Improving management of sexually transmitted infections in primary care: feasibility and acceptability of a new patient management tool for clinicians
Sally B. Rose 1 2 , Susan M. Garrett 1 , Susan R. H. Pullon 11 Department of Primary Health Care and General Practice, University of Otago, Wellington, PO Box 7343, Wellington South, New Zealand.
2 Corresponding author. Email: sally.rose@otago.ac.nz
Journal of Primary Health Care 13(2) 171-179 https://doi.org/10.1071/HC20051
Published: 24 May 2021
Journal Compilation © Royal New Zealand College of General Practitioners 2021 This is an open access article licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License
Abstract
INTRODUCTION: Routinely following an evidence-based clinical pathway of care for bacterial sexually transmitted infections (STIs) such as chlamydia or gonorrhoea is important to help reduce the spread of infections, prevent reinfections and avoid associated health complications.
AIM: To develop an easy-to-use tool for routine use by primary care clinicians to ensure best practice management of patients tested for and diagnosed with chlamydia or gonorrhoea.
METHODS: The tool (a MedTech Advanced Form) was developed in consultation with seven primary care clinicians and included different tabs for use during the STI care pathway (testing, treatment, advice, partner notification and follow up) with clickable links to relevant online resources. The tool was trialled over 3 months by 19 clinicians in three Wellington primary care clinics – two youth health and a student health service. Outcome measures were frequency of use, completeness of fields related to best practice care and clinician acceptance of the tool (from focus group feedback).
RESULTS: The tool was used for approximately one in four patients who were tested during the trial period, with ‘forgetting’ reported as the most common reason for non-use. Clinician views about the tool were favourable, with most indicating they would like to continue use and would recommend it to colleagues. Documentation of best practice care was excellent; fields to record reasons for testing, discussion of sexual history, provision of treatment and advice given were used for most patients for whom the form was completed.
CONCLUSIONS: Inclusion of this STI management tool in the electronic patient records system appeared to improve primary care clinicians’ delivery and documentation of best practice sexual health care at a practice level. Wider use of a modified version of this tool could facilitate more comprehensive best practice management of bacterial STIs.
KEYwords: Best practice management; Chlamydia trachomatis; Neisseria gonorrhoeae; primary care clinicians; sexually transmitted infections.
| WHAT GAP THIS FILLS |
| What is already known: Best practice sexual health guidelines, including partner notification, are often not systematically followed for people tested for, and diagnosed with bacterial STIs in New Zealand primary care practices. |
| What this study adds: Inclusion of an easy-to-use STI management tool in the electronic patient record system appears to improve primary care clinicians’ delivery and documentation of best practice sexual health care at the practice level. |
Introduction
Ongoing high rates of bacterial sexually transmitted infections (STIs) continue to contribute to a substantial but preventable burden of morbidity in New Zealand. Chlamydia trachomatis and Neisseria gonorrhoeae are the most commonly diagnosed bacterial STIs1 and are often asymptomatic.2,3 Symptoms vary by sex and anatomical site of infection and may include vaginal discharge or intermenstrual bleeding, cervicitis, abdominal or pelvic pain, urethritis and testicular pain.3,4 Regardless of whether symptoms are present, untreated infections can result in long-term complications including pelvic inflammatory disease, ectopic pregnancy and infertility; they can be passed on in pregnancy or during delivery and increase the risk of HIV acquisition and transmission.5,6 Gonorrhoea is on the ‘deadliest superbug list’ of the World Health Organization because of increasing concerns about antimicrobial resistant strains that are harder to treat or resistant to known antibiotics.7 Testing at-risk asymptomatic individuals with extragenital sampling (pharyngeal and anorectal) where indicated by patient sexual history, is an important way to diagnose infection and reduce the risk of complications. With rising rates of syphilis both in New Zealand and internationally,8 offering opportunistic testing for syphilis and HIV at the time of chlamydia and gonorrhoea testing is also recommended.9
Young people (particularly rangatahi Māori and Pasifika youth), low-income communities and men who have sex with men are disproportionately affected by STIs.10 These inequities persist despite highly effective antibiotic treatment and best practice guidelines that outline an evidence-based pathway of care, including partner notification and follow up for treatable bacterial STIs such as chlamydia or gonorrhoea.11 These guidelines should be universally applied to the management of STIs with sampling from appropriate anatomical sites as guided by patient sexual histories, timely and appropriate antibiotic treatment, advice about reinfection risk reduction and the importance of treating partners, post-treatment phone follow up and a 3-month test of reinfection.11
Despite evidence that effective clinical management helps to reduce the spread of infections, prevent reinfections and avoid associated health complications,12,13 international research has not yet been able to demonstrate how best to achieve routine best practice management.12 Our past research has found that poor documentation and gaps in care along the STI management pathway are common in primary care – the setting in which the most cases are detected in New Zealand.14–16 A review of primary care case-notes for patients diagnosed with bacterial STIs revealed that only 50% had any documentation of their recent sexual history, 65% had documented evidence of a partner notification plan, and 20% had documentation that partners had been notified.14 Multiple studies have now shown that the recommended 3-month test of reinfection following chlamydia and gonorrhoea treatment is also low.15,17,18
Competing demands on clinicians’ time and lack of practice-wide systems and resources for clinicians are some of the factors limiting routine delivery of best practice care.16 Multiple health encounters are typically needed for the full cycle of STI care. These often involve multiple members of the primary care team, so documenting relevant sexual history, partner notification management and a follow-up plan are key to ensuring patients receive appropriate and seamless care.11,19 Primary care survey respondents recognised that gaps existed in their practice and strongly agreed that practical strategies could help facilitate provision of best practice care, including ‘reminders to discuss partner notification and retesting’, ‘what to say and document’ and ‘printable resources for patients and their partners’.16
The current feasibility study was undertaken to address some of these gaps by developing and then trialling an electronic patient consultation tool to provide a framework for delivery and documentation of best practice care. The tool focused on chlamydia and gonorrhoea as the most common bacterial STIs, but included prompts to consider other STI testing where appropriate. To determine whether the tool would work in the context of a future intervention study, we first sought to collect data on the acceptability and functionality of the tool in a small number of settings.
Methods
Study design and participants
Seven Wellington primary care clinicians (sexual health advisor, nurses, nurse practitioner, general practitioner (GP)) participated in phase 1 (development of the patient consultation tool) and 19 clinicians (14 nurses, two nurse practitioners and three doctors) participated in phase 2 (3-month trial of the tool). The trial ran in three primary care clinics in the Wellington region (two youth health and one university student health clinic) with all nursing staff and 3 of 13 GPs from these clinics taking part. Ten GPs, including locums and very part-time doctors, in the three clinics did not participate. Based on our past research involving these clinics, we expected a minimum of 300 patients to be tested for chlamydia and gonorrhoea and 30–40 cases to be diagnosed over 3 months.14
Outcome measures
Frequency of tool use was measured by the proportion of individuals who had any data entered and were tested during the study period.
Use of key best practice fields were measured for patients diagnosed. This included counts of the tool components: reason for testing, recent sexual history, treatment given, partner notification plan made, treatment compliance and risk-reduction advice given, and advice to return for a 3-month test of reinfection.
Acceptability of the tool to participating clinicians was assessed from focus group feedback.
Phase 1. Development of the STI patient management tool
Primary care clinicians helped to develop a tool that sat in the MedTech electronic patient records system (referred to as the patient management system (PMS); see Supplementary Figure S1). It was designed to closely follow New Zealand Sexual Health Society’s (NZSHS) best practice guidelines for STI screening and subsequent management of chlamydia or gonorrhoea11 and was designed to be inclusive of all genders, sexualities and sexual practices. We met with each clinician one to three times over 3 months to reach agreement on content, field types, response options, order and layout (totalling ~20 h of clinician input).
Each form (containing four tabs) could be used for the complete pathway of care with relevant fields (testing, diagnosis and treatment, and follow up) completed as appropriate at different consultations by different clinicians. Information entered automatically populated patients’ daily records. A resources tab was included with clickable links to relevant NZSHS best practice guidelines,11 HIV pre-exposure prophylaxis (PrEP) eligibility criteria20 and two recent Best Practice Advocacy Centre (BPAC) articles.8,21 Additional information was accessible when hovering over underscored text on the form (when to consider a test of cure, throat and anorectal swabs, syphilis and HIV testing). A consultant with expertise in building advanced forms was contracted to build the form and provided instructions for participating practices to run the installation.
Phase 2. Trialling the tool in primary care
The researchers met with participating clinicians to explain how and when to use the tool, with an accompanying ‘user guide’ left for reference. Clinicians were asked to use the tool routinely when testing for or treating chlamydia and gonorrhoea, with no exclusions specified. They were free to introduce the tool (or not) to patients in any way they deemed appropriate, but it was left to their judgement to determine whether they used it.
Data collection and analysis
One of the research team members visited clinics to collect outcome measures data 3 months after the trial period concluded. A MedTech query identified patients for whom the advanced form had been completed and screenshots were taken of completed forms. Screenshots were saved into a Word document (Microsoft Corporation) for analysis; they did not include clinicians’ names or any patient identifiers (names, National Health Index (NHI) codes, dates of birth, phone numbers or addresses).
Data from screenshots were entered into an Excel spreadsheet (Microsoft Corporation) to record whether each field in the tool had been used. Frequency of use was collated for each field and total numbers and percentages calculated for fields related to main outcomes. Field content was not analysed. Clinics also ran a query to count numbers of patients tested and diagnosed with chlamydia and gonorrhoea during the trial period. This information gave us an estimated denominator from which we calculated the frequency of use (outcome 1: proportion of patients tested or diagnosed for whom the form was used).
Two members of the research team held focus groups with participating clinicians at the end of the trial to assess acceptability of the tool. A short questionnaire was administered to participants followed by an audio-recorded group discussion lasting 30–45 min to seek views on the benefits and drawbacks of using the tool, and ways it could be improved for future use. In the questionnaire, participants were asked to indicate whether they agreed or disagreed with 10 statements presented about the layout, content, ease of use, likelihood of continued use and recommendation to others. The audio was selectively transcribed to identify key comments.
The University of Otago Ethics Committee (Health) approved phase 1 on 18 December 2018 (Ref D18/423) and phase 2 on 24 July 2019 (Ref H109/097).
Results
Frequency of use and completion of best practice fields
The testing tab was completed 139 times for an estimated 528 patients (26%) tested during the trial period (this denominator includes test requests from the 10 clinicians who were not part of the trial so underestimates use by participating clinicians). The treatment tab was used 23 times for an estimated 63 patients diagnosed with chlamydia or gonorrhoea during the study period (equating to 36.5% of all patients diagnosed, and 82% of patients with the testing tab completed who returned a positive diagnosis). The follow-up tab was completed only five times. Table 1 presents the frequency with which fields were completed in each of the three tabs.
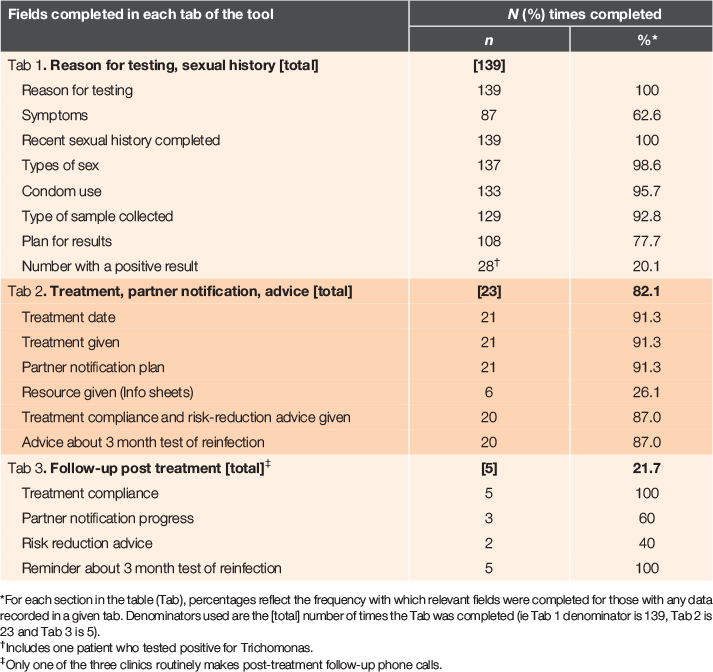
|
Reasons for testing and recent sexual history were completed for all patients who had a form completed (139/139), treatment fields were used for 21 of the 23 positive cases and a partner notification plan completed for 21 of 23 positive cases. Treatment compliance and risk-reduction advice, and advice to return for a 3-month test of reinfection were documented for 20 of 23 cases. Only one of the three clinics routinely makes post-treatment follow-up phone calls, but the nurse responsible for managing these reported continued use of paper forms (having forgotten follow up was included in the tool).
Acceptability of the tool
Data collected in the questionnaire regarding use of the tool are presented in Table 2 for 19 participating clinicians. Of clinicians who had used the tool, estimated frequency of use ranged from 1 to 40 times, summing to 160 times (a slight overestimate of the 139 times it was actually used). Only three clinicians thought the tool increased the duration of their consultations, but none indicated this deterred them from using it. Most participants reported using the tool sometimes or most of the time, with ‘forgetting’ cited as the main reason for non-use (Table 3). Overall responses to the questionnaire were favourable: 80% of participants said they would like to continue using it and 73% indicated they would recommend the tool to a colleague.
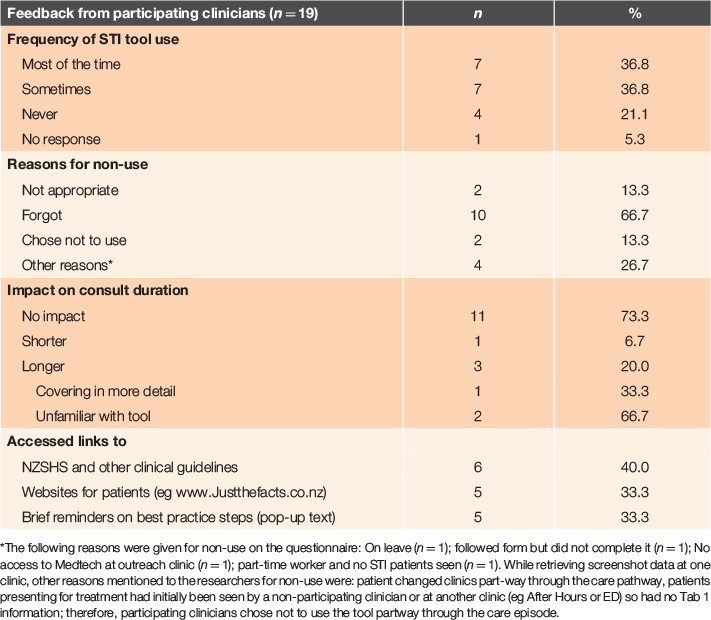
|
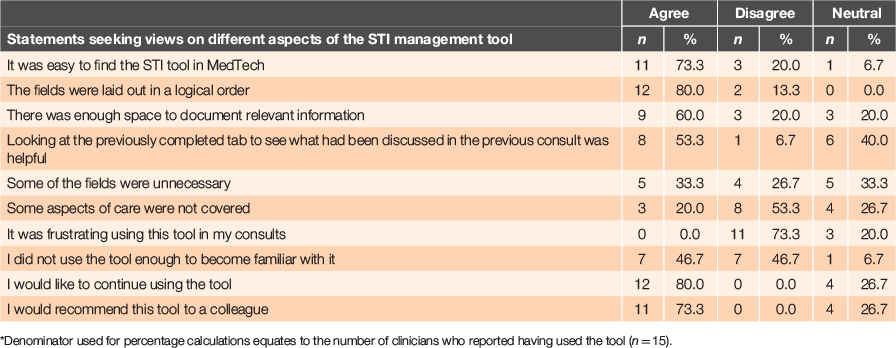
|
Key themes identified from focus group discussions about using the STI tool are presented in Table 4. Advantages of using the tool included: efficiency, promotion of more comprehensive best practice care (‘gold standard care’), more complete and consistent documentation, improved continuity of care, and provision of educational and upskilling opportunities (particularly for new staff). Clinicians also thought it helped to ‘normalise’ the conversation about sex with their patients (eg when patients were told that every patient is asked the same questions) and highlighted the importance of sexual health.
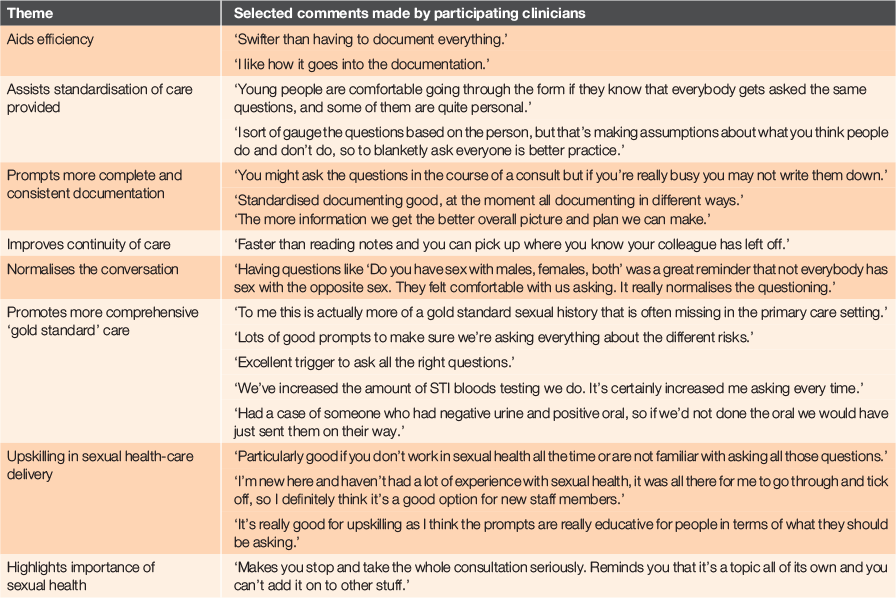
|
Most comments made about negative aspects of the tool related to limitations of MedTech advanced forms and their functionality. For example, clinicians commented on closely spaced layout, small font of text populating the daily record (a default setting that could not be changed), inability to place a link to the form where it could easily be seen and ‘roundabout’ way of accessing the form in the PMS. Other drawbacks related to individual consultation style (eg preference to face the patient during a consultation rather than typing on the computer), and challenges using the tool in broader consultations (eg long-term contraception, cervical smears or infections like thrush and bacterial vaginosis). Several clinicians wondered how patients felt about being asked about numbers of recent partners and types of sexual activity, but noted that no patients refused to answer these questions.
Discussion
Use of this tool reportedly promoted more comprehensive and complete management of STIs at the study practices and prompted consistent and complete documentation of care. Clinician views towards the tool were favourable: most indicated they would like to continue using it and would recommend it to colleagues. Documentation of best practice care was excellent, with most clinicians using the fields provided to record reasons for testing, discussion of sexual history, provision of treatment and advice given. Closer alignment with ‘gold standard’ care11 was identified as a benefit of the tool and it was regarded as improving continuity of care ‘…you can pick up where you know your colleague has left off’. This is an important consideration because STI management typically involves different clinicians over multiple encounters. Despite being acceptable to the clinicians trialling the tool, it was inconsistently used, with approximately one-quarter of patients tested having a form completed. Failure to remember to use the tool was the most common reason for non-use, an aspect of the tool that needs improvement.
The substantial element of end-user consultation is a strength of the study. The tool was developed in consultation with clinicians who are frequently involved in the management of bacterial STIs in response to gaps identified in our past research. Trial clinicians’ feedback was positive overall, and provided clear information about the practicalities, benefits and frustrations associated with use of the tool. Similar tools have been developed for use in primary care (eg cervical screening), but to our knowledge, this is the first report about use of a customised tool for STI management in New Zealand.
The study was not designed to detect changes in clinical outcomes as a result of using this tool, but qualitative data suggested that during the study period, patients received more comprehensive care that aligned with best practice guidelines. We could not accurately estimate the proportion of patients who were tested and treated by participating clinicians and had a form completed. Technical challenges with MedTech meant that the data provided to us by participating clinics did not include the ‘requestor’ details we anticipated receiving, so the denominator of tests and positives rates included non-participating clinicians. Figures provided are therefore an underestimate of the actual frequency of use. No patient feedback was sought in this study, but clinicians reported that patients were typically willing to provide information in more depth than they might have previously given (particularly with respect to sexual history) when they were aware that everyone was being asked the same questions.
To ensure sufficient data in the short project timeframe, practices were chosen for their known high rates of STI testing and diagnosis (primarily due to their youth populations). Further work is needed to determine the acceptability and utility of the tool in mainstream general practice where individual clinicians typically see lower numbers of patients with STIs. Several study clinicians who used it only a few times reported ‘unfamiliarity with the tool’ as a drawback – a similar experience might initially be expected in general practice. By contrast, a participant new to STI testing commented favourably on the role of the tool in providing a clear, easy-to-follow framework for guideline-based provision of care.
Numerous challenges were encountered before installation of the tool into the three participating practices. Researchers spent a substantial amount of time liaising with the Primary Health Organisation (PHO), practice managers, MedTech staff, a private IT provider and the software developer to enable installation in the practices. Funding did not allow development of the tool for more than one patient management system, so only clinics using MedTech 32 were able to participate.
This study highlights the potential for health benefit that could accrue with the introduction of technology-based resources to assist clinicians in their routine delivery of best-practice sexual health care. With minimal intervention, clinicians self-reported delivery of best practice improved by taking more thorough sexual histories, more frequent consideration of syphilis serology and extragenital swabs. Minor suggested changes to the tool design, mostly related to content, could easily be incorporated into a revised version, but a clear limitation of the tool was its lack of immediate visibility in the PMS and reliance on clinician memory to initiate use. A solution might be to include a prompt for completion of the tool upon generating a laboratory request for STI testing. However, differences in clinician consultation style might present challenges for the timeliness of such a reminder as laboratory tests requested should ideally be informed by sexual history assessed while completing the tool. Secure web-based applications are now being used by some PHOs that can be integrated into any PMS and customised to support clinical decision-making. The cost of such a product was prohibitive for the current research, but it would enable development of a more sophisticated tool that could be integrated across multiple platforms, with inbuilt capacity to prompt use for relevant consultations.
Although problems encountered in this feasibility study are likely solvable, they would require buy-in from PHOs and laboratories as well as clinician education and widespread roll out to make a difference at a population level. The rapidly embraced new ways of working in primary care in the face of Covid-1922 might accelerate readiness for, and acceptance of such technology-based resources in future.
Use of this innovative STI management tool appeared to prompt clinicians to take a more thorough approach to documentation and management of patients tested for, and diagnosed with bacterial STIs. With minor modifications to the content and use of a more flexible platform, further evaluation of this tool in general practice settings is warranted. Widespread use of such a tool to promote comprehensive STI case management in primary care could ultimately contribute to a reduction in STI prevalence and associated health consequences.
Competing interests
The authors have no competing interests to declare.
Acknowledgements
We thank the primary care clinicians who provided input into the development of the PMS tool, and the three clinics that participated in the trial phase. We also thank Tū Ora Compass Health, Karo data management, Chris Petersen Consulting and IT Works for sharing their time and expertise during the development and installation of the tool. This project was funded by a Lottery Health Research grant (LHR-2019–99246) and a grant-in-aid from ISTAR limited. The funding bodies played no role in the design, implementation or interpretation of the research.
References
[1] The Institute of Environmental Science and Research Ltd. New Zealand Sexually Transmitted Infection (STI) Surveillance Dashboard 2021; 2021. [cited 2021 April 21]. Available from: https://www.esr.cri.nz/our-services/consultancy/public-health/sti/[2] Stamm WE, editor. Chlamydia trachomatis infections of the adult. New York: McGraw-Hill; 2008.
[3] Centers for Disease Control and Prevention (CDC). Gonorrhoea - CDC fact sheet. Facts and Brochures. Atlanta, Georgia: CDC; 2021. [cited 2021 February 2]. Available from: http://www.cdc.gov/std/gonorrhea/STDFact-gonorrhea-detailed.htm
[4] Centers for Disease Control and Prevention (CDC). Chlamydia - CDC fact sheet. Facts and Brochures. Atlanta, Georgia: CDC; 2021. [cited 2021 February 2]. Available from: http://www.cdc.gov/std/chlamydia/STDFact-chlamydia-detailed.htm
[5] Rowley J, Vander Hoorn S, Korenromp E, et al. Chlamydia, gonorrhoea, trichomoniasis and syphilis: global prevalence and incidence estimates, 2016. Bull World Health Organ. 2019; 97 548–62P.
| Chlamydia, gonorrhoea, trichomoniasis and syphilis: global prevalence and incidence estimates, 2016.Crossref | GoogleScholarGoogle Scholar | 31384073PubMed |
[6] Price MJ, Ades AE, Soldan K, et al. The natural history of Chlamydia trachomatis infection in women: a multi-parameter evidence synthesis. Health Technol Assess. 2016; 20 1–250.
| The natural history of Chlamydia trachomatis infection in women: a multi-parameter evidence synthesis.Crossref | GoogleScholarGoogle Scholar | 27007215PubMed |
[7] World Health Organisation (WHO). Global priority list of antibiotic resistant bacteria to guide research, discovery, and development of new antibiotics. Geneva: WHO; 2015. [cited 2021 February 2]. Available from: http://www.who.int/medicines/publications/WHO-PPL-Short_Summary_25Feb-ET_NM_WHO.pdf?ua=1
[8] Best Practice Advocacy Centre (BPAC) New Zealand. Syphilis rates continue to rise. 2019. [cited 2019 May 13]. Available from: https://bpac.org.nz/2019/syphilis.aspx
[9] New Zealand Sexual Health Society. Sexual health check guideline. 2017. [cited 2019 May 17]. Available from: https://www.nzshs.org/docman/guidelines/principles-of-sexual-health-care/148-sexual-health-check-summary/file
[10] The Institute of Environmental Science and Research Ltd. Sexually Transmitted Infections in New Zealand: Annual Surveillance Report 2016. Porirua, New Zealand: ESR; 2019. [cited 2019 August 20]. Available from: https://surv.esr.cri.nz/PDF_surveillance/STISurvRpt/2016/FINAL_2016_STI_AnnualReport.pdf
[11] New Zealand Sexual Health Society. NZSHS STI Management Guidelines for use in primary care. 2017. [cited 2019 November 15]. Available from: http://www.nzshs.org/guidelines.html
[12] Unemo M, Bradshaw CS, Hocking JS, et al. Sexually transmitted infections: challenges ahead. Lancet Infect Dis. 2017; 17 e235–79.
| Sexually transmitted infections: challenges ahead.Crossref | GoogleScholarGoogle Scholar | 28701272PubMed |
[13] World Health Organization (WHO). Guidelines for the management of sexually transmitted infections. Geneva: World Health Organization; 2003. [cited 2019 November 15]. Available from: http://apps.who.int/iris/bitstream/handle/10665/42782/9241546263_eng.pdf?sequence=1
[14] Rose SB, Garrett SM, Kennedy J, et al. Partner notification and retesting for Chlamydia trachomatis and Neisseria gonorrhoea: a case-note review in New Zealand primary care. J Prim Health Care. 2018; 10 132–39.
| Partner notification and retesting for Chlamydia trachomatis and Neisseria gonorrhoea: a case-note review in New Zealand primary care.Crossref | GoogleScholarGoogle Scholar | 30068468PubMed |
[15] Rose SB, Garrett SM, Stanley J, et al. Retesting and repeat positivity following diagnosis of Chlamydia trachomatis and Neisseria gonorrhoea in New Zealand: a retrospective cohort study. BMC Infect Dis. 2017; 17 526
| Retesting and repeat positivity following diagnosis of Chlamydia trachomatis and Neisseria gonorrhoea in New Zealand: a retrospective cohort study.Crossref | GoogleScholarGoogle Scholar | 28754106PubMed |
[16] Rose SB, Garrett SM, Pullon SRH. Overcoming challenges associated with partner notification following chlamydia and gonorrhoea diagnosis in primary care: a postal survey of doctors and nurses. J Prim Health Care. 2017; 9 136–44.
| Overcoming challenges associated with partner notification following chlamydia and gonorrhoea diagnosis in primary care: a postal survey of doctors and nurses.Crossref | GoogleScholarGoogle Scholar | 29530225PubMed |
[17] Morgan J, Woodhall S. Repeat chlamydia testing across a New Zealand district: 3 years of laboratory data. Sex Transm Infect. 2013; 89 28–31.
| Repeat chlamydia testing across a New Zealand district: 3 years of laboratory data.Crossref | GoogleScholarGoogle Scholar | 22454551PubMed |
[18] Rose SB, Garrett SM, Stanley J, et al. Chlamydia trachomatis and Neisseria gonorrhoeae retesting and reinfection rates in New Zealand Health Care settings: implications for sexually transmitted infection control. Sex Transm Dis. 2020; 47 151–7.
| Chlamydia trachomatis and Neisseria gonorrhoeae retesting and reinfection rates in New Zealand Health Care settings: implications for sexually transmitted infection control.Crossref | GoogleScholarGoogle Scholar | 31880741PubMed |
[19] Best Practice Advocacy Centre New Zealand (BPAC) A “how-to guide” for a sexual health check-up. Best Pract. 2013; 52 . [cited 2020 March 12] http://www.bpac.org.nz/BPJ/2013/April/how-to-guide-sexual-health.aspx
[20] Best Practice Advocacy Centre (BPAC) New Zealand. HIV Pre-Exposure Prophylaxis (PrEP): a how-to guide. 2019. [cited 2019 May 13]. Available from: https://bpac.org.nz/2019/prep.aspx
[21] Best Practice Advocacy Centre (BPAC) New Zealand. Emerging issues in the management of chlamydia and gonorrhoea. 2019. [cited 2019 May 13]. Available from: https://bpac.org.nz/2019/chlamydia-gonorrhoea.aspx
[22] Royal New Zealand College of General Practitioners. RNZCGP College updates: What does Level 2 mean for general practice? 2020. [cited 2020 May 20]. Available from: https://www.rnzcgp.org.nz/Covid19/College_support/College_updates/What_Level_2_looks_like/Covid19/College_support/What_level_2_looks_like.aspx?hkey=393f8ba8-b821-434e-ab84-830a09845b39.


