It is time for a more targeted approach to prediabetes in primary care in Aotearoa New Zealand
Christine Barthow 1 * , Sue Pullon
1 * , Sue Pullon  2 , Eileen McKinlay
2 , Eileen McKinlay  3 , Jeremy Krebs 1
3 , Jeremy Krebs 1
1 Department of Medicine, University of Otago, Wellington, PO Box 7343, Wellington South 6242, New Zealand.
2 Department of Primary Health Care & General Practice, University of Otago, Wellington, PO Box 7343, Wellington South 6242, New Zealand.
3 Centre for Interprofessional Education, University of Otago, PO Box 56, Dunedin, New Zealand.
Journal of Primary Health Care 14(4) 372-377 https://doi.org/10.1071/HC22089
Published: 11 November 2022
© 2022 The Author(s) (or their employer(s)). Published by CSIRO Publishing on behalf of The Royal New Zealand College of General Practitioners. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
Type 2 diabetes (T2DM), its related morbidities and entrenched diabetes‐related inequities pose significant challenges for health care delivery systems in Aotearoa New Zealand (NZ). Primary care services undertake the majority of diabetes prevention work by initially detecting and managing those with prediabetes. In this viewpoint, we present available NZ data to highlight NZ trends in prediabetes and consider the current NZ clinical guidelines and the prediabetes care pathway. Multiple areas for improvement are identified to optimise diabetes prevention, potentially reduce T2DM inequities, and sustain more effective prediabetes management in primary care in NZ.
Keywords: cardiovascular disease, clinical guidelines, epidemiology, New Zealand, prediabetes, prevention, primary health care, progression, renal disease, type 2 diabetes.
Introduction
Type 2 diabetes mellitus (T2DM) is increasingly common in Aotearoa New Zealand (NZ), and substantial inequities in care exist for those of Māori, Pacific or Asian ethnicity.1 Crucially, T2DM is 2.6-fold more likely to occur in the most deprived compared with the least deprived groups in NZ.1 The detection of prediabetes provides an early opportunity to intervene to prevent or delay the onset of T2DM and associated morbidities. Although extensive research supports the efficacy of long-term lifestyle interventions in those with prediabetes,2–6 individuals find adopting and sustaining lifestyle changes difficult.7–12 These interventions are time-intensive and challenging for health providers to deliver.3 In NZ, primary care clinicians identify uncertainties about who is most likely to progress to T2DM and question when and how to intervene effectively.13,14 They struggle to fit prediabetes care into time-constrained appointments.13,14 Finding effective responses to these issues is critical for effective diabetes prevention and health equity.
Prevalence and epidemiological trends related to prediabetes in NZ
NZ lacks up-to-date national data on the prevalence of prediabetes, which is classified as glycated haemoglobin (HbA1c) ≥ 41–49 mmol/mol or fasting glucose 6.1–6.9 mmol/L or 2-h glucose tolerance test 7.8–11.0 mmol/L. However, crude prediabetes estimates for the population aged ≥15 years from the Auckland Metropolitan region are 26% (2014) and approximately 21% in 2021 (pers. comm., Wing Cheuk Chan, Te Whatu Ora Counties Manukau). Most of these people would have been diagnosed using HbA1c, and the rate of undetected prediabetes is unknown. Māori and Pacific Peoples have higher rates of prediabetes,15 developing it at a younger age than others, with relative risks for Māori and Pacific versus non-Māori/non-Pacific of 1.97 for those aged <50 years and 1.42 for those aged ≥50 years.16 Similarly, the rates of prediabetes among NZ youth and children appear to be increasing in line with the rising rates of T2DM in these populations.17 For example, among adolescents with obesity (body mass index (BMI) ≥ 30 kg/m2), 53% had prediabetes,18 and recently, 16% of NZ children aged 8–12 years were found to have prediabetes.19 At a population level, the prevalence of T2DM and prediabetes increases with age;4 however, conversely, longer exposure to elevated glucose levels puts those who develop prediabetes/T2DM earlier in life at greater risk for adverse outcomes.20,21
Prediabetes-related health outcomes
The likelihood of progressing from prediabetes to T2DM is variable. One NZ study22 found 5.0% (95% CI: 4.53–5.42) of adults with prediabetes developed T2DM over 3 years. Progression was greater in men, younger people, and those with higher HbA1c and BMI levels. Specifically, those aged 35–44 years progressed three-fold more frequently than those aged >65 years. Baseline HbA1c and BMI were both independently associated with progression to diabetes with dose-response relationships: HbA1c (41 vs 49 mmol/mol) relative risk (RR) 55.6, [95% CI, 33.3–90.9]; and BMI (<25 vs 40+ kg/m2) RR 3.68 [95% CI, 1.39–9.72]. Although overall progression rates were greater in Māori and Pacific peoples, this was explained by higher baseline HbA1c. Importantly, these data are limited to 3 years, and progression was higher among groups outside the sex/ethnic/age criteria for cardiovascular risk assessment (CVRA). Therefore, a reliance on screening for prediabetes that routinely occurs as part of CVRA processes may miss higher-risk groups most needing interventions. At the opposite end of the age spectrum, a prospective cohort study of older adults with prediabetes in the United States found many more regressed to normal glucose control or died from other causes than progressed to T2DM and, therefore, one would question the value of this diagnosis for those who are of an older age.23 These differences between younger and older adults with prediabetes likely reflect the heterogeneous pathogenesis of T2DM and have important implications for targeting interventions.24
Arguably, the name ‘prediabetes’ is unhelpful, as it may foster the perception that this condition only confers an increased risk for developing T2DM. However, those with prediabetes are also at increased risk of developing cardiovascular disease (CVD) and renal disease without frank T2DM. We found no published NZ data on this; however, a recent UK study examined outcomes related to HbA1c 39–48 mmol/mol over 11 years and found a gradual increase of CVD and renal disease across the spectrum of HbA1c levels, with most disease occurring in those with HbA1c <48 mmol/mol. More than 66% of those with prediabetes who developed CVD or renal disease did not develop T2DM.25 Our exploratory NZ study26 found that 74% of a small sample (n = 153) of adults with prediabetes had metabolic syndrome, and there was considerable variability in the severity of this condition. Together, these findings are a timely reminder of the artificial nature of diagnostic cut-off points and the importance of assessing a comprehensive range of risk factors in those with prediabetes.
Current guidance
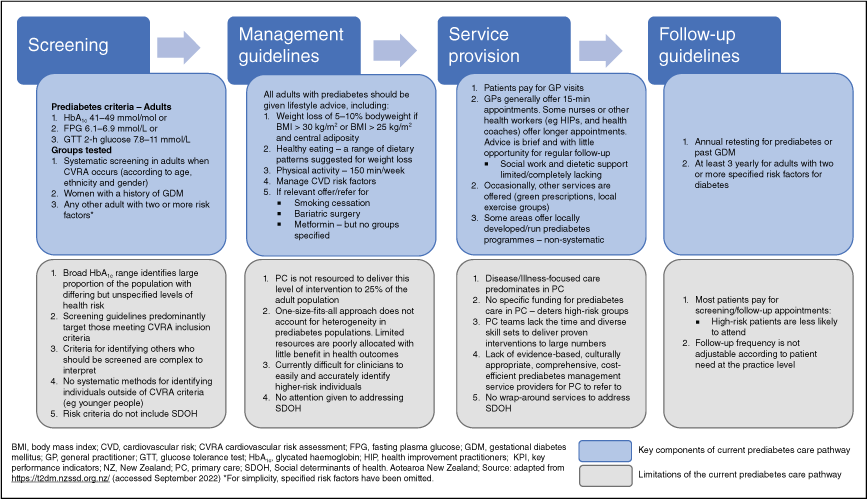
Current NZ clinical guidelines suggest a ‘one-size-fits-all’ approach to prediabetes management in primary care. All adults with prediabetes should receive lifestyle modification advice, metformin may be prescribed, and the active management of CVD risk factors is advised.27 However, given the prevalence of prediabetes and the variability of risks indicated in the data reviewed above, we argue that this approach is inadequate and unsustainable for primary care, and a more refined approach is required.
Fig. 1 illustrates the current NZ prediabetes pathway based on the NZ clinical guidelines and the most frequent models of service provision within primary care settings. Using the data presented above, a critical review of the current NZ clinical guidelines, the views of primary care clinicians, and previous research, we identify limitations to implementing high-quality and targeted prediabetes care across the pathway.
There are numerous opportunities to improve diabetes prevention work, which requires work at multiple levels. Table 1 identifies and suggests potential changes needed at policy, practice, and research levels to improve diabetes prevention in NZ.

|
Conclusion
Prediabetes is common, affecting 21–26% of adults; however, the associated health risks are multifaceted, highly variable, and not fully understood in NZ populations. Current prediabetes guidelines and management pathways do not reflect this variability. Māori and Pacific Peoples are particularly poorly served and yet have high health needs in this area. Current guidelines are poorly optimised for targeting interventions and addressing the sustainability of service delivery within primary care. Widespread and multilevel change is urgently required to address these issues and reduce diabetes-related health inequities.
Data availability
The primary source of data for this paper are published in academic literature.
Conflicts of interest
The authors declare no conflicts of interest.
Declaration of funding
Christine Barthow was supported by a University of Otago Postgraduate Publishing Bursary.
References
[1] Ministry of Health. Annual Data Explorer 2019/20: New Zealand health survey. 2020. Available at https://minhealthnz.shinyapps.io/nz-health-survey-2019-20-annual-data-explorer/ [Accessed 10 November 2021].[2] Uusitupa M, Khan TA, Viguiliouk E, et al. Prevention of type 2 diabetes by lifestyle changes: a systematic review and meta-analysis. Nutrients 2019; 11 2611
| Prevention of type 2 diabetes by lifestyle changes: a systematic review and meta-analysis.Crossref | GoogleScholarGoogle Scholar |
[3] Galaviz KI, Weber MB, Straus A, et al. Global diabetes prevention interventions: a systematic review and network meta-analysis of the real-world impact on incidence, weight, and glucose. Diabetes Care 2018; 41 1526–34.
| Global diabetes prevention interventions: a systematic review and network meta-analysis of the real-world impact on incidence, weight, and glucose.Crossref | GoogleScholarGoogle Scholar |
[4] Echouffo-Tcheugui JB, Selvin E. Prediabetes and what it means: the epidemiological evidence. Annu Rev Public Health 2021; 42 59–77.
| Prediabetes and what it means: the epidemiological evidence.Crossref | GoogleScholarGoogle Scholar |
[5] McManus E, Meacock R, Parkinson B, et al. Population level impact of the NHS Diabetes Prevention Programme on incidence of type 2 diabetes in England: an observational study. Lancet Reg Heal Eur 2022; 19 100420
| Population level impact of the NHS Diabetes Prevention Programme on incidence of type 2 diabetes in England: an observational study.Crossref | GoogleScholarGoogle Scholar |
[6] Marsden AM, Bower P, Howarth E, et al. ‘Finishing the race’ – a cohort study of weight and blood glucose change among the first 36,000 patients in a large-scale diabetes prevention programme. Int J Behav Nutr Phys Act 2022; 19 7
| ‘Finishing the race’ – a cohort study of weight and blood glucose change among the first 36,000 patients in a large-scale diabetes prevention programme.Crossref | GoogleScholarGoogle Scholar |
[7] Barry E, Greenhalgh T, Fahy N. How are health-related behaviours influenced by a diagnosis of pre-diabetes? A meta-narrative review. BMC Med 2018; 16 121
| How are health-related behaviours influenced by a diagnosis of pre-diabetes? A meta-narrative review.Crossref | GoogleScholarGoogle Scholar |
[8] Wild CEK, Rawiri NT, Willing EJ, et al. Challenges of making healthy lifestyle changes for families in Aotearoa/New Zealand. Public Health Nutr 2021; 24 1906–15.
| Challenges of making healthy lifestyle changes for families in Aotearoa/New Zealand.Crossref | GoogleScholarGoogle Scholar |
[9] Vanstone M, Rewegan A, Brundisini F, et al. Diet modification challenges faced by marginalized and nonmarginalized adults with type 2 diabetes: a systematic review and qualitative meta-synthesis. Chronic Illn 2017; 13 217–35.
| Diet modification challenges faced by marginalized and nonmarginalized adults with type 2 diabetes: a systematic review and qualitative meta-synthesis.Crossref | GoogleScholarGoogle Scholar |
[10] Abel S, Whitehead LC, Coppell KJ. Making dietary changes following a diagnosis of prediabetes: a qualitative exploration of barriers and facilitators. Diabet Med 2018; 35 1693–9.
| Making dietary changes following a diagnosis of prediabetes: a qualitative exploration of barriers and facilitators.Crossref | GoogleScholarGoogle Scholar |
[11] Abel SL, Whitehead LC, Tipene-Leach DC, et al. Proximal and distal influences on dietary change among a diverse group with prediabetes participating in a pragmatic, primary care nurse-led intervention: a qualitative study. Public Health Nutr 2021; 24 6015–26.
| Proximal and distal influences on dietary change among a diverse group with prediabetes participating in a pragmatic, primary care nurse-led intervention: a qualitative study.Crossref | GoogleScholarGoogle Scholar |
[12] Howells K, Bower P, Burch P, et al. On the borderline of diabetes: understanding how individuals resist and reframe diabetes risk. Health Risk Soc 2021; 23 34–51.
| On the borderline of diabetes: understanding how individuals resist and reframe diabetes risk.Crossref | GoogleScholarGoogle Scholar |
[13] McKinlay E, Hilder J, Hood F, et al. Uncertainty and certainty: perceptions and experiences of prediabetes in New Zealand primary care – a qualitative study. J Prim Health Care 2022; 14 138–45.
| Uncertainty and certainty: perceptions and experiences of prediabetes in New Zealand primary care – a qualitative study.Crossref | GoogleScholarGoogle Scholar |
[14] Barthow C, Krebs J, McKinlay E. Disincentivised and De-Prioritised Mahi: A Multiple Case Study of Pre-Diabetes Care Undertaken by General Practice in Aotearoa/New Zealand. Oral presentation at the New Zealand Society for the study of diabetes - Annual scientific meeting, 13–14 May 2022, Wellington. Available at https://www.ivvy.com.au/event/NZSSD22/welcome.html
[15] Coppell KJ, Mann JI, Williams SM, et al. Prevalence of diagnosed and undiagnosed diabetes and prediabetes in New Zealand: findings from the 2008/09 adult nutrition survey. N Z Med J 2013; 126 23–42.
[16] Gu Y, Warren J, Kennelly J, et al. Incidence rate of prediabetes: an analysis of New Zealand primary care data. In: Georgiou A, Grain H, Schaper LK, editors. Driving Reform: Digital Health is Everyone’s Business. IOS Press, Incorporated; 2015. pp. 81–6.
[17] Sjardin N, Reed P, Albert B, et al. Increasing incidence of type 2 diabetes in New Zealand children <15 years of age in a regional-based diabetes service, Auckland, New Zealand. J Paediatr Child Health 2018; 54 1005–10.
| Increasing incidence of type 2 diabetes in New Zealand children <15 years of age in a regional-based diabetes service, Auckland, New Zealand.Crossref | GoogleScholarGoogle Scholar |
[18] Leong KSW, Jayasinghe TN, Wilson BC, et al. High prevalence of undiagnosed comorbidities among adolescents with obesity. Sci Rep 2020; 10 20101
| High prevalence of undiagnosed comorbidities among adolescents with obesity.Crossref | GoogleScholarGoogle Scholar |
[19] Mazahery H, Gammon CS, Lawgun D, et al. Pre-diabetes prevalence and associated factors in New Zealand school children: a cross-sectional study. N Z Med J 2021; 134 76–90.
[20] Nanayakkara N, Curtis AJ, Heritier S, et al. Impact of age at type 2 diabetes mellitus diagnosis on mortality and vascular complications: systematic review and meta-analyses. Diabetologia 2021; 64 275–87.
| Impact of age at type 2 diabetes mellitus diagnosis on mortality and vascular complications: systematic review and meta-analyses.Crossref | GoogleScholarGoogle Scholar |
[21] Chan JCN, Lau ESH, Luk AOY, et al. Premature mortality and comorbidities in young-onset diabetes: a 7-year prospective analysis. Am J Med 2014; 127 616–24.
| Premature mortality and comorbidities in young-onset diabetes: a 7-year prospective analysis.Crossref | GoogleScholarGoogle Scholar |
[22] Teng A, Blakely T, Scott N, et al. What protects against pre-diabetes progressing to diabetes? Observational study of integrated health and social data. Diabetes Res Clin Pract 2019; 148 119–29.
| What protects against pre-diabetes progressing to diabetes? Observational study of integrated health and social data.Crossref | GoogleScholarGoogle Scholar |
[23] Rooney MR, Rawlings AM, Pankow JS, et al. Risk of progression to diabetes among older adults with prediabetes. JAMA Intern Med 2021; 181 511–9.
| Risk of progression to diabetes among older adults with prediabetes.Crossref | GoogleScholarGoogle Scholar |
[24] Defronzo RA. Pathogenesis of type 2 diabetes mellitus. In: DeFronzo R, Ferrannini E, Zimmet P, et al., editors. International Textbook of Diabetes Mellitus, 4th edn. Chichester, England: John Wiley & Sons, Ltd.; 2015. pp. 371–400.
[25] Honigberg MC, Zekavat SM, Pirruccello JP, et al. Cardiovascular and kidney outcomes across the glycemic spectrum. J Am Coll Cardiol 2021; 78 453–64.
| Cardiovascular and kidney outcomes across the glycemic spectrum.Crossref | GoogleScholarGoogle Scholar |
[26] Barthow C, Pullon S, Weatherall M, et al. They’re sicker than we think: an exploratory study profiling the cardio-metabolic health in a sample of adults with pre-diabetes in Aotearoa New Zealand. J Prim Health Care 2022; 14 221–8.
| They’re sicker than we think: an exploratory study profiling the cardio-metabolic health in a sample of adults with pre-diabetes in Aotearoa New Zealand.Crossref | GoogleScholarGoogle Scholar |
[27] Ministry of Health, New Zealand Society for the Study of Diabetes. Type 2 diabetes management guidance. 2021. Available at https://t2dm.nzssd.org.nz/Home.html#carousel_1d48 [Accessed 12 November 2021].
[28] Hill-Briggs F, Adler NE, Berkowitz SA, et al. Social determinants of health and diabetes: a scientific review. Diabetes Care 2021; 44 258–79.
| Social determinants of health and diabetes: a scientific review.Crossref | GoogleScholarGoogle Scholar |
[29] Holder-Pearson L, Chase JG. Socio-economic inequity: diabetes in New Zealand. Front Med 2022; 9 1–5.
| Socio-economic inequity: diabetes in New Zealand.Crossref | GoogleScholarGoogle Scholar |
[30] Wilson N, Morenga LT, Mackay S, et al. Food taxes and subsidies to protect health: relevance to Aotearoa New Zealand. N Z Med J 2020; 133 71–85. Available at http://www.ncbi.nlm.nih.gov/pubmed/32161423
[31] NICE. Type 2 diabetes prevention: population and community-level interventions. NICE; 2011. Available at www.nice.org.uk/guidance/ph35
[32] Bell K, Shaw JE, Maple-Brown L, et al. A position statement on screening and management of prediabetes in adults in primary care in Australia. Diabetes Res Clin Pract 2020; 164 108188
| A position statement on screening and management of prediabetes in adults in primary care in Australia.Crossref | GoogleScholarGoogle Scholar |
[33] Barthow CA. Diabetes prevention amongst those with pre-diabetes in Aotearoa New Zealand. Doctor of Philosophy Thesis, University of Otago, New Zealand; 2022.
[34] Te Karu L, Harwood M, Bryant L, et al. Compounding inequity: a qualitative study of gout management in an urban marae clinic in Auckland. J Prim Health Care 2021; 13 27–35.
| Compounding inequity: a qualitative study of gout management in an urban marae clinic in Auckland.Crossref | GoogleScholarGoogle Scholar |
[35] NICE. Type 2 diabetes: prevention in people at high risk Public health guideline. National Institute for Health and Care Excellence; 2017. Available at https://www.nice.org.uk/guidance/ph38/chapter/glossary
[36] Howarth E, Bower PJ, Kontopantelis E, et al. ‘Going the distance’: an independent cohort study of engagement and dropout among the first 100 000 referrals into a large-scale diabetes prevention program. BMJ Open Diabetes Res Care 2020; 8 e001835
| ‘Going the distance’: an independent cohort study of engagement and dropout among the first 100 000 referrals into a large-scale diabetes prevention program.Crossref | GoogleScholarGoogle Scholar |
[37] Barry E, Roberts S, Oke J, et al. Efficacy and effectiveness of screen and treat policies in prevention of type 2 diabetes: systematic review and meta-analysis of screening tests and interventions. BMJ 2017; 356 i6538
| Efficacy and effectiveness of screen and treat policies in prevention of type 2 diabetes: systematic review and meta-analysis of screening tests and interventions.Crossref | GoogleScholarGoogle Scholar |
[38] 3. Prevention or delay of type 2 diabetes: standards of medical care in diabetes -2021. Diabetes Care 2021; 44 S34–9.
| 3. Prevention or delay of type 2 diabetes: standards of medical care in diabetes -2021.Crossref | GoogleScholarGoogle Scholar |
[39] Coppell KJ, Abel SL, Freer T, et al. The effectiveness of a primary care nursing-led dietary intervention for prediabetes: a mixed methods pilot study. BMC Fam Pract 2017; 18 106
| The effectiveness of a primary care nursing-led dietary intervention for prediabetes: a mixed methods pilot study.Crossref | GoogleScholarGoogle Scholar |
[40] Connor D, Coppell K, Gray A, et al. A cost-effectiveness analysis of the prediabetes intervention package (PIP) in primary care: a New Zealand pilot programme. N Z Med J 2019; 132 24–34.
[41] Coppell KJ, Tipene-Leach DC, Pahau HLR, et al. Two-year results from a community-wide diabetes prevention intervention in a high risk indigenous community: the Ngati and healthy project. Diabetes Res Clin Pract 2009; 85 220–7.
| Two-year results from a community-wide diabetes prevention intervention in a high risk indigenous community: the Ngati and healthy project.Crossref | GoogleScholarGoogle Scholar |
[42] Tupai-Firestone R, Matheson A, Prapavessis D, et al. Pasifika youth empowerment programme: a potential public health approach in tackling obesity-health related issues. Altern Int J Indig Peoples 2018; 14 63–72.
| Pasifika youth empowerment programme: a potential public health approach in tackling obesity-health related issues.Crossref | GoogleScholarGoogle Scholar |
[43] Firestone R, Cheng S, Dalhousie S, et al. Exploring Pasifika wellbeing: findings from a large cluster randomised controlled trial of a mobile health intervention programme. N Z Med J 2020; 133 82–101.
[44] Bell R, Smith C, Hale L, et al. Understanding obesity in the context of an Indigenous population—A qualitative study. Obes Res Clin Pract 2017; 11 558–66.
| Understanding obesity in the context of an Indigenous population—A qualitative study.Crossref | GoogleScholarGoogle Scholar |
[45] Anderson YC, Wild CEK, Hofman PL, et al. Participants’ and caregivers’ experiences of a multidisciplinary programme for healthy lifestyle change in Aotearoa/New Zealand: a qualitative, focus group study. BMJ Open 2021; 11 e043516
| Participants’ and caregivers’ experiences of a multidisciplinary programme for healthy lifestyle change in Aotearoa/New Zealand: a qualitative, focus group study.Crossref | GoogleScholarGoogle Scholar |
[46] Wild CEK, Rawiri NT, Willing EJ, et al. What affects programme engagement for Māori families? A qualitative study of a family‐based, multidisciplinary healthy lifestyle programme for children and adolescents. J Paediatr Child Health 2021; 57 670–6.
| What affects programme engagement for Māori families? A qualitative study of a family‐based, multidisciplinary healthy lifestyle programme for children and adolescents.Crossref | GoogleScholarGoogle Scholar |
[47] Glover M, Wong SF, Taylor RW, et al. The complexity of food provisioning decisions by Māori caregivers to ensure the happiness and health of their children. Nutrients 2019; 11 994
| The complexity of food provisioning decisions by Māori caregivers to ensure the happiness and health of their children.Crossref | GoogleScholarGoogle Scholar |
[48] Mercer C, Riini D, Hamerton H, et al. Evaluating a healthy eating, healthy action program in small Mãori communities in Aotearoa, New Zealand. Aust J Prim Health 2013; 19 74–80.
| Evaluating a healthy eating, healthy action program in small Mãori communities in Aotearoa, New Zealand.Crossref | GoogleScholarGoogle Scholar |
[49] Tane T, Selak V, Hawkins K, Lata V, Murray J, Nicholls D, Peihopa A, Rice N, Harwood M, et al. Māori and Pacific peoples’ experiences of a Māori-led diabetes programme. N Z Med J 2021; 134 79–89.
[50] Henwood W. Māori knowledge: a key ingredient in nutrition and physical exercise health promotion programmes for Māori. Soc Policy J New Zeal 2007; 32 155–64.
[51] Masters-Awatere B, Cassim S, Tamatea J, et al. He Pikinga Waiora Kimi Ora lifestyle programme: case study of a successful Community-Based Indigenous diabetes intervention. N Z Med J 2021; 134 68–78.
[52] Whitehead L, Glass C, Coppell K. The effectiveness of goal setting on glycaemic control for people with type 2 diabetes and prediabetes: a systematic review and meta-analysis. J Adv Nurs 2022; 78 1212–27.
| The effectiveness of goal setting on glycaemic control for people with type 2 diabetes and prediabetes: a systematic review and meta-analysis.Crossref | GoogleScholarGoogle Scholar |
[53] Coppell KJ, Abel S, Whitehead LC, et al. A diagnosis of prediabetes when combined with lifestyle advice and support is considered helpful rather than a negative label by a demographically diverse group: a qualitative study. Prim Care Diabetes 2022; 16 301–6.
| A diagnosis of prediabetes when combined with lifestyle advice and support is considered helpful rather than a negative label by a demographically diverse group: a qualitative study.Crossref | GoogleScholarGoogle Scholar |
[54] Chatzi G, Mason T, Chandola T, et al. Sociodemographic disparities in non‐diabetic hyperglycaemia and the transition to type 2 diabetes: evidence from the English Longitudinal Study of Ageing. Diabet Med 2022; 37 1536–44.
| Sociodemographic disparities in non‐diabetic hyperglycaemia and the transition to type 2 diabetes: evidence from the English Longitudinal Study of Ageing.Crossref | GoogleScholarGoogle Scholar |
[55] Dunbar JA. Diabetes prevention in Australia: 10 years results and experience. Diabetes Metab J 2017; 41 160–7.
| Diabetes prevention in Australia: 10 years results and experience.Crossref | GoogleScholarGoogle Scholar |
[56] Baker IDI Heart and Diabetes Institute. The Australian Type 2 Diabetes Risk Assessment Tool (AUSDRISK). May 2010; Available at https://www.health.gov.au/resources/apps-and-tools/the-australian-type-2-diabetes-risk-assessment-tool-ausdrisk/tool