The assessment of acute chest pain in New Zealand rural hospitals utilising point-of-care troponin
Rory Miller 1 , Garry Nixon 21 Otago Medical School, Dunedin School of Medicine, Dunedin, New Zealand
2 Programme Director of the Rural Postgraduate Programme, Dunedin School of Medicine, Dunedin, New Zealand
Correspondence to: Rory Miller, 317 Linton Crescent, Whangamata, New Zealand. Email: rory.miller@otago.ac.nz
Journal of Primary Health Care 10(1) 90-92 https://doi.org/10.1071/HC18007
Published: 29 March 2018
Journal Compilation © Royal New Zealand College of General Practitioners 2018.
This is an open access article licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
In response to a Ministry of Health directive, New Zealand District Health Boards have developed emergency department Accelerated Diagnostic Chest Pain Pathways (ACPPs) combining objective scoring, ECG and high sensitivity troponin (hsTn) to facilitate the safe and early discharge of patients who present with suspected cardiac origin chest pain.1,2
Much of rural New Zealand lacks timely laboratory based hsTn and instead relies on point-of-care troponin (POCTn), with much lower sensitivity rendering these ACPPs unsuitable for use in these areas.3,4
There is no evidence for the use of POCTn in high-risk populations for rural hospital use, although an ACPP incorporating POCTn in a low-risk urban population has been shown to be safe.5,6 This is being validated in a rural New Zealand General Practice setting.7
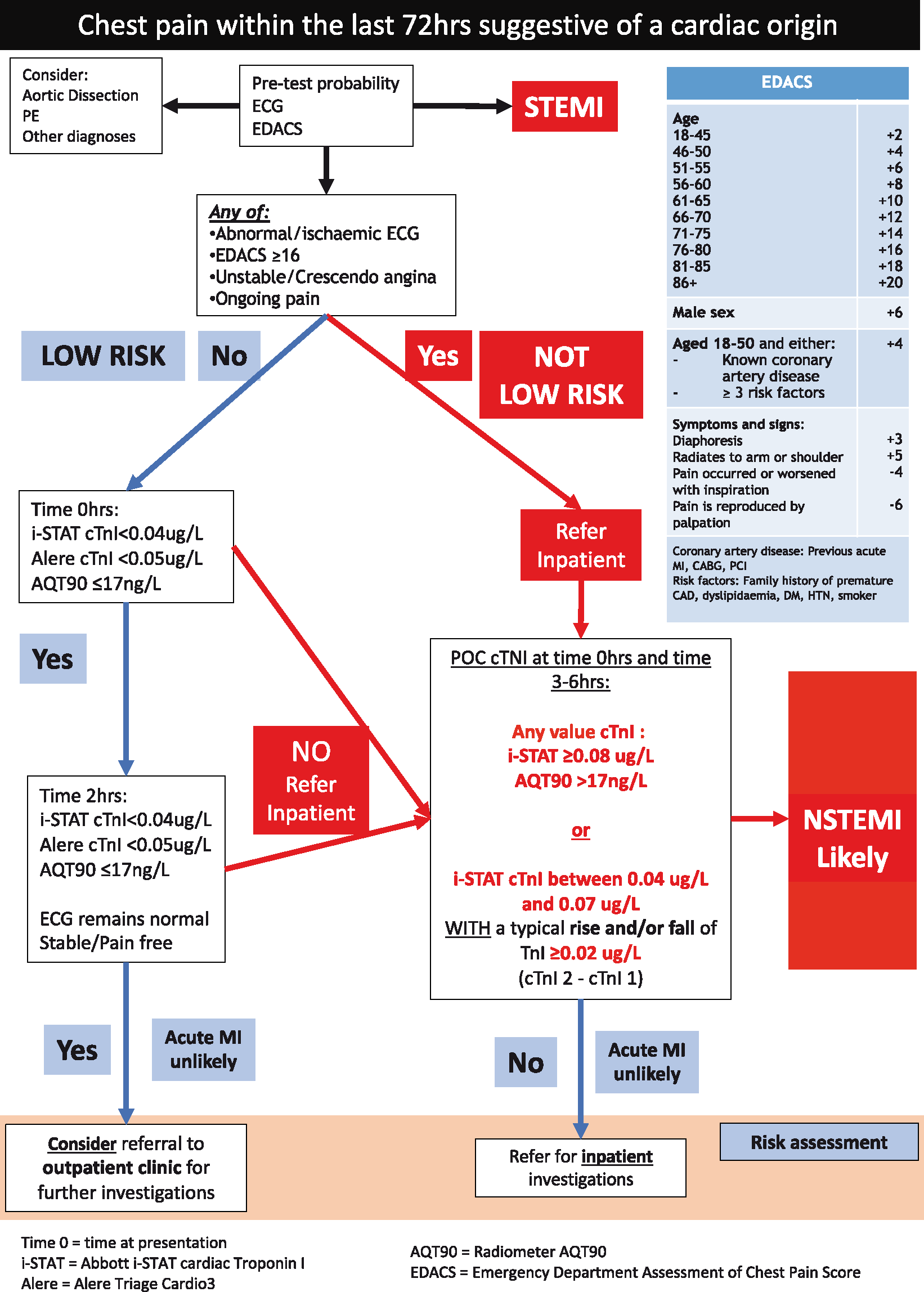
Adapting these data and consensus guidelines from the Australasian Association of Biochemists we recommend the attached pathway for use in rural areas reliant on POCTn (Fig. 1).5–10
The Emergency Department Assessment of Chest Pain (EDACS) and an ECG are used to categorise patients into either ‘low-risk’ or ‘not low-risk’ groups.9,10
Low-risk (rural general practice and rural hospitals): POCTn is performed at presentation and repeated two hours later. Patients are able to be discharged if both POCTn levels are less than 0.04 ug/L (Abbott i-STAT) or 0.05 ug/L (Alere Triage Cardio–3) with appropriate referral for urgent outpatient risk assessment.5
Not low-risk: Patients are admitted to a rural hospital. Two negative POCTn tests performed at presentation and between three and six hours later effectively excludes a myocardial infarction and these patients are referred for inpatient risk assessment.8,9
To improve sensitivity, we endorse using a POCTn cut-off below the manufacturer’s recommendation.4,8,11,12 However, in an effort to maintain specificity, a typical rise and fall of troponin is required for a positive test at low but detectable levels.8 The change in troponin occurs reliably in the hours immediately following a cardiac event but there may be little change between the first and second levels several hours after the onset of chest pain.13 Therefore, a persistently high troponin without alternate explanation should not be ignored. Qualitative POCTn assays lack sensitivity to exclude myocardial infarction and we do not recommend their use.14,15
We expect this pathway to miss less than 1% of major adverse cardiac events (MACE) in keeping with other ACPP.2,5,16 We recommend further research to validate this pathway, preferably with a newer higher sensitivity POCTn.
Competing interest
None
References
[1] Than M, Aldous S, Lord SJ, et al. A 2-Hour diagnostic protocol for possible cardiac chest pain in the emergency department a randomized clinical trial. JAMA Intern Med. 2014; 174 51–8.[2] Munro AR, Jerram T, Morton T, Hamilton S. Use of an accelerated diagnostic pathway allows rapid and safe discharge of 70% of chest pain patients from the emergency department. N Z Med J. 2015; 128 62–71.
[3] Pickering J. 2014 update of: Availability of troponin testing for cardiac patients in New Zealand 2002 to 2011: implications for patient care. 2015 [cited 2017 October 21]; Available from: https://www.hiirc.org.nz/page/52757/troponin-assays-in-use-in-nz-dec–2014/?tab=7536{\&}contentType=251{\&}section=41562
[4] Schneider HG, Ablitt P, Taylor J. Improved sensitivity of point of care troponin I values using reporting to below the 99th percentile of normals. Clin Biochem. 2013; 46 979–82.
| Improved sensitivity of point of care troponin I values using reporting to below the 99th percentile of normals.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXovVWgsb8%3D&md5=0a37eb2d7d0285dc921791f105199d0eCAS |
[5] Than M, Cullen L, Reid CM, et al. A 2-h diagnostic protocol to assess patients with chest pain symptoms in the Asia-Pacific region (ASPECT): A prospective observational validation study. Lancet. 2011; 377 1077–84.
| A 2-h diagnostic protocol to assess patients with chest pain symptoms in the Asia-Pacific region (ASPECT): A prospective observational validation study.Crossref | GoogleScholarGoogle Scholar |
[6] Aldous S, Mark Richards A, George PM, et al. Comparison of new point-of-care troponin assay with high sensitivity troponin in diagnosing myocardial infarction. Int J Cardiol. 2014; 177 182–6.
| Comparison of new point-of-care troponin assay with high sensitivity troponin in diagnosing myocardial infarction.Crossref | GoogleScholarGoogle Scholar |
[7] Scott-Jones J, Norman T, Than M, Devlin G, Egan G, George P, et al. Measured Implementation of an Accelerated Chest Pain Diagnostic Pathway in Primary Care. Heart Lung Circ. 2017; 26 S41
| Measured Implementation of an Accelerated Chest Pain Diagnostic Pathway in Primary Care.Crossref | GoogleScholarGoogle Scholar |
[8] AACB: Australasian Associations Biochemists. Recommendations for Use of Point-of-Care (POC) Troponin Assays in Assessment of Acute Coronary Syndrome. 2016;(December):1–33.
[9] Than M, Flaws D, Sanders S, Doust J, Glasziou P, Kline J, et al. Development and validation of the emergency department assessment of chest pain score and 2h accelerated diagnostic protocol. EMA -. Emerg Med Australas. 2014; 26 34–44.
| Development and validation of the emergency department assessment of chest pain score and 2h accelerated diagnostic protocol. EMA -.Crossref | GoogleScholarGoogle Scholar |
[10] Roche T, Jennings N, Clifford S, O’connell J, Lutze M, Gosden E, et al. Review article: Diagnostic accuracy of risk stratification tools for patients with chest pain in the rural emergency department: A systematic review. EMA -. Emerg Med Australas. 2016; 28 511–24.
| Review article: Diagnostic accuracy of risk stratification tools for patients with chest pain in the rural emergency department: A systematic review. EMA -.Crossref | GoogleScholarGoogle Scholar |
[11] Abbott Point of Care Inc. Cardiac troponin I (cTnI). 2013; pp. 1–10.
[12] Diercks DB, Peacock WF, Hollander JE, Singer AJ, Birkhahn R, Shapiro N, et al. Diagnostic accuracy of a point-of-care troponin I assay for acute myocardial infarction within 3 hours after presentation in early presenters to the emergency department with chest pain. Am Heart J. 2012; 163 74–80.e4.
| Diagnostic accuracy of a point-of-care troponin I assay for acute myocardial infarction within 3 hours after presentation in early presenters to the emergency department with chest pain.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhs1WjsrjE&md5=d20d2441ccaf51585beb2b78cd4fe64aCAS |
[13] Ali N, Le Jeune I, Simmonds M, et al. Use and interpretation of cardiac troponin testing. Br J Hosp Med. 2015; 76 C135–40.
| Use and interpretation of cardiac troponin testing.Crossref | GoogleScholarGoogle Scholar |
[14] Sørensen JT, Terkelsen CJ, Steengaard C, Lassen JF, Trautner S, Christensen EF, et al. Prehospital troponin T testing in the diagnosis and triage of patients with suspected acute myocardial infarction. Am J Cardiol. 2011; 107 1436–40.
| Prehospital troponin T testing in the diagnosis and triage of patients with suspected acute myocardial infarction.Crossref | GoogleScholarGoogle Scholar |
[15] Schuchert A, Hamm C, Scholz J, et al. Prehospital testing for troponin T in patients with suspected acute myocardial infarction. Am Heart J. 1999; 138 45–8.
| Prehospital testing for troponin T in patients with suspected acute myocardial infarction.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK1MzktlGisg%3D%3D&md5=d02d9739336607ae37cc2d3f7ba78f4bCAS |
[16] Venge P, van Lippen L, Blaschke S, Christ M, Geier F, Giannitsis E, et al. Equal clinical performance of a novel point-of-care cardiac troponin I (cTnI) assay with a commonly used high-sensitivity cTnI assay. Clin Chim Acta. 2017; 469 119–25.
| Equal clinical performance of a novel point-of-care cardiac troponin I (cTnI) assay with a commonly used high-sensitivity cTnI assay.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2sXlvFWmtLg%3D&md5=7ce1ba536be40a6cdff06d5737a9d523CAS |