Point-of-Care Haematology Analyser Quality Assurance Programme: a rural nursing perspective
Catherine Beazley 1 4 , Katharina Blattner 1 2 , Geoffrey Herd 31 Hokianga Health Enterprise Trust, Parnell Street, Northland, New Zealand
2 Dunedin School of Medicine, Otago University, Dunedin, New Zealand
3 Northland District Health Board, Whangarei, Northland, New Zealand
4 Corresponding author. Email: catherine.beazley@hokiangahealth.org.nz
Journal of Primary Health Care 13(1) 84-90 https://doi.org/10.1071/HC20080
Published: 23 March 2021
Journal Compilation © Royal New Zealand College of General Practitioners 2021 This is an open access article licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License
Abstract
BACKGROUND AND CONTEXT: Rural health services without an onsite laboratory lack timely access to haematology results. Set in New Zealand’s far north, this paper provides a rural nursing perspective on how a health service remote from a laboratory introduced a haematology analyser suitable for point-of-care use and established the associated quality assurance programme.
ASSESSMENT OF PROBLEM: Five broad areas were identified that could impact on successful implementation of the haematology analyser: quality control, staff training, physical resources, costs, and human resource requirements.
RESULTS: Quality control testing, staff training and operating the haematology analyser was more time intensive than anticipated. Finding adequate physical space for placement and operation of the analyser was challenging and costs per patient tests were higher than predicted due to low volumes of testing.
STRATEGIES FOR IMPROVEMENT: Through a collaborative team approach, a modified quality assurance programme was agreed on with the supplier and regional point-of-care testing co-ordinator, resulting in a reduced cost per test. The supplier provided dedicated hours of staff training. Allocated time was assigned to run point-of-care testing quality assurance.
LESSONS: Having access to laboratory tests can reduce inequalities for rural patients, but natural enthusiasm to introduce new point-of-care technologies and devices needs to be tempered by a thorough consideration of the realities on the ground. Quality assurance programmes need to fit the locality while being overseen and supported by laboratory staff knowledgeable in point-of-care testing requirements. Associated costs need to be sustainable in both human and physical resources.
KEYwords: Haematology bench top analyser; point-of-care; quality assurance; rural health service; rural hospital; rural laboratory testing; rural nursing
| WHAT GAP THIS FILLS |
| What is already known: The theoretical requirements of quality assurance for a point-of-care testing programme close to a laboratory are well established. |
| What this study adds: This paper describes grass-roots nursing experience of implementing a quality assurance programme for a bench top haematology analyser in a rural setting remote from a laboratory. |
Background and context
A full blood count (FBC) is a commonly ordered blood test in the assessment of acutely unwell patients.1 For many rural and remote health services without an onsite laboratory, there can be long delays in obtaining FBC results. Point-of-care testing laboratory devices allow testing at or near the patient beside, providing rapid results and improving patient outcomes.2–5 Point-of-care testing has been shown to have benefits in rural health care, including reduced turnaround time for results and increased diagnostic certainty.6–8 Most point-of-care testing haematology devices internationally and in New Zealand (NZ) are used in large urban hospital laboratories and operated by trained laboratory staff.9 When the nearest laboratory is a long distance away, non-laboratory staff (eg doctors and nurses) who generally have little or no prior laboratory experience, can become responsible for operating the point-of-care testing devices and maintaining the essential quality assurance programme.2,9–11
When committing to point-of-care testing, it is necessary to ensure the reliability of test devices to enable confidence for users to act on the results. This is achieved through a quality assurance programme that involves a set of procedures to check the point-of-care testing function (accuracy, repeatability and stability of testing).3 A quality assurance programme comprises internal and external requirements, including certification and ongoing staff training. The internal quality control assurance includes running quality control solutions and system checks, whereas the external quality assurance involves tasks such as precision testing and whole blood sample correlation. A more detailed breakdown of the components to be considered in a quality assurance programme are shown in Table 1.

|
Hokianga Health Enterprise Trust (Hokianga Health) is a community-owned, not-for-profit rural health-care provider serving a high-health needs, mainly Māori population of ~6500 people distributed over 1520 km2.12 Hokianga Health integrates community-based primary health care with inpatient services at Hokianga hospital. The hospital has 10 acute beds as well as maternity and residential care beds. The Hokianga Health service nursing team provides 24-h, 7 days a week acute and emergency care. Hospital registered nurses are rostered in three shifts (two day and one night shift) and are responsible for in-patients, the residential ward and any acute and emergency presentations. There is no onsite laboratory in Hokianga Health and access to the nearest laboratory (located 120 km away in Whangarei) is limited to a once daily courier delivery on weekdays. This results in a long turnaround time for laboratory results; 8 h on weekdays but up to 72 h on weekends and public holidays. Hokianga Health patients requiring specialist care are referred to Whangarei base hospital, Northland District Health Board (Northland DHB).
Twelve years ago, Hokianga Health, with support from the Northland DHB point-of-care testing coordinator, introduced a point-of-care testing device (Abbott i-STAT analyser from Abbott Diagnostics, Illinois, USA) to provide basic biochemistry tests.13 The i-STAT analyser was shown to have a positive impact on patient care at Hokianga Health by improving diagnostic certainty and reducing hospital transfers, and it was cost-effective.6,7 However, the absence of timely FBC results remained an issue for acute clinical care at Hokianga Health until 2016, when a haematology bench top analyser (Abbott) suitable for point-of-care testing became available (initially on a trial basis).
The impact of the haematology analyser at Hokianga Health on patient care, the acceptability of the device for medical and nursing staff and a cost benefit analysis are detailed elsewhere.14
The aim of this paper is to describe, from a local rural nursing perspective, the implementation of a point-of-care testing haematology analyser and its associated quality assurance programme, including the challenges encountered, lessons learnt and recommendations for others considering implementing a similar service.
Ethics approval was granted by the NZ Northern B Health and Disability Ethics Committee in January 2016 (16/NTB/2).
Assessment of problems
A Hokianga Health team was established to set up the haematology analyser. The team included the clinical nurse manager and experienced local clinicians (KB, CB). The team was given guidance by the Northland DHB point-of-care testing coordinator (GH) who had extensive prior experience in point-of-care testing and was well known to the local team. External support was also provided by Abbott Diagnostics, particularly regarding the set up and validation of the analyser, guidelines on the specifications of the quality assurance programme and training.3 It was anticipated that the haematology analyser would be used in cases of acute patient management where prompt FBC results may impact on clinical care. Routine FBC testing would continue using the usual pathway.
There was a limited choice of available haematology analysers that were suitable for point-of-care haematology testing in our remote rural setting. The Hokianga Health team had determined that in addition to haemoglobin and red blood cell measures, a five-part white cell differential (including separate neutrophil counts) and platelet counts were required to meet their clinical needs. These haematology requirements, plus the need for appropriate service and support from the supplier, meant that the Emerald 22 haematology analyser (Abbott Diagnostics) was chosen.
The team at Hokianga Health had previous experience of point-of-care testing implementation and maintenance, so context-specific challenges were anticipated. However, team discussions also uncovered uncertainties. For example, although local nursing staff had been successfully trained to operate the i-STAT point-of-care testing analyser, it was unclear whether this previous exposure would make it easier to train nursing staff to operate a second point-of-care testing device.
To assess and evaluate progress during set-up, we held regular team meetings, both formal and informal. In addition, a field diary was maintained by the lead nurse (CB) to record practical issues encountered. Staff were regularly encouraged to provide feedback on any issues that arose, either by phone, in person or by writing in the ward communications book that was checked on a daily basis by a team member.
Results of the assessment
After the point-of-care haematology analyser arrived, five main issues were identified: meeting requirements for quality control testing; local nurse training; physical resources; costs per patient test; and human resource requirements.
Meeting requirements for quality control testing
The recommended internal quality control requirements for the haematology analyser were more time intensive and technically complicated than those of the (by now familiar) i-STAT. For example, each day, an uninterrupted period of ~15 min of nurse time was required to run operational checks and confirm parameters were correct for patient testing. This proved to be a major extra task for the nursing staff who were already undertaking quality control testing for the other (i-STAT) point-of-care testing analyser. The responsibility for undertaking the quality control testing was assigned to the night duty nursing staff to complete during their regular shift. This lack of ring-fenced time to attend to quality control tasks resulted in frequent interruptions to the quality control testing.
The quality control testing solutions were analysed each day. Quality control data are used to confirm the precision and reproducibility of the results produced by the analyser. The point-of-care haematology analyser will flag if a test result exceeds specified limits. An important element of the quality control programme for the point-of-care haematology analyser is regular review of the quality control data. The quality control data can also help to identify problems with the analyser before the analysis of patient samples.3 This daily assessment of analytical precision using the quality control material helps to ensure the clinical reliability of the patient test results at Hokianga Health. Table 1 provides more detail of quality control tasks and time required at Hokianga Health.
Local nurse training
The nurse training required for operating a second point-of-care testing device was substantial despite the staff’s prior exposure to another device. A ‘train-the-trainer’ approach was initially adopted to keep disruption to clinical duties to a minimum. Two ‘super users’ were fully trained in the use of the analyser, including the quality assurance programme procedures. The super users then opportunistically trained other staff and acted as an ongoing resource. However, this level of support proved insufficient and resulted in uncertainty, confusion, and feelings of inadequacy for the nursing staff. In particular, staff felt ill-prepared to undertake some of the more technical point-of-care associated tasks; for example, loading quality control information for new lot numbers of quality control material before use on the analyser.
Physical resources
The haematology analyser was a much larger device than expected and was not easily portable, requiring more workspace than anticipated. The point-of-care i-STAT analyser was a handheld and portable device, but the haematology analyser and printer combined required a space of ~0.75m × 0.5m and was not portable to the patient bedside; consequently, the haematology analyser is referred to as a ‘bench-top’ analyser. In addition, there were technical challenges with the device relating to the storage and printing of results, storage of reagents and waste management.
Costs per patient test
At Hokianga Health, the average number of point-of-care FBC tests performed each week was seven, with no patient tests performed some days (reflecting the small population served). The haematology supplier had assumed a much higher usage when calculating cost per patient test for the analyser. The lower frequency of use at Hokianga Health therefore had flow-on effects on costs; for example, the cost of quality control solutions that were to be changed weekly in accordance with the manufacturer’s recommendations, was incorporated into the per patient test cost (costing $125 NZD per set of solutions). At Hokianga Health, sometimes the haematology analyser was not used for patient testing between quality control checks. Consequently, the cost per patient test (including quality controls, reagents and diluents) increased from a predicted $15 NZD to approximately $23 NZD.
Human resource requirements
The time required by nursing staff to run the daily quality control and oversee the quality assurance programme proved much greater than estimated, and eventually required specific ring-fenced staff time. Examples of challenges that influence time taken to complete quality control tasks are presented in Table 2.

|
Initially, analyser-related issues were raised by staff at least once or twice per week, but these decreased in frequency over time as the haematology analyser became part of ‘business as usual’.
Strategies for improvement
After reviewing the requirements for quality control, the supplier and the point-of-care testing coordinator agreed on a quality assurance programme modified to fit the Hokianga Health context, which reduced the frequency of quality control tasks. The point-of-care testing coordinator provided ongoing guidance and assistance (including regular site visits) during which external quality assurance programme requirements were attended to.
In response to staff feedback regarding the point-of-care testing haematology analyser operations, a more formal approach was taken to staff training. The supplier provided onsite training over 3 days, allowing staff easy access to training sessions during their scheduled work shifts. At these sessions, staff were orientated to the analyser set up, functions, method of sampling, start-up quality control testing and printing of results. Eighty percent of the nursing staff were able to attend training sessions and an additional two staff were given 2 h of specific training to become super users. Super users were trained in problem solving, updating analyser settings, loading new quality control files and carrying out bleach cleaning and training in how to assist with precision testing. The additional training and improved access to support resulted in fewer staff queries and fewer reports of operational problems.
To accommodate the point-of-care testing haematology analyser, a designated corner of bench space was allocated specifically to the analyser and an extension built to accommodate the associated printer and allow storage of quality control-related results, cartridges, and paper. Quality control results were filed once a week and stored securely for a period of at least 5 years.
Discussion was held with the supplier and the Northland DHB point-of-care testing coordinator regarding the unexpectedly high cost per patient test related to the low volume usage. After this discussion, it was agreed that the lifespan of each set of controls could be extended to 2 weeks provided the quality control performance data were satisfactory. This proved to be the case, with the quality control results indicating the analyser was performing well and it became possible to reduce the cost per patient test to approximately $12 NZD.
Based on information provided in Table 1, a report detailing the tasks and staff time required for all tasks associated with the quality assurance programme, including quality control, was presented to Hokianga Health management. This resulted in the allocation of 8 h per week of protected time for staff to undertake these tasks, as well as oversee operation of the other point-of-care testing devices at Hokianga Health.
Lessons and messages
Having prompt access to a FBC result by using a point-of-care device reduces inequalities for rural patients and eases stress for patients and their families by providing diagnostic certainty and avoiding unnecessary transfers that take patients away from their families and local community supports.5,15 Early identification and treatment in acute presentations can also positively influence better health outcomes for Māori living in rural areas.16 However, the natural enthusiasm to introduce point-of-care testing laboratory devices and related technologies in the rural setting needs to be tempered by an in-depth consideration of the practical realities of everyday use.7
The importance of carrying out a thorough evaluation of the potential set-up and sustained operating costs, before introducing a point-of-care testing device into a rural practice environment, is highlighted in this study and concurs with previous research.8,15 Particular attention needs to be given to hidden costs such as dedicated staff time and the additional per test costs in quality control, time and hardware associated with running point-of-care testing in a rural, low volume setting. In the future, generic quality assurance programme recommendations should be adapted for low volume, non-laboratory, rural sites.
In hindsight, an earlier and more in-depth review by the clinical team and specific to the haematology analyser would have identified some of the issues encountered. However, it is difficult to ascertain the full impact in different environments ahead of implementation, particularly in rural low-resource settings that rely on small teams.
Strengths of the Hokianga Health team’s approach included their previous exposure to the i-STAT point-of-care testing device, a well-established relationship with the regional point-of-care testing co-ordinator and knowledge of the realities of rural practice. These factors combined with local team and Northland DHB point-of-care coordinator aspiration and commitment to achieving equity for the Hokianga community patients, were critical to the successful implementation of the haematology analyser at Hokianga Health.
For Hokianga Health, the changes made to practice by implementing the haematology analyser and its quality assurance programme have been sustained. At the time of writing, the haematology analyser remains operational and is now considered a routine diagnostic tool in patient assessments, improving equity of access for rural patients.
Recommendations for other organisations
Table 3 provides a set of questions to consider for others looking at embarking on point-of-care test implementation in rural settings where no onsite laboratory services are available.
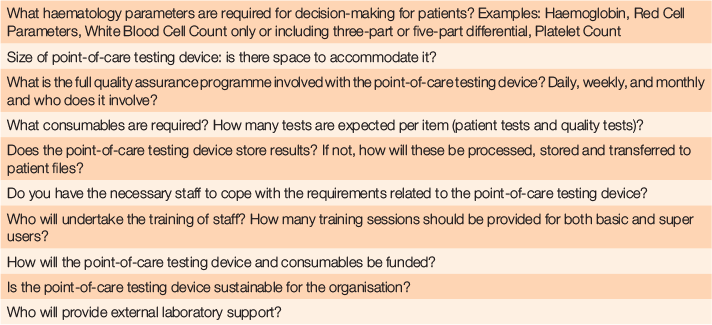
|
Competing interests
The authors declare no competing interests.
Funding
This research did not receive any specific funding.
Acknowledgements
The authors would like to thank the nursing staff at Hokianga Hospital who were involved with the introduction of the haematology analyser.
References
[1] Briggs C, Kimber S, Green L. Where are we at with point-of-care in haematology? Br J Haematol. 2012; 158 679–90.| Where are we at with point-of-care in haematology?Crossref | GoogleScholarGoogle Scholar | 22765160PubMed |
[2] St John A. The evidence to support point-of-care testing. Clin Biochem Rev. 2010; 31 111–9.
| 24150515PubMed |
[3] New Zealand Point of Care Testing Advisory Group. New Zealand Best Practice Guidelines for Point-of-Care Testing; 2014. [cited 2019 February]. Available from: www.nzimls.org.nz/user/file/760/2014%20New%20Zealand%20Best%20Practice%20POCT%20Guidelines.pdf.
[4] Sumita NM, Ferreira CES, Martino MDV, et al. Clinical applications of point-of-care testing in different conditions. Clin Lab. 2018; 64 1105–12.
| Clinical applications of point-of-care testing in different conditions.Crossref | GoogleScholarGoogle Scholar | 30146832PubMed |
[5] Nixon GH. Improving access to diagnostic testing for rural communities in Aotearoa/New Zealand. PhD Dissertation, University of Auckland, Auckland; 2019.
[6] Blattner K, Nixon G, Dovey S, et al. Changes in clinical practice and patient disposition following the introduction of point-of-care testing in a rural hospital. Health Policy. 2010; 96 7–12.
| Changes in clinical practice and patient disposition following the introduction of point-of-care testing in a rural hospital.Crossref | GoogleScholarGoogle Scholar | 20034696PubMed |
[7] Blattner K, Nixon G, Jaye C, Dovey S. Introducing point-of-care testing into a rural hospital setting: thematic analysis of interviews with providers. J Prim Health Care. 2010; 2 54–60.
| Introducing point-of-care testing into a rural hospital setting: thematic analysis of interviews with providers.Crossref | GoogleScholarGoogle Scholar | 20690403PubMed |
[8] Wong HY, Marcu LG, Bezak E, Parange NA. Review of health economics of point-of-care testing worldwide and its efficacy of implementation in the primary health care setting in remote Australia. Risk Manag Healthc Policy. 2020; 13 379–86.
| Review of health economics of point-of-care testing worldwide and its efficacy of implementation in the primary health care setting in remote Australia.Crossref | GoogleScholarGoogle Scholar | 32440241PubMed |
[9] Herd G, Musaad S. Clinical governance and point-of-care testing at health provider level. N Z Med J. 2015; 128 41–6.
| 26149903PubMed |
[10] Shaw JLV. Practical challenges related to point of care testing. Pract Lab Med. 2016; 4 22–9.
| Practical challenges related to point of care testing.Crossref | GoogleScholarGoogle Scholar | 28856189PubMed |
[11] Shephard MD, ed. A practical guide to global point-of-care testing. Melbourne, Vic.: CSIRO Publishing; 2016.
[12] Hokianga Health Enterprise Trust. Annual report June 2019. Rawene: Hokianga Health Enterprise Trust; 2019.
[13] Abbott. Point of care: iSTAT handheld. [updated 2019]. Princeton, NJ, USA: Abbott; 2016. [cited 2020 March 16]. Available from: https://www.pointofcare.abbott/int/en/offerings/istat/istat-handheld.
[14] Blattner K, Beazley C, Nixon G, et al. The impact of the introduction of a point-of-care haematology analyser in a New Zealand rural hospital with no onsite laboratory. Rural Remote Health. 2019; 19 4934
| The impact of the introduction of a point-of-care haematology analyser in a New Zealand rural hospital with no onsite laboratory.Crossref | GoogleScholarGoogle Scholar | 31035770PubMed |
[15] Dahm MR, McCaughey E, Li L, et al. Point-of-Care testing across rural and remote emergency departments in Australia: staff perceptions of operational impact. Stud Health Technol Inform. 2017; 239 28–34.
| 28756433PubMed |
[16] Ministry of Health. Mātātuhi Tuawhenua: Health of Rural Māori 2012. Wellington: Ministry of Health; 2012.


