Towards new forms of communication and surveillance: a mixed methods study of rapid respiratory virus assessment in general practice during the SARS-CoV-2 pandemic
Anthony Dowell 1 , Sue Huang 2 , Christine McIntosh 3 , Michelle Balm 4 , Isabella Cheung 5 , Lorraine Castelino
1 , Sue Huang 2 , Christine McIntosh 3 , Michelle Balm 4 , Isabella Cheung 5 , Lorraine Castelino  6 * , Nikki Turner 5 6
6 * , Nikki Turner 5 6
1
2
3
4
5
6
Abstract
Improvements in diagnostic test accuracy across multiple pathogens have resulted in multi-viral point-of-care testing (POCT) via a rapid antigen test (RAT).
This study aimed to describe general practice practitioners’ reactions to a pilot respiratory virus surveillance programme during the SARS-CoV-2 pandemic, which enabled surveillance for influenza and other respiratory viruses alongside POCT for SARS-CoV-2.
Participating general practices collected viral swabs between May and December 2022. Nasopharyngeal swabs were taken for both an immediate COVID-19 RAT and a polymerase chain reaction (PCR) for testing SARS-CoV-2, influenza, respiratory syncytial virus (RSV) and other respiratory viruses. A questionnaire explored practitioners’ experiences and perceptions, addressing project setup, swabbing process and perceived overall value.
Of 4135 swabbed patients, 54% were positive for one of the tested viruses. Involved nurses and doctors reported high adaptability to the swabbing process. Clinicians valued obtaining rapid diagnostic information for patient management and patient communication. While no significant barriers were identified, practitioners acknowledged additional time requirements and potential challenges with swabbing young children.
The study demonstrated the feasibility and clinical utility of using POCT swabbing for immediate RAT and subsequent PCR testing for respiratory viruses in general practices when managing a viral pandemic. The data assisted in identifying community transmission of respiratory viruses, provided information for patient management and reinforced positive health messages about viral illnesses. The study suggests potential benefits for both individual patient care and population-based surveillance. The study also identified the potential value of multi-viral POCT testing via a RAT.
Keywords: antimicrobial prescribing, COVID-19 pandemic, general practice, physician–patient communication, point of care testing, respiratory virus surveillance, SARS-CoV-2 pandemic, viral respiratory infection.
| WHAT GAP THIS FILLS |
| What is already known: The introduction of polymerase chain reaction (PCR) has been seen as a rapid and sensitive method for respiratory virus surveillance, and the COVID-19 pandemic highlighted the critical need for rapid diagnosis of SARS-CoV-2 and the importance of using PCR testing for an accurate assessment. |
| What this study adds: This study demonstrated the feasibility and clinical utility of using point-of-care test (POCT) swabbing for immediate rapid antigen test (RAT) and subsequent PCR testing for respiratory viruses in general practices in the middle of managing a viral pandemic. |
Introduction
Accurate diagnosis and assessment of influenza and other respiratory illnesses in primary care are important for surveillance, potential treatment advances1 and patient communication. Point-of-care testing (POCT) has long been integral in health care,2 initially in hospital care then extended to primary care,3 with tests such as blood glucose monitoring and pregnancy tests.
Rapid reporting in respiratory infection is a relatively recent development, initially using C-reactive protein (CRP),4 with much of POCT use being focused on antibiotic and more recently, anti-viral prescribing.5,6 Polymerase chain reaction (PCR) testing is recognised as a rapid and sensitive method for respiratory virus surveillance7,8 with studies demonstrating POCT being both feasible and having an antimicrobial prescribing impact.9,10
While PCR testing is the ‘gold standard’ test for virus identification, it requires centralised laboratory-based testing, and creates delays in clinical decision making. Multi-viral rapid antigen testing (RAT) has enabled testing for multiple respiratory viruses on one sample, but early platforms had low sensitivity and specificity.11 Despite primary care internationally showing enthusiasm for POCT, reservations remained about test accuracy and results interpretation.12,13
The Aotearoa New Zealand (NZ) Ministry of Health funds the Institute of Environmental Science and Research (ESR) to undertake winter surveillance for influenza like illness (ILI) using weekly nasopharyngeal swabs for PCR testing from sentinel general practices using the World Health Organization international standard for ILI.14
The arrival of COVID-19 highlighted the critical need for rapid diagnosis and genetic sample collection for a novel respiratory virus.15,16 To effectively identify SARS-CoV-2, primary care needed to adapt traditional acute respiratory illness (ARI) screening17 to recognise a broader list of respiratory symptoms. Therefore, a pragmatic solution was needed to combine screening for SARS-CoV-2 alongside influenza viruses without overburdening an already strained workforce.18
With improvements in the diagnostic accuracy of tests across multiple pathogens,19 we determined it appropriate to pilot using a RAT enabling an immediate test for SARS-CoV-2 alongside PCR-based testing for multiple respiratory viruses. We also aimed to explore facilitators and barriers to its use,20 and to understand the type and incidence of respiratory viruses among consultation-seeking patients with an ARI.
Methods
We recruited a purposive sample of six general practices with a range of geographical and socio-demographic characteristics. Nasopharyngeal viral swab collection commenced between May and June 2022 and ended on 11 December 2022. An electronic collection template for virology and clinical data entry was developed for recording viral swabs, clinical details and swab results.
Practices were informed that the ARI surveillance would use a routine nasopharyngeal swab (at that time current practice indicated for COVID-19 testing – ie no change in practice) to test for influenza and non-influenza respiratory viruses and SARS-CoV-2 with a single PCR swab. A RAT test for immediate COVID-19 results was undertaken by using the same PCR swab to allow for immediate feedback for the SARS-CoV-2 results.
The case definition for ARI consisted of any acute respiratory symptoms such as new or worsening cough, fever (at least 38°C), shortness of breath, sore throat, coryza (runny nose), anosmia and dysgeusia (altered sense of taste). Patients presenting with less typical symptoms (eg headache, myalgia) were also recommended for testing, as were patients with any other symptoms consistent with COVID-19.
A nasopharyngeal/mid-turbinate swab was taken from the patient and inserted into liquid transport media. The liquid media was sampled using a RAT swab, processed on site for SARS-CoV-2 and the remaining viral transport media was sent to a central laboratory for multiplex PCR testing. The samples were tested there for SARS-CoV-2, influenza A and B and non-influenza respiratory viruses (respiratory syncytial virus, parainfluenza virus types 1, 2, 3 and 4, rhinovirus, adenovirus and human metapneumovirus (hMPV). PCR test results for SARS-CoV-2 were available on the practice management system (PMS) within 48 h and for the other viral tests within 7 days.
A proof of concept validation for using a single viral liquid media for both RAT and laboratory-based PCR testing was undertaken using 60 nasopharyngeal/mid-turbinate swabs, comparing RAT results with PCR results. The good correlation of results in samples where the cycle threshold (Ct) value was <25 confirmed that the same sample could be used for both tests.
A questionnaire with 27 questions exploring the introduction of the viral testing programme and facilitators and barriers to its use was completed by the practice manager or a lead clinician in each practice at the end of the study period (questionnaire outline is included in Appendix 1).
Ethics approval was obtained from the Health and Disability Ethics Committees NTX/11/11/102/AM66.
Results
Overall 4135 patients met ARI eligibility criteria and 2233 (54%) tested positive for at least one of the viruses tested. Table 1 shows the results of testing.
| Respiratory viruses | Practice | Total | ||||||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | |||
| Number of specimens tested | 753 | 741 | 346 | 644 | 447 | 1204 | 4135 | |
| Number of positive specimens (%) | 316 (42.0) | 386 (52.1) | 188 (54.3) | 371 (57.6) | 251 (56.2) | 721 (59.9) | 2233 (54) | |
| Influenza A | 35 | 4 | 34 | 86 | 11 | 153 | 323 | |
| Influenza B | 0 | 1 | 0 | 0 | 0 | 0 | 1 | |
| Respiratory syncytial virus (RSV) | 12 | 34 | 17 | 18 | 30 | 48 | 159 | |
| Parainfluenza 1 (PIV1) | 0 | 0 | 0 | 1 | 0 | 0 | 1 | |
| Parainfluenza 2 (PIV2) | 3 | 15 | 4 | 9 | 12 | 29 | 72 | |
| Parainfluenza 3 (PIV3) | 54 | 45 | 20 | 25 | 27 | 60 | 231 | |
| Rhinovirus (RV) | 142 | 162 | 74 | 143 | 100 | 214 | 835 | |
| Adenovirus (AdV) | 19 | 52 | 11 | 23 | 19 | 76 | 200 | |
| Human metapneumovirus (hMPV) | 41 | 85 | 33 | 57 | 64 | 102 | 382 | |
| Enterovirus | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| SARS-CoV-2 | 32 | 29 | 16 | 43 | 27 | 101 | 248 | |
| Single virus detection (number and % of positive specimens) | 294 (93.0) | 349 (90.4) | 167 (88.8) | 341 (91.9) | 214 (85.3) | 664 (92.1) | 2029 (90.9) | |
| Multiple virus detection (number and % of positive specimens) | 22 (7.0) | 37 (9.6) | 21 (11.2) | 30 (8.1) | 37 (14.7) | 57 (7.9) | 204 (9.1) | |
Rhinovirus was the most common (835/4135, 20.2%) followed by hMPV (382/4135, 9.2%).
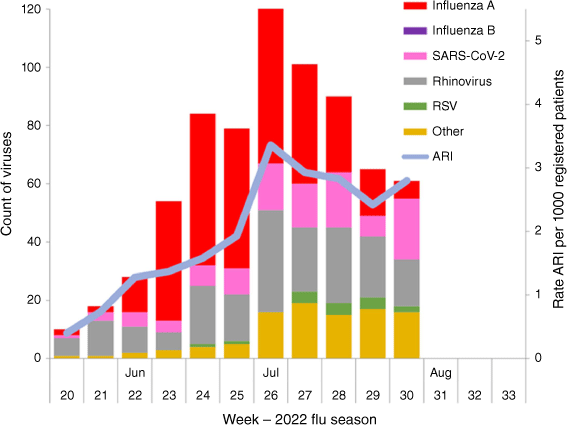
The results were used as part of the weekly reporting from ESR during this period providing a valuable addition to other sources of virology surveillance (Fig. 1).
Clinician responses from questionnaire
All sentinel practices (n = 6) completed the questionnaire. One questionnaire was completed by either the practice manager, clinical nurse lead or GP of each practice. Responses were divided into a number of theme areas including project setup, the process of taking the swabs and perceptions of the overall value of the approach.
1. Effectiveness of the systems. All practices successfully implemented ARI swabbing into practice without difficulty. The clinical reporting template was easily integrated into the practice management system.
2. Acceptability of undertaking swabbing. Swabbing was performed by doctors and nurses in the practices, with most clinical team members having some input into swabbing. There was a high patient uptake of the combined RAT and PCR swab, with most practices reporting over 70% participation.
3. Usefulness of identification of respiratory viruses. Clinicians appreciated the immediacy of RAT results especially when COVID-19 was rapidly circulating. The availability of multiplex PCR results for other respiratory viruses within a clinically useful timeframe was also considered of great benefit for decision making.
It gave a diagnosis that we would otherwise not have had. (participating general practitioner)
Practices used the additional information to reinforce patient knowledge about viral illnesses including transmission and enabled understanding about treatment and non-prescription of antibiotics. Patients also appreciated having information regarding the aetiology of their illness. The multiplex PCR results provided information about prevalence of circulating respiratory viruses, which had been absent in the community for 2 years due to border closures
4. Extra time involvement. While there were no significant barriers identified in the process, clinicians were aware of the additional time required for result follow-up due to staggered result availability.
5. Innovative outcome – combining into a single event: The use of a single mid-turbinate nasal swab for both RAT and PCR testing was perceived as an advantage by practices.
6. Delay in receiving results: The delay in time between the immediate COVID result from the RAT and the wait for the PCR result was seen as a disadvantage.
We would need to receive the results within 2–3 days to be clinically helpful for that patient and be used to reduce antibiotic prescribing, etc.
Because processing was taking place within a research setting, increased time was required for the additional transport from practices to a central lab. If locally processed and the assays were run daily, a 2 day turnaround could be achievable.
Discussion
This project demonstrated the feasibility of using standard primary care practice to collect national surveillance data during a pandemic. The use of a single sampling event was acceptable and advantageous for practices and patients. The data assisted national surveillance in identifying community transmission of ILIs and documenting the relative prevalence of circulating respiratory viruses, including influenza and COVID-19, at a time of uncertain and changing viral patterns.
In keeping with recent overseas studies evaluating the use of POCT molecular testing in general practices, our practices perceived benefits to having increased diagnostic information regarding viral aetiology leading to potential changes in prescribing and communication strategies with patients.13,21–23
The strength of our study is the incorporation of the process and technology into routine practice at a time of significant workload pressure from respiratory illness. Enthusiastic participation was achieved in a range of different types and settings, including a Māori health provider serving a high needs population.
Limitations included questions about the feasibility of swabbing infants and young children within routine clinical practice.
We have demonstrated the feasibility of introducing routine POCT RAT for respiratory viral infections in general practice with reported satisfaction from both clinicians and patients with the information gained.
With the introduction of multi-viral RAT technology, there is the potential to have a single swab provide immediate diagnostic information on a range of different viruses with utility both for individual patient management and also for population based surveillance of influenza, SARS-CoV-2 and other viral pathogens.24
Data availability
The data that supports this study cannot be publicly shared due to ethical and privacy reasons.
Declaration of funding
We thank Flu Lab for funding the SHIVERS-V team to undertake research encompassed by the project called ‘Influenza in a post-COVID world’ which has allowed researchers in Aotearoa New Zealand to collect crucial data on influenza and produce local innovations and have international impact with a Southern Hemisphere ‘population laboratory’.
Acknowledgements
We wish to thank and acknowledge the participating practices for supporting this study.
References
1 Van Elden LJ, Van Essen GA, Boucher CA, et al. Clinical diagnosis of influenza virus infection: evaluation of diagnostic tools in general practice. Br J Gen Pract 2001; 51(469): 630-4.
| Google Scholar | PubMed |
2 Liu X, Zhu X, Kost GJ, et al. The creation of point-of-careology. Point Care 2019; 18(3): 77-84.
| Crossref | Google Scholar |
3 Blattner K, Nixon G, Jaye C, et al. Introducing point-of-care testing into a rural hospital setting: thematic analysis of interviews with providers. J Prim Health Care 2010; 2(1): 54-60.
| Google Scholar | PubMed |
4 Diederichsen HZ, Skamling M, Diederichsen A, et al. Randomised controlled trial of CRP rapid test as a guide to treatment of respiratory infections in general practice. Scand J Prim Health Care 2000; 18(1): 39-43.
| Crossref | Google Scholar | PubMed |
5 Brendish NJ, Malachira AK, Armstrong L, et al. Routine molecular point-of-care testing for respiratory viruses in adults presenting to hospital with acute respiratory illness (ResPOC): a pragmatic, open-label, randomised controlled trial. Lancet Respir Med 2017; 5(5): 401-11.
| Crossref | Google Scholar | PubMed |
6 Smedemark SA, Aabenhus R, Llor C, et al. Biomarkers as point‐of‐care tests to guide prescription of antibiotics in people with acute respiratory infections in primary care. Cochrane Database Syst Rev 2022; 10(10): CD010130.
| Crossref | Google Scholar | PubMed |
7 Wallace LA, Collins TC, Douglas JD, et al. Virological surveillance of influenza-like illness in the community using PCR and serology. J Clin Virol 2004; 31(1): 40-5.
| Crossref | Google Scholar | PubMed |
8 Zumla A, Al-Tawfiq JA, Enne VI, et al. Rapid point of care diagnostic tests for viral and bacterial respiratory tract infections—needs, advances, and future prospects. Lancet Infect Dis 2014; 14(11): 1123-35.
| Crossref | Google Scholar | PubMed |
9 Brigadoi G, Gastaldi A, Moi M, et al. Point-of-Care and rapid tests for the etiological diagnosis of respiratory tract infections in children: a systematic review and meta-analysis. Antibiotics 2022; 11(9): 1192.
| Crossref | Google Scholar | PubMed |
10 Lee JJ, Verbakel JY, Goyder CR, et al. The clinical utility of point-of-care tests for influenza in ambulatory care: a systematic review and meta-analysis. Clin Infect Dis 2019; 69(1): 24-33.
| Crossref | Google Scholar | PubMed |
11 Bruning AH, de Kruijf WB, van Weert HC, et al. Diagnostic performance and clinical feasibility of a point-of-care test for respiratory viral infections in primary health care. Fam Pract 2017; 34(5): 558-63.
| Crossref | Google Scholar | PubMed |
12 Howick J, Cals JW, Jones C, et al. Current and future use of point-of-care tests in primary care: an international survey in Australia, Belgium, The Netherlands, the UK and the USA. BMJ Open 2014; 4(8): e005611.
| Crossref | Google Scholar | PubMed |
13 Jones CH, Howick J, Roberts NW, et al. Primary care clinicians’ attitudes towards point-of-care blood testing: a systematic review of qualitative studies. BMC Fam Pract 2013; 14: 117.
| Crossref | Google Scholar | PubMed |
14 World Health Organization. WHO surveillance case definitions for ILI and SARI. 2014. Available at https://www.who.int/teams/global-influenza-programme/surveillance-and-monitoring/case-definitions-for-ili-and-sari
15 Velavan TP, Meyer CG. COVID-19: a PCR-defined pandemic. Int J Infect Dis 2021; 103: 278-9.
| Crossref | Google Scholar | PubMed |
16 Kim H, Hong H, Yoon SH. Diagnostic performance of CT and reverse transcriptase polymerase chain reaction for coronavirus disease 2019: a meta-analysis. Radiology 2020; 296(3): E145-55.
| Crossref | Google Scholar | PubMed |
17 Te Whatu Ora (Health New Zealand). About COVID-19. 2024. Available at https://info.health.nz/conditions-treatments/infectious-diseases/covid-19/about-covid-19/
18 Pledger M, Mohan N, Silwal P, et al. The enrolment gap and the COVID-19 pandemic: an exploration of routinely collected primary care enrolment data from 2016 to 2023 in Aotearoa New Zealand. J Prim Health Care 2023; 15(4): 316-23.
| Crossref | Google Scholar | PubMed |
19 Zhang Z, Ma P, Ahmed R, et al. Advanced point-of-care testing technologies for human acute respiratory virus detection. Adv Mater 2022; 34(1): e2103646.
| Crossref | Google Scholar | PubMed |
20 Corker E, Lorencatto F, Anderson N, et al. Acceptability and facilitators of and barriers to point‐of‐care HIV testing in a homeless‐focused service in Gloucestershire: a qualitative evaluation. HIV Med 2022; 23(3): 237-48.
| Crossref | Google Scholar | PubMed |
21 Khalid TY, Duncan LJ, Thornton HV, et al. Novel multi-virus rapid respiratory microbiological point-of-care testing in primary care: a mixed-methods feasibility evaluation. Fam Pract 2021; 38(5): 598-605.
| Crossref | Google Scholar | PubMed |
22 de Lusignan S, Hoang U, Liyanage H, et al. Integrating molecular point-of-care testing for influenza into primary care: a mixed-methods feasibility study. Br J Gen Pract 2020; 70(697): e555-62.
| Crossref | Google Scholar | PubMed |
23 Wood F, Brookes-Howell L, Hood K, et al. A multi-country qualitative study of clinicians’ and patients’ views on point of care tests for lower respiratory tract infection. Fam Pract 2011; 28(6): 661-9.
| Crossref | Google Scholar | PubMed |
Appendix 1
Questionnaire to practices
Question 1: What practice management system do you have at your practice?
Question 2: Were there any difficulties in integrating the ARI surveillance request into the existing laboratory request form?
Question 3: How did you inform the practice team about the project?
Question 4: How many members of the clinical team regularly participated in swabbing and associated activities?
Question 6: How many of these participating members were nurses?
Question 7. Was this all done via a respiratory clinic?
Question 8: Did all your ARI presentations receive RAT or swab tests?
Question 9: If all your ARI presentations did not receive RAT or swab tests, who were likely to have them (eg children)?
Question 10: What were the main reasons for not having a RAT or PCR?
Question 11: Can you estimate how many of your enrolled patients may have received a RAT and not a swab for combined RAT/PCR?
Question 12: What would be the most common reasons for patients receiving a RAT and not a swab for combined RAT/PCR?
Question 13: What proportion of all presenting respiratory cases do you think you managed to capture with a PCR swab test?
Question 14: What strengths/advantages did your staff find in the process of obtaining a PCR test?
Question 15: What barriers did your staff find in the process of obtaining a PCR test?
Question 16: Were staff able to follow the protocol ARI eligibility criterion?
Question 17: Were there any difficulties with following the protocol ARI eligibility criterion?
Question 18: What is your opinion on the use of the single mid-turbinate nasal (ie deep nasal) swab for both RAT testing and PCR?
Question 19: What is your preference towards the previous two swabs method as compared to the one-swab procedure?
Question 20: Do you have any comments on your preference between the one and two-swab methods mentioned above?
Question 21: Were there any difficulties in performing the rapid antigen test (RAT) compared to the viral PCR swab?
Question 22: Do you have any comments about the difficulties in performing the rapid antigen test (RAT) compared to the viral PCR swab?
Question 23: Did you find the additional information from the swabbing useful clinically?
Question 24: Do you have comments about your above answer on the usefulness of the additional information from the swabbing?
Question 25: Did you give the swab information back to patients?
Question 26: Did you receive any feedback from patients about this project? If yes, then can you please describe the feedback?
Question 27: Your participation in a future project?