Pre-diagnostic routes to colorectal cancer in Central New Zealand: factors that lead to emergency presentation and longer diagnostic intervals at primary and secondary level care
Melissa Warren 1 2 * , Jon Emery 3 , Mei Krishnasamy 1 4 5 , Anne O'Donnell 6 , Karla Gough 1 71 Department of Nursing, Faculty of Medicine, Dentistry and Health Sciences, The University of Melbourne, Parkville, Vic. 3010, Australia.
2 Breast Cancer Foundation New Zealand.
3 Department of General Practice, Faculty of Medicine, Dentistry and Health Sciences, The University of Melbourne, Vic., Australia.
4 VCCC Alliance, Melbourne, Vic., Australia.
5 Academic Nursing Unit, Peter MacCallum Cancer Centre, Melbourne, Vic., Australia.
6 Department of Medical Oncology, Capital and Coast District Health Board, Wellington, New Zealand.
7 Department of Health Services Research, Peter MacCallum Cancer Centre, Melbourne, Vic., Australia.
Journal of Primary Health Care 14(1) 48-56 https://doi.org/10.1071/HC21107
Published: 13 April 2022
© 2022 The Author(s) (or their employer(s)). Published by CSIRO Publishing on behalf of the Royal New Zealand College of General Practitioners. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
Introduction: Although international large-scale studies have investigated routes to diagnosis for colorectal cancer, there is limited information on how New Zealanders seek help for bowel symptoms across different pre-diagnostic routes.
Aim: To better understand pre-diagnostic routes for colorectal cancer, including the characteristics of patients and key events associated with each route.
Methods: This study was a retrospective audit of hospital administrative and medical records for 120 patients with a confirmed diagnosis of colorectal cancer between 2016 and 2017. All patients were receiving care at one of two hospitals in central New Zealand; one urban and one rural. Extracted data were used to: categorise pre-diagnostic routes for colorectal cancer; describe the characteristics of people who presented by each route; and compare key events in the diagnostic and treatment intervals for people who presented by each route.
Results: Six routes to the diagnosis of colorectal cancer were identified. The three main routes included: routine general practitioner (GP) referral (28%, 95% CI: 21–37%), emergency presentation (27%, 95% CI: 20–35%), and other outpatient services (26%, 95% CI: 19–34%). Patients diagnosed by routine GP referral had the longest time to diagnosis, impacting on timeliness of treatment.
Discussion: This study has generated detailed insights about pre-diagnostic routes for colorectal cancer in New Zealand and shown consistency with findings from previously published international research. The granular findings can now inform areas for person- and system-level interventions that, in turn, could be tested in future studies to minimise emergency department and late presentations for colorectal cancer treatment in New Zealand.
Keywords: Bowel symptoms; cancer diagnosis; colorectal cancer; diagnosis delay; general practice; health-care access; hospital care; New Zealand.
Introduction
Colorectal cancer is the second most common cancer in both men and women and the second highest cause of cancer death in New Zealand.1,2 Few studies have investigated pre-diagnostic patient pathways, or routes, to diagnosis for colorectal cancer in New Zealand.3–5 Different routes can affect timeliness of care and clinical outcomes.6
Early diagnosis is considered to be central to improving colorectal cancer outcomes in New Zealand patients.1 The free National Bowel Screening Programme for men and women aged 60–74 years was introduced in New Zealand in July 2017 as part of a staged roll-out programme to help detect bowel cancer at an early stage. Although the Bowel Screening Programme will help to reduce morbidity and mortality, prompt symptomatic diagnosis also remains a priority, as most colorectal cancers are detected symptomatically.6,7
Pathways to cancer diagnosis have been defined as having four main time points: date of first symptom; date of first presentation; date of referral (for specialist investigation); and date of diagnosis. There are therefore four main intervals: appraisal; help-seeking; diagnostic; and pre-treatment.8 The appraisal interval, or the time taken to interpret bodily changes and symptoms, and the help-seeking interval, or the time taken to seek help from a health professional such as a general practitioner (GP), are estimated to represent the greatest proportion of the total time in the pathway to diagnosis for colorectal cancer.4,6,7
| WHAT GAP THIS FILLS |
| What is already known: Time taken to interpret bowel symptoms and to seek help from a health professional such as a general practitioner are estimated to represent the greatest proportion of the total time in the pre-diagnostic pathway for colorectal cancer diagnosis. |
| What this study adds: This study investigated pre-diagnostic patient pathways to diagnosis of colorectal cancer in New Zealand. The largest proportion of patients diagnosed with colorectal cancer in hospital started their colorectal cancer diagnosis and treatment pathway by general practitioner referral, either as a routine or urgent 2-week wait referral. Primary healthcare professionals are pivotal in the early detection and timely referral of patients with colorectal cancer to secondary level care, reducing diagnostic delay and contributing to optimal health outcomes. |
The diagnostic interval includes primary care, referral, and secondary care intervals.9 Throughout the diagnostic interval, delays in diagnosis arise from healthcare system factors such as waiting times for investigations, specialist appointments, and inadequate integration between primary and secondary level care.10 Patients’ first point of contact with health services is usually in primary health care, so primary health-care teams have an important role in the early detection and diagnosis of cancer.11 GPs are often considered ‘gatekeepers’ to hospital-led services, facilitating appropriate and timely referral of patients from primary level care into secondary level care specialist services.11
Timely access to specialist cancer services and reducing diagnostic delays in the health-care system has prompted many countries, including New Zealand, to implement ‘fast-track’ cancer referral routes for patients with a high likelihood of cancer. Although fast-track referral routes can help with timely access to specialist services and reduce diagnostic delay,11 the challenge for the health system is that many patients with colorectal cancer will present with single or subtle symptoms that are not strongly predictive of colorectal cancer, so they do not meet the urgent referral criteria that hospitals use.5
The 2015 PIPER (Presentations, Investigations, Pathways, Evaluation and Rx) study1 investigated differences in survival after diagnosis with colorectal cancer by rurality, ethnicity and socioeconomic deprivation in New Zealand and provided comprehensive insights into the outcome and management of New Zealanders with colorectal cancer. A key finding from the PIPER study was the high rates of emergency presentation, accounting for one-third of patients with colon cancer. Māori and Pacific peoples were more likely to present acutely at an emergency department,1 a route associated with higher morbidity and mortality than non-emergency presentation routes.12–14 Investigation of other pre-diagnostic pathways was not part of the scope of this study.
A scoping review of original research investigating the pre-diagnostic period for colorectal cancer in New Zealand identified eight studies published between 2009 and 2019, highlighting the paucity of research carried out in New Zealand to investigate pre-diagnostic pathways for colorectal cancer.5 Of these eight studies, most were >5 years, qualitative, and focused on screening.
The main aim of this study was to better understand pre-diagnostic routes for colorectal cancer, including the characteristics of patients and key intervals associated with each route. The objectives were to categorise pre-diagnostic routes for people receiving care for colorectal cancer at one urban and one rural hospital in central New Zealand, describe the characteristics of people who presented by each route, and compare key events in the diagnostic and treatment intervals for people who presented by each pre-diagnostic route.
Methods
We used a retrospective case file audit and process mapping for 120 patients with a confirmed diagnosis of colorectal cancer from first pre-diagnostic health-care provider presentation to first treatment.
Patient sample
The target sample was 120 patients with a confirmed diagnosis of colorectal cancer who had received treatment at one urban and one rural hospital in New Zealand. Patients were identified from the New Zealand Cancer Registry. Patients with a confirmed diagnosis of colorectal cancer between 2016 and 2017 from one urban hospital (Wellington Hospital) and one rural hospital (Masterton Hospital) in central New Zealand were selected from the registry data.2 All Māori and Pasifika patients were purposefully selected (Māori, n = 21; Pasifika, n = 8). The remaining patients (non-Māori, n = 91) were sampled consecutively. Consecutive sampling included all non-Māori patients who met the inclusioncriteria; that is, they had a confirmed diagnosis of colorectal cancer and data were available from first pre-diagnostic health-care provider presentation to first treatment. It was important to capture pre-diagnostic routes for Māori, as they are more likely to be diagnosed following presentation to an emergency department.1
Data collection
Patient characteristics were collected and recorded using Research Electronic Data Capture (REDCap; Vanderbilt University), a web-based application to support data capture and management. Information gathered from medical records included patient demographics (age, sex, ethnicity, marital status, living situation, and geographical area of home address) and main presenting lower gastro-intestinal symptoms. Triage prioritisation at secondary level care was informed by the New Zealand Ministry of Health (MoH) Referral Criteria for Direct Outpatient Colonoscopy or CT Colonography: 2-week category (rapid diagnosis), and 6-week category (semi-urgent).15 Geographical areas of patients’ home addresses were converted using the New Zealand Department 2013 Index of Deprivation to provide a measure of relative socioeconomic deprivation.16
The Elliss–Brookes ‘Routes to Diagnosis’ (Box 1) were used to categorise each patient’s pre-diagnostic route for colorectal cancer.17 These routes were developed from information about 739 667 individuals diagnosed with cancer in England in 2006–08. The National Bowel Screening Programme was initiated at the rural Masterton Hospital in July 2017, but at the time of the study had not been launched at the urban Wellington Hospital. The Model of Pathways to Treatment was designed to inform the measurement, description, and interpretation of times to diagnosis and treatment initiation,18,19 and guided the choice and definition of key events for process mapping (detection of bodily changes, patient perceives reason to discuss symptom with health-care professional, first consultation with health-care professional, diagnosis, and start of treatment).
| Box 1. Routes to diagnosis described by Elliss–Brookes et al.17 | ||||||||||||||||||
| ||||||||||||||||||
Timely access to services was based on MoH Faster Cancer Treatment Indicators.20 The 31- day indicator is that patients with a confirmed cancer receive their first cancer treatment (or other management) within 31 days of a decision to treat. The 62- day indicator is that patients referred urgently with a high suspicion of cancer receive their first treatment within 62 days of the referral being received by the hospital.
Information needed to determine whether patients received timely access to services was based on number of days from date of receipt of referral (in study hospitals) to diagnosis, decision to treat and first treatment. In practice, District Health Boards collect the 62-day target data (when there is high suspicion of cancer and the hospital doctor receiving the referral believes there is a need for an appointment within 2 weeks) for reporting to the MoH only for patients on the urgent GP 2-week wait route. For this study, the 62-day target was applied to all pre-diagnostic routes to explore in-depth the wait times to first specialist appointment, diagnostic investigation, and first treatment for each of the routes.
Statistical analysis
Descriptive statistics were used to summarise data about pre-diagnostic routes to diagnosis for colorectal cancer. They were also used to summarise patient demographics, main presenting symptoms and disease stage by pre-diagnostic route. Descriptive statistics included counts and percentages for nominal variables and medians and interquartile ranges for continuous variables. Confidence intervals were estimated using the Agresti–Coull method.21
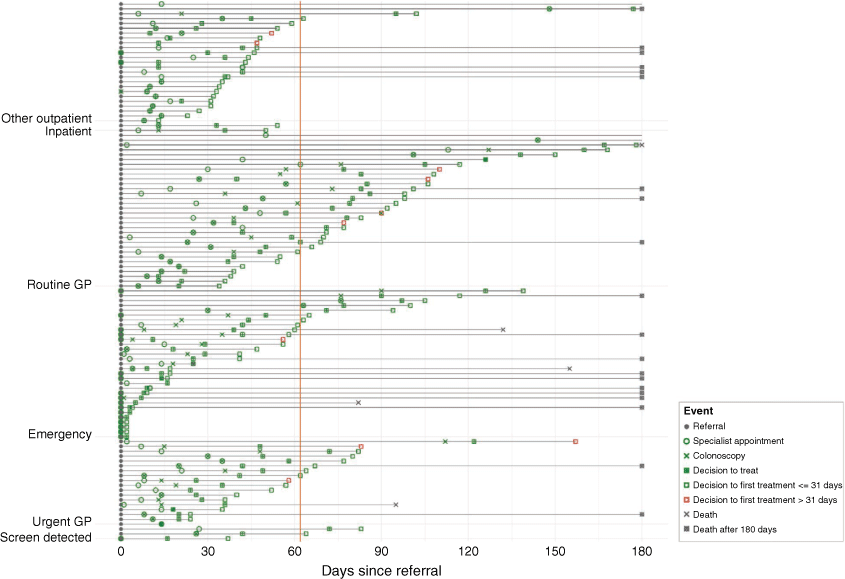
Process mapping the sequence of steps or events that occur within a specified time frame22 was carried out to highlight and compare key events in the diagnostic and treatment intervals of people presenting by each route. We prepared a simplified event plot, sorted by route to diagnosis, to depict the sequencing of key events and delays in the first 180 days following referral for hospital care. Days were calculated using dates from referral to first specialist appointment, colonoscopy, and decision to treat, from decision to treat to first treatment, and from referral to death (before first 180 days). All analyses were performed in R version 3.6.1.23 The event plot was also created in R using the ‘ggplot2’ package.24 The study was not designed or powered for comparisons between patients who presented via different routes.
Ethics
Ethical approval for this study was granted by the New Zealand Health and Disability Ethics Committee (18/STH/77).
Results
Patient characteristics
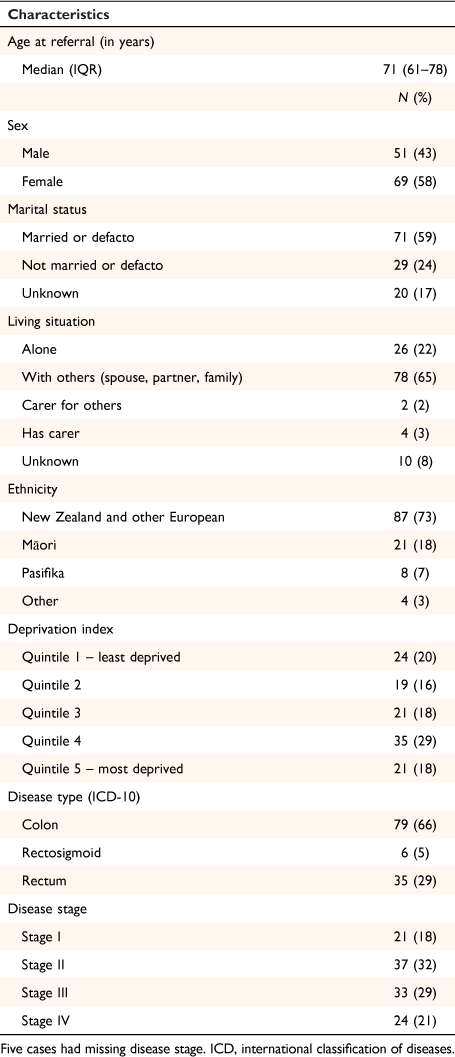
Demographic and clinical characteristics for the sample are presented in Table 1. Twenty-one (18%) patients were Māori, 8 (7%) Pasifika, and 91 (76%) non-Māori.
|
Routes to diagnosis
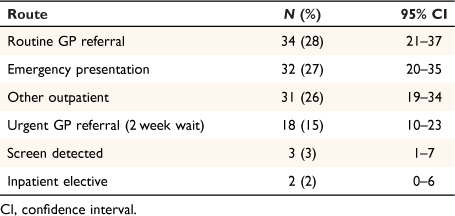
Table 2 shows the number of patients in each route to colorectal cancer diagnosis. Patients presented were from six of the eight pre-diagnostic routes previously described.17 The three main pre-diagnostic routes were: routine GP referral (37 patients (28%, 95% CI: 21–37%)); emergency presentation (32 patients (27%, 95% CI: 20–35%)); and other outpatient (an elective route starting with an outpatient appointment, following self-referral, consultant-to-consultant, other or unknown referral; 31 patients (26%, 95% CI: 19–34%)). Eighteen of the 52 patients presenting via a GP referral route came via the urgent GP 2-week wait route.
|
Patient characteristics by pre-diagnostic route
Patient characteristics by pre-diagnostic route are shown in Table 3. More urban hospital patients followed an emergency (69%) presentation route than rural patients (31%). More rural patients followed an urgent GP 2- week wait route (67%) than urban patients (33%). More women (56%) had an emergency presentation than men (44%), and one-third (31%) of Māori and Pasifika patients (31%) had an emergency presentation. More patients living in areas with high levels of deprivation presented via an emergency route than patients living in least deprived areas.

|
Presenting symptoms by pre-diagnostic route
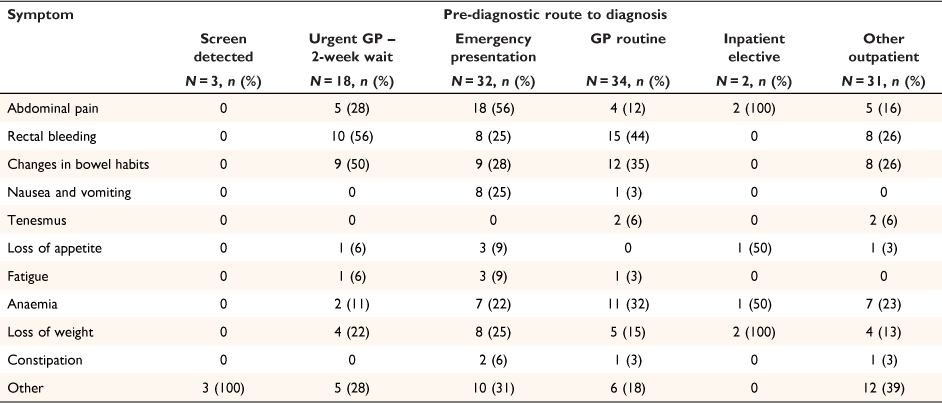
Presenting symptoms by pre-diagnostic route are shown in Table 4. The greatest proportion of pre-diagnostic symptoms occurred for patients on the emergency presentation route, followed by patients on the urgent GP 2- week wait and GP routine routes. Abdominal pain, rectal bleeding and changes in bowel habits were the main presenting symptoms. For patients presenting via an emergency pathway, 56% experienced abdominal pain and 56 and 44% of those presenting via the urgent GP 2- week wait or GP routine routes reported rectal bleeding.
|
Faecal occult blood testing in primary care
Local agreement with Wellington hospital meant that urban GPs did not routinely request faecal occult blood tests as part of their urgent or routine referrals. In contrast, faecal occult blood tests were carried out by rural GPs for 58% (7/12) of patients referred on the urgent GP route and 53% (9/17) of patients on the routine GP referral route.
Disease stage by route to diagnosis
Fifty-five of 120 patients (46%) were diagnosed with stage I–II disease and 58 patients (48%) were diagnosed with stage III–IV disease. Disease stage was unknown for seven patients. Among patients presenting as emergencies, 63% (20/32) of patients had late-stage disease compared with 50% (9/18) of patients who presented by the urgent GP route and 45% (15/34) of patients presenting via the routine GP referral route. Of 31 patients who presented via the other outpatient route, 11 (35%) had late-stage disease. Of three patients diagnosed via the National Bowel Screening Programme, one had late-stage disease and both patients diagnosed on the inpatient elective route also had late-stage cancer.
Key events by route to diagnosis
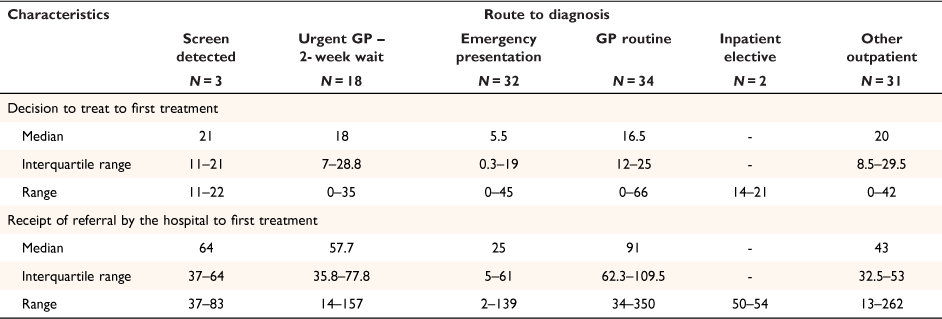
In Fig. 1, a sequence of key events (and delays) for the first 180 days is presented by pre-diagnostic route. The orange line on the event plot highlights the MoH’s Faster Cancer 62-day target. Irrespective of pre-diagnostic route, most patients received their first treatment within 31 days from decision to treat (31-day indicator). Only 10 patients (8%) (cases with red squares on the event plot) received their first treatment > 31 days from the decision to treat.
Compared to patients referred via other pre-diagnostic routes, patients referred via the routine GP pathway had longer wait times to first specialist appointment and colonoscopy investigation. Most patients who presented via the emergency route received their first treatment before the 31-day indicator; however, emergency presentation is considered a sub-optimal pathway, with poorer clinical outcomes than experienced by patients diagnosed through screening or non-emergency routes. Of 34 patients presenting via the routine GP route, 24 (71%) exceeded the 62-day indicator from referral receipt by the hospital to first treatment, with 17 (50%) patients taking >90 days to first treatment (Table 5). Most patients on the routine GP referral route (29/34; 85%) presented with one red flag symptom (unexplained rectal bleeding, iron deficiency, or altered bowel habit for >6 weeks), and were triaged as semi-urgent by hospital specialties (gastroenterology or general surgery).
|
Discussion
Despite colorectal cancer being the second most commonly diagnosed cancer in New Zealand, few studies have explored pre-diagnostic routes for New Zealanders affected by colorectal cancer.5 This study used Routes to Diagnosis17 to categorise pre-diagnostic routes, and our data demonstrated strong alignment with the routes previously identified in England. The only major differences between the two studies were proportions for urgent GP referral (15% in this study compared to 26% in the England study17) and other outpatient routes (26% in this study compared to 10% in the England study17).
Although there is robust evidence to demonstrate that the urgent GP 2- week wait referral route has contributed to shorter diagnostic intervals for colorectal cancer,25 it relies on patients having ‘red flag’ symptoms to meet the criteria for urgent GP fast-track referral. Another crucial factor is the variation in local health services. Access to diagnostic tests and to specialist advice for suspected cancer in primary care patients is more limited and slower in New Zealand than in other high-income countries.26 Evidence from the United Kingdom (UK) shows that variation in referral and cancer detection rates by GPs may have less to do with individual GP behaviours or practice characteristics and more to do with local health system capability, such as availability of diagnostic services and specialist service providers.27 Barriers to primary and secondary care collaboration also contributed to differential pathways to diagnosis and clinical outcomes.27 Differences in referral processes, internal assessment and triage of referrals for suspected colorectal cancer were also demonstrated in our study.
More patients living in areas with high levels of deprivation presented as emergencies than patients living in areas with lower levels of deprivation, confirming findings from earlier research that demonstrate an association between social determinants of health, socio-demographic inequalities, and emergency presentation routes.28 Presentation as an emergency is strongly associated with poorer cancer outcomes13 and patients who presented via an emergency route in this study were more likely to be diagnosed with late-stage disease.
Regardless of ethnicity, patients on the routine GP referral route had the longest time interval to diagnosis and first treatment. One reason for this is that patients on this route were not perceived to have a high suspicion of cancer and were triaged as semi-urgent for further investigation. Many patients (82%) on the GP routine referral route presented with a single ‘red flag’ colorectal symptom and were aged >60 years, placing them at higher risk for colorectal cancer. Two-thirds of new colorectal cancer registrations in New Zealand are for people aged ≥65 years.2
The New Zealand MoH Referral Criteria for Direct Outpatient Colonoscopy or computed tomography (CT) Colongraphy15 includes two referral categories for GPs and non-gastrointestinal specialists: 2-week category (rapid diagnosis), and 6-week category (semi-urgent). Although the 6-week referral category includes patients with ‘red flag’ symptoms, triage priority at secondary level care is given to patients referred with a combination of symptoms over patients with just one lower-gastrointestinal symptom.
Recent studies in the UK and Australia29–33 have examined how referral and triage of high- and lower-risk bowel cancer symptoms in primary and secondary care can be enhanced by improving the time taken to interpret bodily changes and symptoms, the risk stratification for symptoms suggestive of bowel cancer and the use of faecal immunochemical testing (FIT). In Australia,32 triage prioritisation criteria to determine patients most likely to benefit from urgent colonoscopy investigation have been developed. Triage prioritisation to identify people more likely to have colorectal cancer takes into account both single and combinations of symptoms in addition to positive immunohistochemical faecal occult blood test (iFOBT (+)), anaemia, and age >60 years.33
There is growing interest internationally in the use of FIT to triage symptomatic patients. Studies in the UK29–31 have shown that FIT can improve the sensitivity of triage of patients with new bowel symptoms and can safely and objectively determine a patient’s risk of significant bowel disease. Patients with low-risk and single symptoms seldom meet urgent referral criteria. FIT has been shown to perform well in this lower-risk primary care population and could be used to rule in patients who require prompt investigation. A key finding from our study was that many patients on the GP routine referral route did not meet criteria for a high suspicion for cancer, yet were ultimately diagnosed with colorectal cancer. These are the patients for whom FIT could improve triage.
Data collected for our study (2016 and 2017) precedes review of the referral and triage process, and timeliness of the diagnostic pathway at primary and secondary level care across the country. At a national level, the MoH has recently updated the guidance and criteria that allow GPs to refer patients directly for outpatient bowel investigation (colonoscopy or CT colonography) if they have symptoms or signs suggestive of bowel cancer and meet the referral criteria for investigation without first seeing a gastroenterologist or general surgeon.15 The MoH has published Bowel Cancer Quality Performance Indicators34 that will help identify the proportion of people with bowel cancer who are diagnosed following a referral to a clinic, screening, or presentation to emergency department, and assess the timeliness of treatment (from first histological diagnosis to first treatment), through standardised measurement of performance. These measures may prompt system change.
Our study has limitations. Case file audit (retrospective examination of patients’ medical records) and process mapping is considered an important source of information to augment and validate study of the routes to diagnosis for colorectal cancer. The main limitations of case file audit are the accuracy of the information in the case notes (medical records) and limited information on the pre-diagnostic pathway at primary care level. Another potential limitation is the small sample size. Small samples may produce unreliable estimates leading to incorrect inferences.35 Nevertheless, proportions of patients on each route to diagnosis in this study were compatible with those reported by others.1,3,6,14,17 We provide confidence intervals to give information about the degree of uncertainty, consistent with best practice.35
Despite its small sample size, this study has provided insight to the main pre-diagnostic routes for colorectal cancer in central New Zealand. Findings have shown that nearly half of patients had followed a GP routine or urgent (2-week wait) route to diagnosis for colorectal cancer, highlighting the important role GPs and primary health-care teams have in optimising early detection and diagnosis of cancer. To further improve outcomes from colorectal cancer, intervention is needed at a system level to strengthen the interface between primary and secondary level care, enhancing opportunity for standardised referral and triage processes for all patients affected by colorectal cancer.
Data availability
The data that support this study are available in the article.
Conflicts of interest
The authors declare they have no conflicts of interest.
Declaration of funding
This PhD study was supported by the Cancer Research Trust New Zealand – Cancer Nurse Fellowship (Award code GOT-1633-NF).
Acknowledgements
Melissa Warren wishes to thank Cancer Research Trust New Zealand.
References
[1] Jackson C, Sharples K, Firth M, et al. The PIPER Project: an internal examination of colorectal cancer management in New Zealand. Cancer trials New Zealand. Ministry of Health; 2015.[2] New Zealand Ministry of Health. Cancer new registrations and deaths 2013. Wellington, New Zealand: Ministry of Health; 2016.
[3] Windner Z, Crengle S, de Graaf B, et al. New Zealanders’ experiences and pathways to a diagnosis of bowel cancer: a cross-sectional descriptive study of younger cohort. NZ Med J 2018; 131 30–39.
[4] Blackmore T, Norman K, Kidd J, et al. Barriers and facilitators to colorectal cancer diagnosis in New Zealand: a qualitative study. BMC Fam Pract 2020; 21 206
| Barriers and facilitators to colorectal cancer diagnosis in New Zealand: a qualitative study.Crossref | GoogleScholarGoogle Scholar | 33003999PubMed |
[5] Firth M, Blackmore T, Chepulis L, et al. Why does New Zealand have such poor outcomes from colorectal cancer?: the importance of the pre-diagnostic period. J Prim Health Care 2021; 13 15–16.
| Why does New Zealand have such poor outcomes from colorectal cancer?: the importance of the pre-diagnostic period.Crossref | GoogleScholarGoogle Scholar | 33785107PubMed |
[6] Hall N, Birt L, Banks J, et al. Symptom appraisal and healthcare-seeking for symptoms suggestive of colorectal cancer: a qualitative study. BMJ Open 2015; 5 e008448
| Symptom appraisal and healthcare-seeking for symptoms suggestive of colorectal cancer: a qualitative study.Crossref | GoogleScholarGoogle Scholar | 26453591PubMed |
[7] Renzi C, Lyratzopoulos G, Card T, et al. Do colorectal cancer patients diagnosed as an emergency differ from non-emergency patients in their consultation patterns and symptoms? A longitudinal data-linkage study in England. Br J Cancer 2016; 115 866–875.
| Do colorectal cancer patients diagnosed as an emergency differ from non-emergency patients in their consultation patterns and symptoms? A longitudinal data-linkage study in England.Crossref | GoogleScholarGoogle Scholar | 27537389PubMed |
[8] Weller D, Vedsted P, Rubin G, et al. The Aarhus statement: improving design and reporting studies on early cancer diagnosis. Br J Cancer 2012; 106 1262–1267.
| The Aarhus statement: improving design and reporting studies on early cancer diagnosis.Crossref | GoogleScholarGoogle Scholar | 22415239PubMed |
[9] Olesen F, Hansen RP, Vedsted P. Delay in diagnosis: the experience in Denmark. Br J Cancer 2009; 101 S5–S8.
| Delay in diagnosis: the experience in Denmark.Crossref | GoogleScholarGoogle Scholar | 19956163PubMed |
[10] Brown S, Castelli M, Hunter DJ. How might healthcare systems influence speed of cancer diagnosis: a narrative review. Soc Sci Med 2014; 116 56–63.
| How might healthcare systems influence speed of cancer diagnosis: a narrative review.Crossref | GoogleScholarGoogle Scholar | 24980792PubMed |
[11] Emery JD, Shaw K, Williams B, et al. The role of primary care in early detection and follow-up of cancer. Nat Rev Clin Oncol 2014; 11 38–48.
| The role of primary care in early detection and follow-up of cancer.Crossref | GoogleScholarGoogle Scholar | 24247164PubMed |
[12] Samson P, O’Grady G, Keating J. An international comparison study of stage of colorectal cancer diagnosis: how does New Zealand compare? NZ Med J 2009; 122 74–83.
[13] McArdle CS, Hole DJ. Emergency presentation of colorectal cancer is associated with poor 5-year survival. Br J Surg 2004; 91 605–609.
| Emergency presentation of colorectal cancer is associated with poor 5-year survival.Crossref | GoogleScholarGoogle Scholar | 15122613PubMed |
[14] Zhou Y, Abel GA, Hamilton W. Diagnosis of cancer as an emergency: a critical review of current evidence. Nat Rev Clin Oncol 2017; 14 45–56.
| Diagnosis of cancer as an emergency: a critical review of current evidence.Crossref | GoogleScholarGoogle Scholar | 27725680PubMed |
[15] New Zealand Ministry of Health NZ. Referral criteria for direct access outpatient colonoscopy or computed tomography colonography. Wellington, New Zealand: Ministry of Health; 2019.
[16] Atkinson J, Salmond C, Crampton P. NZDep: 2013 Index of Deprivation. Dunedin: University of Otago; 2014.
[17] Elliss-Brookes L, McPhail S, Ives A, et al. Routes to diagnosis for cancer: determining the patient journey using multiple routine data sets. Br J Cancer 2012; 107 1220–1226.
| Routes to diagnosis for cancer: determining the patient journey using multiple routine data sets.Crossref | GoogleScholarGoogle Scholar | 22996611PubMed |
[18] Scott SE, Walter FM, Webster A, et al. The model of pathways to treatment: conceptualization and integration with existing theory. Br Health Psychol 2013; 18 45–65.
| The model of pathways to treatment: conceptualization and integration with existing theory.Crossref | GoogleScholarGoogle Scholar |
[19] Walter F, Webster A, Scott S, et al. The Andersen model of total patient delay: a systematic review of its application in cancer diagnosis. J Health Serv Res Policy 2012; 17 110–118.
| The Andersen model of total patient delay: a systematic review of its application in cancer diagnosis.Crossref | GoogleScholarGoogle Scholar | 22008712PubMed |
[20] New Zealand Ministry of Health. Faster cancer treatment indicators: business rules and data definitions. Wellington, New Zealand: Ministry of Health; 2014.
[21] Brown LD, Cai TT, DasGupta A. Interval estimation for a binomial proportion. Stat Sci 2001; 16 101–117.
| Interval estimation for a binomial proportion.Crossref | GoogleScholarGoogle Scholar |
[22] Molassiotis A, Wilson B, Brunton L, et al. Mapping patients’ experiences from initial change in health to cancer diagnosis: a qualitative exploration of patient and system factors mediating this process. Eur J Cancer Care 2010; 19 98–109.
| Mapping patients’ experiences from initial change in health to cancer diagnosis: a qualitative exploration of patient and system factors mediating this process.Crossref | GoogleScholarGoogle Scholar |
[23] R Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2019. Available at https://www.R-project.org/ [Accessed 28 February 2022]
[24] Wickham H. ggplot2: Elegant graphics for data analysis. New York, NY: Springer-Verlag; 2016.
[25] Neal RD, Din NU, Hamilton W, et al. Comparison of cancer diagnostic intervals before and after implementation of NICE guidelines: analysis of data from the UK General Practice Research Database. Br J Cancer 2014; 110 584–592.
| Comparison of cancer diagnostic intervals before and after implementation of NICE guidelines: analysis of data from the UK General Practice Research Database.Crossref | GoogleScholarGoogle Scholar | 24366304PubMed |
[26] Htun HW, Elwood JM, Ioannides SJ. Investigations and referral for suspected cancer in primary care in New Zealand – a survey linked to the International Cancer Benchmarking Partnership. Eur J Cancer Care 2017; 26 e12634
| Investigations and referral for suspected cancer in primary care in New Zealand – a survey linked to the International Cancer Benchmarking Partnership.Crossref | GoogleScholarGoogle Scholar |
[27] Burton C, O’Neill L, Oliver P, et al. Contribution of primary care organisation and specialist care provider to variation in GP referrals for suspected cancer: ecological analysis of national data. BMJ Qual Saf 2020; 29 296–303.
| Contribution of primary care organisation and specialist care provider to variation in GP referrals for suspected cancer: ecological analysis of national data.Crossref | GoogleScholarGoogle Scholar | 31586938PubMed |
[28] Lyratzopoulos G, Abel GA, Brown CH, et al. Socio-demographic inequalities in stage of cancer diagnosis: evidence from patients with female breast, lung, colon, rectal, prostate, renal, bladder, melanoma, ovarian and endometrial cancer. Ann Oncol 2013; 24 843–850.
| Socio-demographic inequalities in stage of cancer diagnosis: evidence from patients with female breast, lung, colon, rectal, prostate, renal, bladder, melanoma, ovarian and endometrial cancer.Crossref | GoogleScholarGoogle Scholar | 23149571PubMed |
[29] Mowat C, Digby J, Strachan JA, et al. Impact of introducing a faecal immunochemical test (FIT) for haemoglobin into primary care on the outcome of patients with new bowel symptoms: a prospective cohort study. BMJ Open Gastroenterol 2019; 6 e000293
| Impact of introducing a faecal immunochemical test (FIT) for haemoglobin into primary care on the outcome of patients with new bowel symptoms: a prospective cohort study.Crossref | GoogleScholarGoogle Scholar | 31275586PubMed |
[30] Thompson M, O’Leary D, Heath I, et al. Have large increases in fast-track referrals improved bowel cancer outcomes in UK? BMJ 2020; 371 m3273
| Have large increases in fast-track referrals improved bowel cancer outcomes in UK?Crossref | GoogleScholarGoogle Scholar | 33172846PubMed |
[31] Bailey SER, Abel GA, Atkins A, et al. Diagnostic performance of a faecal immunochemical test for patients with low-risk symptoms of colorectal cancer in primary care: an evaluation in the South West of England. Br J Cancer 2021; 124 1231–1236.
| Diagnostic performance of a faecal immunochemical test for patients with low-risk symptoms of colorectal cancer in primary care: an evaluation in the South West of England.Crossref | GoogleScholarGoogle Scholar | 33462361PubMed |
[32] Victoria State Government. Colonoscopy categorisation guidelines. Melbourne, Australia: Victoria State Government; 2017.
[33] Emery JD, Kyriakides M, Faragher I, et al. Validation of Australian and Victorian guidelines for colonoscopy triage. Intern Med J 2021; 51 1457–1462.
| Validation of Australian and Victorian guidelines for colonoscopy triage.Crossref | GoogleScholarGoogle Scholar | 33462903PubMed |
[34] Ministry of Health. Bowel cancer quality performance indicators: descriptions. Wellington, New Zealand: Ministry of Health; 2019.
[35] Cumming G. The new statistics: why and how. Psychol Sci 2014; 25 7–29.
| The new statistics: why and how.Crossref | GoogleScholarGoogle Scholar | 24220629PubMed |