consent before data were collected. The study was approved by the New Zealand Health and Disability Ethics Committee (No. 20/NTB/120/AM01).
Sociodemographic and health data, height, body weight and composition, nutrition risk status, depressive symptoms, and quality of life were assessed at baseline and following the 18-week intervention. Sociodemographic and health data were obtained, with permission during the consent process, from the residents’ clinical notes. Muscle and fat mass were assessed using portable bioelectrical impedance (InBodyS10, Inbody Co. Ltd., Seoul, Korea) validated against dual‐energy X‐ray absorptiometry (DEXA).11 Nutrition risk was assessed using the MNA-SF; malnourished (score 0–7), at risk of malnutrition (score 8–11), or normal nutrition status (score 12–14).12 Depressive symptoms were assessed using the validated 15-item Geriatric Depression Scale (GDS): normal to no depressive symptoms (score 0–4), mild depression (score 5–9), and moderate to severe depression (score 10–15).13 Quality of life was assessed using the Medical Outcomes Study 12-item Short Form Survey (SF-12) tool to describe physical and mental functioning, which indicates disability as being: absent (score >50), mild (score 40–50), moderate (score 30–40), or severe (score <40).14
Participants received neutral-flavoured Fortisip™ Compact Protein (Danone Nutricia, Australia) four times daily for 18 weeks, which provided 576 kcal energy and 35 g protein daily in addition to usual dietary intake. The ONS (60 mL) was dispensed in small glasses by registered nurses during the medication rounds at 8 am, 12 pm, 5 pm, and 7 pm. Participant compliance was monitored by nurses who also noted any reason for not consuming the full serving.
Statistical analysis was carried out using SPSS statistical software (Version 27; SPPS Inc., Chicago, IL, USA). Normality of data was assessed using Shapiro–Wilk and Kolmogorov–Smirnov tests. Parametric data are presented as mean and standard deviation and non-parametric data as median with 25th and 75th percentiles. Pre- and post-intervention measurements were compared using dependent sample t-tests for parametric data and Wilcoxon signed-rank test for non-parametric data. Significance was set at P = 0.05.
Results
Of 50 residents who met the eligibility criteria, 32 provided consent and 20 completed the study (12 dropouts: 2 participant burden, 3 removed by clinical management, 2 unacceptable weight gain, 1 appetite suppressed, 4 died).
Participants’ characteristics and health status are presented in Table 1. Participants were 50% women and had a mean (±s.d.) age of 86.7 ± 6.8 years. The MNA-SF score median [25th, 75th percentile] was 9.0 [7.3, 10.0], with 20% of participants assessed as malnourished and 80% as at risk of malnutrition. Participants had 6.0 ± 2.7 co-morbidities and took 7.5 ± 3.3 medications.

|
Overall, 98.6% of the prescribed ONS was consumed over the 18-week intervention. The most common reasons for not consuming the whole dose were disliking the taste, being unwell or asleep.
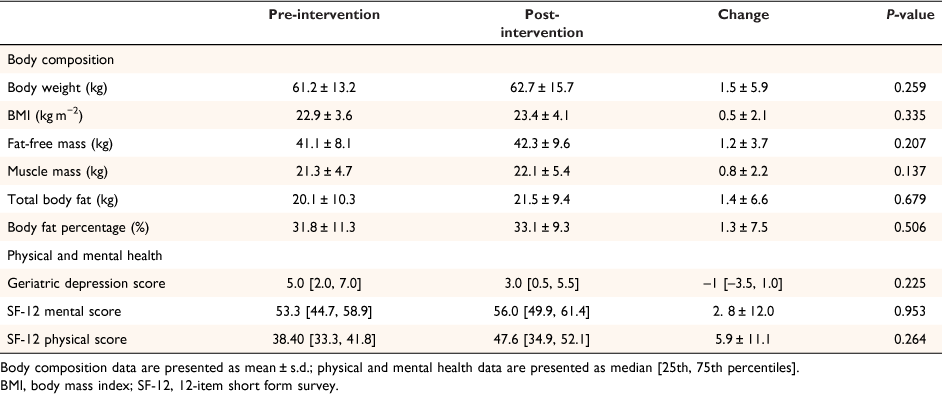
Although the ONS did not significantly improve most body composition or any health and wellbeing measures (Table 2), it resulted in an increase in body weight and BMI in 65% (13/20) of participants. Total body fat mass and percentage increased in 63% (10/16) and muscle mass increased in 56% (9/16) of participants. An increase in total body fat mass and percentage was observed in a higher proportion of participants aged ≥90 (83%) than <90 (50%) years. Seventy-seven percent of residents whose nutrition risk status improved also had an increase in body weight and BMI compared to only 43% whose nutritional status did not improve (P = 128 for both weight and BMI).
|
The MNA-SF score increased in 65% (13/20) of participants in response to the intervention, resulting in nutrition risk status of: malnourished 10%, at risk of malnutrition 65%, and normal nutrition 25%. Non-significant changes in the GDS (−1 [−3.5, 1.0]), SF-12 mental component (+2.8 ± 12.0), and SF-12 physical component (+5.9 ± 11.1) scores were observed.
Discussion
This pilot study is the first to report outcomes of, and compliance to (98.6%), a compact, energy-dense ONS using the Med Pass protocol among RAC residents in New Zealand. The high compliance to the 18-week intervention demonstrates its acceptability among RAC residents. A similar intervention in Germany among RAC residents who were malnourished or at risk of malnutrition reported 73% compliance to a 12-week intervention.15 Although the total volume of ONS in that study matched the current study, it was served as 2×125 mL bottles of ONS (Fortimel Compact, Nutricia) rather than 4 × 60 mL of ONS served in glasses as in the current study. The study also reported that high compliance (≥80%) led to significantly greater weight gains than low compliance (≤30%), highlighting the importance of compliance to promote body weight maintenance and reduce risk of sarcopenia.
BMI is a significant predictor of sarcopenia risk among RAC residents,16 hence the importance of identifying and treating malnutrition in RAC. Despite non-significant findings (P > 0.05), the current study resulted in improved physical measures over the 18-week intervention. Over half the participants showed improvements in body weight, BMI, fat-free mass, muscle mass, and nutrition risk status. Significant increases in muscle mass following similar ONS interventions have been reported in Taiwan and USA.17–19 An increase in muscle mass from baseline to post-intervention was the measure nearest to reaching significance and showed a small–medium effect size. This finding suggests, with a larger sample of residents, significance may have been reached and body composition changes could be improved. Nutrition risk (MNA-SF) scores improved by 10%, resulting in fewer participants assessed as malnourished (10%) or at risk of malnutrition (65%), and a resultant one-quarter (25%) of participants returning to normal nutrition risk status.
This pilot study had several limitations. First, during the final 30 days of the intervention, New Zealand was under COVID-19 lockdown restrictions. As a result, residents were prevented from undertaking usual levels of physical activity and from having visitors. These factors may have adversely affected residents’ appetite, usual dietary intake, and psychological wellbeing. Also, due to COVID-19 restrictions within the RAC facility, we were unable to engage non-intervention residents as a control group. Although small–medium effect sizes were observed, the small sample size did not allow the findings to reach significance. Although the inclusion of cognitively impaired residents reduced the overall response rate to the SF-12 and GDS questionnaires, their inclusion helped to improve the generalisability of the findings.
Conclusion
Overall, we observed an improvement in participant nutrition risk status, body weight, and muscle mass. Providing a nutrient and energy-dense ONS using the Med Pass protocol may be an effective method to improve nutrition risk status in RAC residents and warrants further investigation among a larger sample.
Data availability
The data that support this study will be shared upon reasonable request to the corresponding author.
Conflicts of interest
The authors declare no conflicts of interest.
Declaration of funding
This research was supported by a product grant from Nutricia.
References
[1] Yeung SSY, Reijnierse EM, Pham VK, et al. Sarcopenia and its association with falls and fractures in older adults: a systematic review and meta‐analysis. J Cachexia Sarcopenia Muscle 2019; 10 485–500.| Sarcopenia and its association with falls and fractures in older adults: a systematic review and meta‐analysis.Crossref | GoogleScholarGoogle Scholar |
[2] Bone AE, Hepgul N, Kon S, et al. Sarcopenia and frailty in chronic respiratory disease: lessons from gerontology. Chron Respir Dis 2017; 14 85–99.
| Sarcopenia and frailty in chronic respiratory disease: lessons from gerontology.Crossref | GoogleScholarGoogle Scholar |
[3] Liu P, Hao Q, Hai S, et al. Sarcopenia as a predictor of all-cause mortality among community-dwelling older people: a systematic review and meta-analysis. Maturitas 2017; 103 16–22.
| Sarcopenia as a predictor of all-cause mortality among community-dwelling older people: a systematic review and meta-analysis.Crossref | GoogleScholarGoogle Scholar |
[4] Cruz-Jentoft AJ, Bahat G, Bauer J, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing 2019; 48 16–31.
| Sarcopenia: revised European consensus on definition and diagnosis.Crossref | GoogleScholarGoogle Scholar |
[5] Mithal A, Bonjour J-P, Boonen S, et al. Impact of nutrition on muscle mass, strength, and performance in older adults. Osteoporos Int 2013; 24 1555–66.
| Impact of nutrition on muscle mass, strength, and performance in older adults.Crossref | GoogleScholarGoogle Scholar |
[6] Wham C, Fraser E, Buhs-Catterall J, et al. Malnutrition risk of older people across district health board community, hospital and residential care settings in New Zealand. Australas J Ageing 2017; 36 205–11.
| Malnutrition risk of older people across district health board community, hospital and residential care settings in New Zealand.Crossref | GoogleScholarGoogle Scholar |
[7] Volkert D, Beck AM, Cederholm T, et al. ESPEN guideline on clinical nutrition and hydration in geriatrics. Clin Nutr 2019; 38 10–47.
| ESPEN guideline on clinical nutrition and hydration in geriatrics.Crossref | GoogleScholarGoogle Scholar |
[8] Canadian Agency for Drugs and Technologies in Health. The Medication Pass Nutritional Supplement Program in Patients Receiving Medication: A Review of Clinical Effectiveness and Guidelines. Ottawa (ON): Canadian Agency for Drugs and Technologies in Health; 2015. Available at https://www.ncbi.nlm.nih.gov/books/NBK304827/
[9] Jukkola K, MacLennan P. Improving the efficacy of nutritional supplementation in the hospitalised elderly. Australas J Ageing 2005; 24 119–24.
| Improving the efficacy of nutritional supplementation in the hospitalised elderly.Crossref | GoogleScholarGoogle Scholar |
[10] Jessica J. Tackling malnutrition in Residential Aged Care (RAC) with a new compact Oral Nutritional Supplement (ONS). MSc Thesis, Massey University, New Zealand; 2022.
[11] Ling CHY, de Craen AJM, Slagboom PE, et al. Accuracy of direct segmental multi-frequency bioimpedance analysis in the assessment of total body and segmental body composition in middle-aged adult population. Clin Nutr 2011; 30 610–5.
| Accuracy of direct segmental multi-frequency bioimpedance analysis in the assessment of total body and segmental body composition in middle-aged adult population.Crossref | GoogleScholarGoogle Scholar |
[12] Kaiser MJ, Bauer JM, Ramsch C, et al. Validation of the Mini Nutritional Assessment Short-Form (MNA®-SF): a practical tool for identification of nutritional status. J Nutr Health Aging 2009; 13 782
| Validation of the Mini Nutritional Assessment Short-Form (MNA®-SF): a practical tool for identification of nutritional status.Crossref | GoogleScholarGoogle Scholar |
[13] Yesavage JA, Brink TL, Rose TL, et al. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res 1982; 17 37–49.
| Development and validation of a geriatric depression screening scale: a preliminary report.Crossref | GoogleScholarGoogle Scholar |
[14] Jakobsson U, Westergren A, Lindskov S, et al. Construct validity of the SF‐12 in three different samples. J Eval Clin Pract 2012; 18 560–6.
| Construct validity of the SF‐12 in three different samples.Crossref | GoogleScholarGoogle Scholar |
[15] Jobse I, Liao Y, Bartram M, et al. Compliance of nursing home residents with a nutrient- and energy-dense oral nutritional supplement determines effects on nutritional status. J Nutr Health Aging 2015; 19 356–64.
| Compliance of nursing home residents with a nutrient- and energy-dense oral nutritional supplement determines effects on nutritional status.Crossref | GoogleScholarGoogle Scholar |
[16] Darroch P, O’Brien WJ, Mazahery H, et al. Sarcopenia prevalence and risk factors among residents in aged care. Nutrients 2022; 14 1837
| Sarcopenia prevalence and risk factors among residents in aged care.Crossref | GoogleScholarGoogle Scholar |
[17] Lee LC, Tsai AC, Wang JY. Need-based nutritional intervention is effective in improving handgrip strength and Barthel Index scores of older people living in a nursing home: a randomized controlled trial. Int J Nurs Stud 2015; 52 904–12.
| Need-based nutritional intervention is effective in improving handgrip strength and Barthel Index scores of older people living in a nursing home: a randomized controlled trial.Crossref | GoogleScholarGoogle Scholar |
[18] Lee LC, Tsai AC, Wang JY, et al. Need-based intervention is an effective strategy for improving the nutritional status of older people living in a nursing home: a randomized controlled trial. Int J Nurs Stud 2013; 50 1580–8.
| Need-based intervention is an effective strategy for improving the nutritional status of older people living in a nursing home: a randomized controlled trial.Crossref | GoogleScholarGoogle Scholar |
[19] Chen L, Nelson DR, Zhao Y, et al. Relationship between muscle mass and muscle strength, and the impact of comorbidities: a population-based, cross-sectional study of older adults in the United States. BMC Geriatr 2013; 13 74
| Relationship between muscle mass and muscle strength, and the impact of comorbidities: a population-based, cross-sectional study of older adults in the United States.Crossref | GoogleScholarGoogle Scholar |


