Decision-making on listing new medicines for public funding in New Zealand: the case of ‘new’ type 2 diabetes medications
Farzana Sarkisova 1 , Charon Lessing
1 , Charon Lessing  1 * , Caroline Stretton
1 * , Caroline Stretton  1
1
1 School of Public Health and Interdisciplinary Studies, Auckland University of Technology, 90 Akoranga Drive, Northcote 0627, Auckland, New Zealand.
Journal of Primary Health Care 14(1) 13-20 https://doi.org/10.1071/HC21122
Published: 13 April 2022
© 2022 The Author(s) (or their employer(s)). Published by CSIRO Publishing on behalf of The Royal New Zealand College of General Practitioners. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
Introduction: New medicines for the management of type 2 diabetes became available internationally in 2005, yet only in 2018 did the first of these become available in New Zealand. Access to these new medicines in New Zealand is largely dependent on decisions made by the Pharmaceutical Management Agency (PHARMAC).
Aim: This study sought to describe the decision-making processes to better understand access to new medicines in New Zealand.
Methods: We conducted an analysis of publicly accessible information on therapeutic committee deliberations, prices of medicines and registration and formulary listing dates.
Results: Prices for the new diabetes medicines in New Zealand are lower than comparator countries, but access to them takes longer.
Discussion: Given that knowledge on efficacy, safety and quality is widely available to support decision-making on new medicines, differences in access to them between nations appears to depend on the fourth hurdle of cost. However, we suggest that a rush to market is the norm, that activities of the pharmaceutical industry and regulatory agencies are less transparent than desirable, and that greater focus on availability of safety data is required. Deliberations of PHARMAC therapeutic committees are robust yet protracted. Opportunities to expedite decision-making, as well as resolving inequities, may be worthy of examination.
Keywords: access to medicines, diabetes, drug costs, healthcare funding, HTA, PHARMAC, pharmaceuticals, therapeutics committee.
Introduction
Prevalence of type 2 diabetes (T2D) in the New Zealand adult population was 5.9% in 2019–201 and is predicted to reach 7% by 2040.2 The estimated cost of health services provided to people living with T2D in New Zealand is $1.3 billion, with a large proportion spent on the treatment of diabetes-related complications.3
In the early 2000s, new medicines arrived in the marketplace for the pharmacological management of T2D, with suggested advantages for people with diabetes-related comorbidities. Regulatory approval and acceptance of these new medicines requires a minimum of three hurdles to be met – evidence of efficacy, safety, and quality.4 A fourth hurdle, that of cost-effectiveness, is formally evaluated by many countries with publicly funded healthcare systems.
Access to medicines in New Zealand is dependent primarily on two government agencies. Medsafe – the Medical Devices Safety Authority5 – is mandated to ensure ‘medicines meet acceptable standards of safety, quality and efficacy’; the first three hurdles. Medsafe ensures that the final pharmaceutical product on New Zealand shelves has been produced under good manufacturing processes and is safe to use. The Pharmaceutical Management Agency (PHARMAC) is the Crown Agent whose overall statutory objective is to secure ‘the best health outcomes that are reasonably achievable from pharmaceutical treatment and from within the amount of funding provided’; the fourth hurdle of cost-effectiveness.6
Funding decisions of PHARMAC are underpinned by clinical evidence and determined by the Pharmacology and Therapeutics Advisory Committee (PTAC) and specialist advisory sub-committees, with financial decisions ultimately made by the PHARMAC Board.7,8 An application for funding of a new medicine is considered first by the relevant specialist advisory sub-committee (in the case of T2D medicines, clinicians with expertise in diabetes) who scrutinise the available clinical trial evidence and rank the application from low to high priority. The full PTAC (10 clinicians and representation of the PHARMAC Board who meet four times a year to consider all applications) then further considers the application in the context of other funding decisions at hand. In between a recommendation by the PTAC and the final decision to fund a medicine, a sometimes-lengthy round of deliberations, consultations and economic analyses takes place within PHARMAC.
| WHAT GAP THIS FILLS |
| What is already known: The Crown Agent, PHARMAC, has a reputation for achieving value for money with regard to medicines in New Zealand, and for their ability to contain costs. PHARMAC has been criticised for delaying access to new medicines. |
| What this study adds: The relative roles of Medsafe and PHARMAC are examined, with overlap between the two demonstrated. Much of the perceived delay in funding new medicines in New Zealand is due to PHARMAC’s attention to clinical and cost-effectiveness and diligence regarding safety – which in the case of the new diabetes medicines did not become clear for about a decade from product approval. The inclusion of ethnicity as a criterion for enhanced access to diabetes medicines for Māori people is described, needing further investigation to determine any impact. |
Medicines then listed in the Community Pharmaceutical Schedule (‘the Schedule’) can be accessed with full or partial subsidy. Broadly, this means that New Zealand residents will receive subsidised medicines, whereas for medicines not listed in the Schedule, they bear the full market cost of the medicine.9 Certain medicines may have access restricted through the requirement for a Special Authority application to be made for funding.10 Restricted access is a tool used by PHARMAC to limit access to high-cost medicines or where medicines require some degree of monitoring or specialist care. Restricted access is intended to ensure availability for people who would benefit most from receiving the medicine. Some such medicines can still be accessed if patients are willing to pay out-of-pocket for them.
PHARMAC has been criticised for being slow to fund new medicines and for having fewer preparations subsidised than other similar countries, particularly Australia.11–14 In 2018, New Zealanders with diabetes gained access to the first of the new T2D medicines when PHARMAC agreed to fund vildagliptin, a dipeptidyl peptidase-4 (DPP-4) inhibitor.15 In 2021, prescribers had two additional medicines available for use – dulaglutide, a glucagon-like peptide-1 (GLP-1) analogue and empagliflozin, a sodium–glucose co-transporter 2 (SGLT-2) inhibitor.16,17 Access to the latter two medicines is restricted to patients meeting certain Special Authority criteria, which notably includes a landmark decision to include Māori and any Pacific ethnicity among other access eligibility criteria.18
This article examines the seemingly protracted process that led PHARMAC to these funding decisions for the new T2D medicines and the role that decision-making committees have had in these outcomes.
Methods
Approval status of the new T2D entities was determined from information available from Medsafe19 and from the PHARMAC website, including annual reports, notifications, press releases, the PHARMAC Application Tracker tool, and the PTAC committee and Diabetes subcommittee meeting records.8 The timeline of the various applications and related discussions in New Zealand have been synthesised and are attached as Annex 1. Similarly, Australian data were obtained from public summary documents made available from the Pharmaceutical Benefits Advisory Committee (PBAC).20
For price comparisons, publicly available New Zealand prices were used (although agreements between manufacturers and payers may differ, reflecting confidential discounts made by PHARMAC). Comparisons have been drawn with the United States (US), Australia and the United Kingdom of Great Britain (UK) with prices converted to $US using the average yearly exchange rate for 2019.21
Searches for published clinical trial data and national diabetes guidelines were conducted using online medical databases.
Results
Published evidence to support the use of the newer T2D therapies and documentation of adverse effects has increased exponentially. Evidence is accruing to suggest these newer therapies exhibit advantages in T2D patients with concomitant atherosclerotic cardiovascular disease, heart failure, and diabetic kidney disease.22,23 A summary of the mechanisms of action, place in therapy of these medicines and cautions is given in Table 1.22–27

|
GLP-1 analogues (e.g. exenatide, dulaglutide)
The first innovator agent, exenatide (a GLP-1 analogue), gained regulatory approval from the US Food and Drug Administration (FDA) in 2005.28 Two years later in 2007, registration was approved by Medsafe,29 making exenatide available for use in New Zealand, albeit unfunded. Simultaneously, PHARMAC declined the first application for exenatide due to limited evidence of additional benefits over funded treatment options, high cost, and an absence of long-term safety and efficacy data30 (Fig. 1). In 2008, the UK’s National Institute for Health and Care Excellence (NICE) reported a lack of evidence to define a place in therapy for the GLP-1 analogues, specifically the availability of only eight randomised clinical trials for exenatide in addition to the four clinical studies conducted prior to registration by the FDA.31 At the time, NICE recommended exenatide be considered as a third-line option before initiating insulin, also noting a lack of cost-effectiveness over insulin. The American Diabetes Association and the European Association for the Study of Diabetes issued guidance to include the newer therapies as add-on therapy to metformin in 2012, noting a ‘paucity of comparative effectiveness research on long-term treatment outcomes’.32
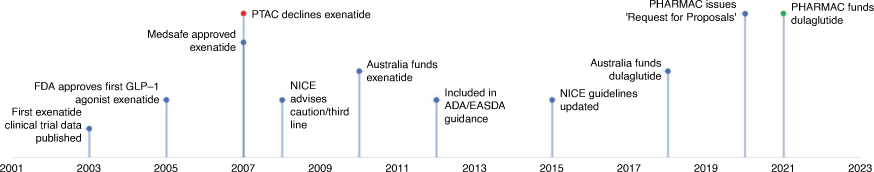
|
Between 2012 and 2015, the PTAC considered applications for funding of GLP-1 analogues a further four times, consistently noting a lack of information on adverse effects and safety concerns, and recommended low or medium–high priority for use of GLP-1 analogues as combination therapy for patients with special criteria restrictions.33 In 2015, PHARMAC signalled an intention to consider funding for all the newer treatment options with a ‘Request for Information’34 and in 2020 commenced commercial negotiations for their supply.35 In 2021, dulaglutide was added to the Schedule, the first funded member of the GLP-1 analogue class.9 Australia listed GLP-1 analogues, exenatide in 201036 and dulaglutide in 2018,37 in the Pharmaceutical Benefits Scheme (PBS).
DPP-4 inhibitors (e.g. sitagliptin, vildagliptin)
In October 2008, Medsafe registered vildagliptin (a DPP-4 inhibitor) for use in New Zealand,38 whereas Australia added sitagliptin to the PBS in 200839 and vildagliptin in August 201036 (Table 240–44).
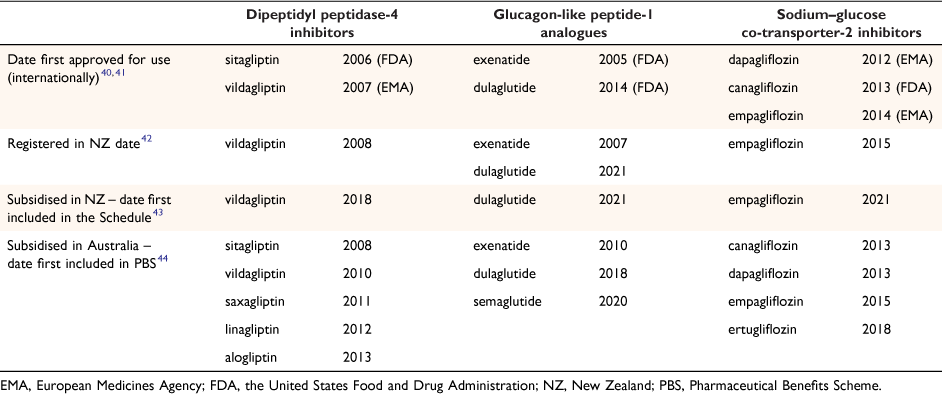
|
In 2009, PHARMAC twice considered an application for vildagliptin and sitagliptin and noted there was ‘insufficient long-term toxicity and clinical outcome data’ and that pivotal studies had been omitted from the application.45,46 Furthermore, they noted a lack of safety data, commenting on an FDA warning of potential adverse effects and high drop-out rates (in some cases around 80% at 2 years), thus echoing a 2008 Cochrane review of 25 studies stating that ‘long‐term data especially on cardiovascular outcomes and safety [was] urgently needed before widespread use’.47 The subcommittee considered the exact place in therapy for DPP-4 inhibitors was unclear at that time, with no superiority for either sitagliptin or vildagliptin over one another.45
Further meetings of the subcommittee and full PTAC between 2010 and 2015 continued the debate on the relative efficacy and safety of the DDP-4 inhibitors.8,33 In 2018, the first of the newer T2D medicines, vildagliptin, was listed in the Schedule in a multi-product funding agreement with the supplier, 9 years from first application to PHARMAC.15
SGLT-2 inhibitors (e.g. canagliflozin, dapagliflozin, empagliflozin)
Similarly, discussions about empagliflozin, particularly the lack of knowledge regarding adverse effects, took around 7 years from year of first application to PHARMAC to funding. Importantly, the committee’s reticence is supported by an investigation into one of the first SGLT-2 inhibitors, canagliflozin. Evidence shows that the parent company knew of potential ketoacidosis during phase 2 clinical trials in 2010 and, despite several warnings from both its own researchers and from the FDA, continued to downplay the recognised adverse effect and instead spent more than US$100 million between 2013 and 2020 to promote the product.48 Australia approved canagliflozin for use in December 2013;49 it having been registered by the FDA in March 2013.50 Medsafe approved empagliflozin for use in New Zealand in April 2015.51 In 2015, 2017 and 2018, the FDA added successive warnings about associated risks of ketoacidosis, bone fracture and Fournier’s gangrene.52–54
Prices of diabetes medicines
Prices of medicines are listed in Table 3.9,44,55–58 The approximate daily cost in New Zealand ranges from cents in the case of metformin and gliclazide to NZ$3 for insulin, and approximately NZ$2–4 for the newer agents. Notably, New Zealand has lower prices for all medicines, except for insulin glargine in the UK. Prices of these medicines are highest in the USA.

|
Discussion
Following the completion of clinical studies, the pharmaceutical industry is naturally eager to gain an international foothold and secure a return on investment. Counter to this, regulatory agencies must balance enabling access to new, potentially life-saving therapies against the risk that these same new therapies are unsafe – a ‘risk versus risk’ dilemma.59 Belief that better health outcomes are gained by rapid access to new medicines must be measured against an examination of true therapeutic advantage via robust technology assessment processes that therapeutic committees offer.60,61 Few ‘innovative’ products demonstrably show superiority to products already available. In a study of all new medicines approved by the European Medicines Agency between 1999 and 2005, only 10% showed a statistically significant difference in the primary clinical endpoint.62
Judgement of the suitability of the new medicines for subsidy in New Zealand, in terms of both potential health gain and budgetary impact, is arguably the remit of both Medsafe and PHARMAC. Medsafe certainly has an obligation to manage the quality and safety hurdles, whereas PHARMAC, through its clinical committees, would naturally seem responsible for both efficacy and cost-effectiveness.
Adequate safety data for most of these medicines, and evidence from the larger landmark clinical trials were available internationally only in the latter half of the 2000s.22,25,26 Medsafe, however, was swift to register these new medicines, endorsing both safety and quality of the final product at least, if not efficacy. The PHARMAC Diabetes advisory committee clearly held concerns around efficacy and safety central throughout.63,64 This is the role of any therapeutics committee – based on the evidence at hand, to consider the efficacy, and the safety, of a new medicine over that which is already available.61 In the infancy of a new medicine’s life, this is necessarily a moving target, particularly for one which is poised to be used in large populations. As evidence from robust clinical trials is published, increasing light is shed on potential adverse outcomes as well as advantages of the medicine. In contrast to New Zealand, Australia moved more swiftly in all instances to add the newer medicines to the PBS, possibly in part due to a larger population-based source of funding available for new therapies and apparently with more confidence in the face of limited evidence of safety.
Restricted access
On top of the ‘risk versus risk’ dilemma, PHARMAC further considers opportunity cost in a prioritisation process. Decisions made within a capped annual budget balance access to new medicines against one another, and against a list of previously ranked medicines awaiting funding. The period of the life cycle from commercial negotiations commencing to funding agreements being reached are veiled and protracted, with both PHARMAC and the industry preferring commercial secrecy. It is not clear when negotiations began for supply of vildagliptin that were achieved in 2018, or for the supply arrangement of empagliflozin and dulaglutide in 2020–21. However, the processes PHARMAC employ can include ‘bundling’ of multiple products from suppliers, as was the case for vildagliptin. This implies a confluence of applications for several different products at the same time and may well explain some of the delay in finalising agreements.
Despite PHARMAC's ability to negotiate financially advantageous deals, the agency estimates that 5 years of funding of empagliflozin and dulaglutide will cost NZ$125 million.65 The higher cost of the newer medicines may require that access be restricted to people who will benefit most, reflected in the discussions and final application of Special Authority access criteria.
The case for ethnicity as a Special Authority criterion
Māori and Pacific peoples are disproportionally affected by diabetes, being at least three-fold more likely to experience the condition and also have a higher risk of diabetes-related complications, with this inequity persisting over decades.1,2,66,67 In 2019, PHARMAC noted that Māori and Pacific peoples have not benefited from medicines in the same way as other ethnicities have, and committed to ‘a bold goal to eliminate inequities in access to medicines by 2025’.68 Special Authority criteria were first proposed by the Diabetes Subcommittee in March 2019 yet, although acknowledging the disproportionate impact on Māori and Pacific peoples, the committee missed an opportunity to include ethnicity as a criterion at this stage, instead using a 5-year cardiovascular disease risk threshold.64 In September 2020, PHARMAC requested feedback on their proposal to fund empagliflozin and dulaglutide, and suggested that they ‘would support the implementation and monitor the uptake of dulaglutide to see whether the medicines are being accessed by those people with highest need (e.g. Māori and Pacific).69 Given that in August 2020, PHARMAC reaffirmed its commitment to uphold Te Tiriti o Waitangi responsibilities,70 the Crown Agent again failed to take the opportunity to redress inequity.71 Extensive feedback delayed the final decision but, importantly, saw PHARMAC include an ethnicity criterion in the Special Authority requirements for Māori and Pacific peoples recognising ‘the heightened risk of cardiovascular and renal outcomes in these ethnic groups’.16
In the first 6 months of empagliflozin being included in the Schedule, PHARMAC reports more than 30 000 people have accessed the medicine, with 47% identifying as Māori or Pacific.18 Although this number affirms the high burden of disease in New Zealand, enhanced access to medicines by Māori cannot be simply extrapolated. PHARMAC has now announced funding of a second medicine (rosuvastatin) with enhanced access for Māori and Pacific people.72
Undoubtedly, PHARMAC have achieved value-for-money in the cost of these medicines. The prices New Zealand pays for medicines are consistently lower than most other Organisation for Economic Co-operation and Development (OECD) countries,73 and the Crown Agency continues to protect the New Zealanders from an expanding pharmaceutical bill, arguably allowing the government to invest in other aspects of health care.74 However, this necessitates a reconsideration of the risk versus risk dilemma – did the delay in funding the new diabetes medicines potentially increase downstream costs to the health budget due to long-term sequelae, or did it prevent serious adverse effects?
Policy implications
The role of Medsafe in ensuring access to (only) safe, efficacious medicines produced under quality conditions appears to have been ceded, in part, to PHARMAC and the relationship between these two agencies lacks clarity. PHARMAC places high emphasis on clinical trial data, taking longer to agree to fund the newer diabetes medicines than Australia and other countries in this case, and consistently achieves lower price agreements. The use of an ethnicity criterion to improve access and reduce inequity needs to be evaluated to ensure it has achieved its goal.
Limitations
This article has attempted to follow the journey of the new diabetes medicines in New Zealand to shed light on a partially veiled process and to contribute to an ongoing discussion on access to medicines. A more robust discussion of the economic impact of delayed decisions would add to the debate. The economic modelling required to undertake such an evaluation is considerable and awaits future research.
Data availability
The data that support this study are available in the article and the accompanying supplementary material.
Competing interests
The authors declare no competing interests.
Declaration of funding
This study was conducted as part of an Auckland University of Technology Summer Scholarship for which Farzana Sarkisova received university funding.
Supplementary material
Supplementary material is available online.
Acknowledgements
The authors would like to acknowledge the assistance of Dr Farai Chinyanganya, Sandra Ponen and Hoda Fahmy in gathering information on medicine prices. The contribution of the article reviewers is also acknowledged.
References
[1] New Zealand Ministry of Health. New Zealand health survey annual data explorer. Indicator: type 2 diabetes (diagnosed when aged 25 years or older used as a proxy for type 2 diabetes). 2020. Available at https://minhealthnz.shinyapps.io/nz‐health‐survey‐2020‐21‐annual‐data‐explorer/_w_7b1c4f48/#!/ [cited 8 September 2021][2] PricewaterhouseCoopers New Zealand. The economic and social cost of type 2 diabetes. 2021. Available at https://healthierlives.co.nz/wp-content/uploads/Economic-and-Social-Cost-of-Type-2-Diabetes-FINAL-REPORT.pdf [cited 5 July 2021]
[3] Krebs J, Coppell K, Cresswell P, et al. Access to diabetes drugs in New Zealand is inadequate. NZ Med J 2016; 129 6–9.
[4] Rawlins MD. Crossing the fourth hurdle. Br J Clin Pharmacol 2012; 73 855–860.
| Crossing the fourth hurdle.Crossref | GoogleScholarGoogle Scholar | 22404227PubMed |
[5] New Zealand Medicines and Medical Devices Safety Authority. About Medsafe. 2020. Available at https://www.medsafe.govt.nz/other/about.asp#pre [cited 8 September 2020]
[6] New Zealand Pharmaceutical Management Agency. Operating Policies and Procedures Manual 4th edn. 2020. Available at https://pharmac.govt.nz/medicine-funding-and-supply/the-funding-process/policies-manuals-and-processes/operating-policies-and-procedures/ [cited 8 September 2021]
[7] New Zealand Pharmaceutical Management Agency. Supporting information about the Factors for Consideration. 2020. Available at https://pharmac.govt.nz/medicine-funding-and-supply/the-funding-process/policies-manuals-and-processes/factors-for-consideration/supporting-information-for-the-factors-for-consideration/ [cited 8 September 2021]
[8] New Zealand Pharmaceutical Management Agency. Pharmacology and Therapeutics Advisory Committee (PTAC). 2021. Available at https://pharmac.govt.nz/about/expert-advice/pharmacology-and-therapeutics-advisory-committee-ptac/ [cited 3 September 2021]
[9] New Zealand Pharmaceutical Management Agency. Online Pharmaceutical Schedule. 2021. Available at https://www.pharmac.govt.nz/wwwtrs/ScheduleOnline.php [cited 8 September 2021]
[10] New Zealand Pharmaceutical Management Agency. Special Authority applications. 2021. Available at https://pharmac.govt.nz/medicine-funding-and-supply/make-an-application/special-authority-forms/ [cited 8 September 2021]
[11] Wonder M, Milne R. Access to new medicines in New Zealand compared to Australia. NZ Med J 2011; 124 12–28.
[12] Taylor C, Wonder M. Exploring the implications of a fixed budget for new medicines: a study of reimbursement of new medicines in Australia and New Zealand. Aust Health Rev 2015; 39 455–461.
| Exploring the implications of a fixed budget for new medicines: a study of reimbursement of new medicines in Australia and New Zealand.Crossref | GoogleScholarGoogle Scholar | 25751688PubMed |
[13] Wonder M, Fisher R. Subsidised access to new melanoma drugs: in need of further innovation? NZ Med J 2016; 129 37–54.
[14] Rawson NS. Comparison of numbers and timing of new medication regulatory approvals in Canada and New Zealand. Regul Toxicol Pharmacol 2019; 101 24–28.
| Comparison of numbers and timing of new medication regulatory approvals in Canada and New Zealand.Crossref | GoogleScholarGoogle Scholar | 30391284PubMed |
[15] New Zealand Pharmaceutical Management Agency. Approval of multi-product funding agreement with Novartis. 2018. Available at https://pharmac.govt.nz/news-and-resources/consultations-and-decisions/approval-of-multi-product-funding-agreement-with-novartis/ [cited 8 September 2021]
[16] New Zealand Pharmaceutical Management Agency. Decision to fund two new medicines for type 2 diabetes – Amended with Q&A. 2021. Available at https://pharmac.govt.nz/news-and-resources/consultations-and-decisions/decision-to-fund-two-new-medicines-for-type-2-diabetes/ [cited 9 September 2021]
[17] New Zealand Society for the Study of Diabetes. Special report: NZSSD type 2 diabetes management guidelines 2021. 2021. Available at https://www.researchreview.co.nz/nz/clinical-area/general-medicine/general-practice/special-report-nzssd-type-2-diabetes-management-g.aspx?hash=d171e98ec4f85f61af1a7e99b0cc2ef826a2f65101e5d8b8ab22216de258abeb [cited 8 September 2021]
[18] New Zealand Pharmaceutical Management Agency. Uptake of medicines for type 2 diabetes. 2021. Available at https://pharmac.govt.nz/news-and-resources/uptake-of-medicines-for-type-2-diabetes/ [cited 8 September 2021]
[19] New Zealand Medicines and Medical Devices Safety Authority. Product/Application Search. 2021. Available at https://www.medsafe.govt.nz/regulatory/DbSearch.asp [cited 8 September 2021]
[20] Australian Government Department of Health: Pharmaceutical Benefits Scheme. Public summary documents by product. 2021. Available at https://www.pbs.gov.au/info/industry/listing/elements/pbac-meetings/psd/public-summary-documents-by-product [cited 6 September 2021]
[21] Organisation for Economic Co-operation and Development. Exchange rates. 2021. Available at https://data.oecd.org/conversion/exchange-rates.htm [cited 8 September 2021]
[22] Davies MJ, D’Alessio DA, Fradkin J, et al. Management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2018; 41 2669–2701.
| Management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD).Crossref | GoogleScholarGoogle Scholar | 30291106PubMed |
[23] American Diabetes Association. Pharmacologic approaches to glycemic treatment: Standards of Medical Care in Diabetes—2021. Diabetes Care 2021; 44 S111–S124.
| American Diabetes Association. Pharmacologic approaches to glycemic treatment: Standards of Medical Care in Diabetes—2021.Crossref | GoogleScholarGoogle Scholar | 33298420PubMed |
[24] New Zealand Formulary. Diabetes mellitus. 2021. Available at https://nzf.org.nz/nzf_3624?searchterm=Diabetes [cited 20 December 2021]
[25] National Institute for Health and Care Excellence. NICE guideline: Type 2 diabetes in adults: management. 2021. Available at https://www.nice.org.uk/guidance/ng28 [cited 12 December 2021]
[26] Buse JB, Wexler DJ, Tsapas A, et al. 2019 update to: Management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2020 43 487–493.
| 2019 update to: Management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD).Crossref | GoogleScholarGoogle Scholar |
[27] New Zealand Ministry of Health. Type 2 Diabetes Management Guidance. 2021. Available at https://t2dm.nzssd.org.nz/ [cited 8 September 2021]
[28] United States Food and Drug Administration. Drug Approval Package: Exenatide. 2005. Available at https://www.accessdata.fda.gov/drugsatfda_docs/nda/2005/021773_ByettaTOC.cfm [cited 8 September 2021]
[29] New Zealand Medicines and Medical Devices Safety Authority. New Zealand data sheet: Byetta. 2021. Available at https://www.medsafe.govt.nz/profs/Datasheet/b/Byettainj.pdf [cited 12 December 2021]
[30] Pharmacology and Therapeutics Advisory Committee. PTAC meeting–8 & 9 August 2007. 2007. Available at https://www.pharmac.govt.nz/assets/ptac-minutes-2007-08.pdf [cited 12 December 2021]
[31] National Collaborating Centre for Chronic Conditions. Type 2 diabetes: national clinical guideline for management in primary and secondary care (update): NICE Clinical Guidelines, No. 66. London: Royal College of Physicians; 2008. Available at https://www.ncbi.nlm.nih.gov/books/NBK53885/
[32] Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2012; 35 1364–1379.
| Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD).Crossref | GoogleScholarGoogle Scholar | 22517736PubMed |
[33] New Zealand Pharmaceutical Management Agency. Specialist advisory committees. 2021. Available at https://pharmac.govt.nz/about/expert-advice/specialist-advisory-committees/ [cited 12 December 2021]
[34] New Zealand Pharmaceutical Management Agency. Request for information on anti-diabetic agents. 2015. Available at https://www.pharmac.govt.nz/assets/rfi-2015-02-12-anti-diabetic-agents.pdf [cited 12 December 2021]
[35] New Zealand Pharmaceutical Management Agency. Request for proposals – supply of diabetes agents: SGLT-2 inhibitors, GLP-1 agonists and DPP-4 inhibitors. 2020. Available at https://www.pharmac.govt.nz/assets/rfp-2010-01-15-diabetes-agents.pdf [cited 12 December 2021]
[36] Australian Government Department of Health: Pharmaceutical Benefits Scheme. PBS Publications Archive: Schedule of Pharmaceutical Benefits. Effective 1 August 2010. 2010. Available at https://www.pbs.gov.au/publication/schedule/2010/2010-08-01-general-schedule.pdf [cited 22 November 2021]
[37] Australian Government Department of Health: Pharmaceutical Benefits Scheme. PBS Publications Archive: Schedule of Pharmaceutical Benefits. Effective 1 June 2018. 2018. Available at https://www.pbs.gov.au/publication/schedule/2018/06/2018-06-01-general-schedule.pdf [cited 9 September 2021]
[38] New Zealand Medicines and Medical Devices Safety Authority. New Zealand data sheet: Galvus tablet. 2020. Available at https://www.medsafe.govt.nz/profs/Datasheet/g/galvustab.pdf [cited 12 December 2021]
[39] Australian Government Department of Health: Pharmaceutical Benefits Scheme. PBS Publications Archive: Schedule of Pharmaceutical Benefits. Effective 1 August 2008. 2008. Available at https://www.pbs.gov.au/publication/schedule/2008/2008-08-01-general-schedule.pdf [cited 22 November 2021]
[40] United States Food and Drug Administration. Drugs@FDA: FDA-Approved Drugs. Available at https://www.accessdata.fda.gov/scripts/cder/daf/ [cited 8 December 2021]
[41] European Medicines Agency. Search medicines on the EMA website. 2021. Available at https://www.ema.europa.eu/en/medicines [cited 8 December 2021]
[42] New Zealand Medicines and Medical Devices Safety Authority. Data sheets and consumer medicine information. 2020. Available at https://www.medsafe.govt.nz/Medicines/infoSearch.asp [cited 8 December 2021]
[43] New Zealand Pharmaceutical Management Agency. Application Tracker. Available at https://connect.pharmac.govt.nz/apptracker/s/ [cited 8 September 2021]
[44] Australian Government Department of Health: Pharmaceutical Benefits Scheme. PBS Publications Archive: Schedule of Pharmaceutical Benefits. 2021. Available at https://www.pbs.gov.au/info/publication/schedule/archive [cited 8 December 2021]
[45] Diabetes Subcommittee of the Pharmacology and Therapeutics Advisory Committee. Diabetes Subcommittee of PTAC: Meeting held 21 August 2009. 2009. Available at https://www.pharmac.govt.nz/assets/ptac-diabetes-subcommittee-minutes-2009-08.pdf [cited 8 September 2021]
[46] Pharmacology and Therapeutics Advisory Committee. PTAC meeting held 12 & 13 November 2009. 2009. Available at https://www.pharmac.govt.nz/assets/ptac-minutes-2009-11.pdf [cited 8 September 2021]
[47] Richter B, Bandeira-Echtler E, Bergerhoff K, Lerch C. Dipeptidyl peptidase-4 (DPP-4) inhibitors for type 2 diabetes mellitus. Cochrane Database Syst Rev 2008;
| Dipeptidyl peptidase-4 (DPP-4) inhibitors for type 2 diabetes mellitus.Crossref | GoogleScholarGoogle Scholar | 18646086PubMed |
[48] Terhune C, Respaut R. J&J kept quiet on popular diabetes drug as red flags multiplied. Medscape. 2021. Available at https://www.medscape.com/viewarticle/964393?spon=34&uac=70499HJ&impID=3874272&sso=true&faf=1&src=WNL_mdpls_211214_mscpedit_fmed
[49] Australian Government Department of Health: Pharmaceutical Benefits Scheme. PBS Publications Archive: Schedule of Pharmaceutical Benefits. Effective 1 December 2013. 2013. Available at https://www.pbs.gov.au/publication/schedule/2013/12/2013-12-01-general-schedule.pdf [cited 22 November 2021]
[50] United States Food and Drug Administration. Drug Approval Package: Invokana. 2013. Available at https://www.accessdata.fda.gov/drugsatfda_docs/nda/2013/204042Orig1s000TOC.cfm [cited 16 December 2021]
[51] New Zealand Medicines and Medical Devices Safety Authority. New Zealand data sheet: Jardiance. 2019. Available at https://www.medsafe.govt.nz/profs/Datasheet/j/jardiancetab.pdf [cited 16 December 2021]
[52] United States Food and Drug Administration. Potential signals of serious risks/new safety information identified by the FDA Adverse Event Reporting System (Faers). 2020. Available at https://www.fda.gov/drugs/questions-and-answers-fdas-adverse-event-reporting-system-faers/october-december-2020-potential-signals-serious-risksnew-safety-information-identified-fda-adverse [cited 16 December 2021]
[53] United States Food and Drug Administration. FDA Drug Safety Podcast: FDA confirms increased risk of leg and foot amputations with the diabetes medicine canagliflozin (Invokana, Invokamet, Invokamet XR). 2017. Available at https://www.fda.gov/drugs/fda‐drug‐safety‐podcasts/fda‐drug‐safety‐podcast‐fda‐confirms‐increased‐risk‐leg‐and‐foot‐amputations‐diabetes‐medicine [cited 19 December 2021]
[54] United States Food and Drug Administration. FDA Drug Safety Communication: FDA revises label of diabetes drug canagliflozin (Invokana, Invokamet) to include updates on bone fracture risk and new information on decreased bone mineral density. 2015. Available at https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-revises-label-diabetes-drug-canagliflozin-invokana-invokamet [cited 19 December 2021]
[55] International Business Machines Corporation. IBM Micromedex Red Book. 2021. Available at https://www.ibm.com/products/micromedex-red-book [cited 20 December 2021]
[56] BMJ group. British National Formulary. 2021. Available at https://www.bnf.org/products/bnf-online/ [cited 17 February 2020]
[57] National Health Service. Electronic Drug Tariff. 2020. Available at http://www.drugtariff.nhsbsa.nhs.uk [cited 17 February 2020]
[58] Pharmacy Retailing (NZ) Ltd. ProPharma. 2020. Available at https://www.propharma.co.nz/Login.aspx [cited 14 February 2020]
[59] Daemmrich A, Krücken G. Risk versus risk: decision-making dilemmas of drug regulation in the United States and Germany. Sci Cult 2000; 9 505–534.
| Risk versus risk: decision-making dilemmas of drug regulation in the United States and Germany.Crossref | GoogleScholarGoogle Scholar |
[60] Evans J, Laking G, Strother M, et al. Mind the gap: an analysis of foregone health gains from unfunded cancer medicines in New Zealand. Semin Oncol 2016; 43 625–637.
| Mind the gap: an analysis of foregone health gains from unfunded cancer medicines in New Zealand.Crossref | GoogleScholarGoogle Scholar | 28061980PubMed |
[61] Vitry A, Shin NH, Vitre P. Assessment of the therapeutic value of new medicines marketed in Australia. J Pharm Policy Pract 2013; 6 2
| Assessment of the therapeutic value of new medicines marketed in Australia.Crossref | GoogleScholarGoogle Scholar | 24764537PubMed |
[62] van Luijn JC, Gribnau FW, Leufkens HG. Superior efficacy of new medicines? Eur J Clin Pharmacol 2010; 66 445–448.
| Superior efficacy of new medicines?Crossref | GoogleScholarGoogle Scholar | 20224944PubMed |
[63] Diabetes Subcommittee of the Pharmacology and Therapeutics Advisory Committee. Diabetes Subcommittee of PTAC: Meeting held 10 October 2016. 2016. Available at https://www.pharmac.govt.nz/assets/ptac-diabetes-subcommittee-minutes-2016-10.pdf [cited 8 September 2021]
[64] Diabetes Subcommittee of the Pharmacology and Therapeutics Advisory Committee. Diabetes Subcommittee of PTAC: Meeting held 19 March 2019. 2019. Available at https://www.pharmac.govt.nz/assets/ptac-diabetes-subcommittee-minutes-2019-03.pdf [cited 8 September 2021]
[65] New Zealand Pharmaceutical Management Agency. Media release: PHARMAC to fund new diabetes medicines with amended Special Authority criteria. 2020. Available at https://pharmac.govt.nz/news-and-resources/news/pharmac-to-fund-new-diabetes-medicines-with-amended-special-authority-criteria/ [cited 8 September 2021]
[66] Yu D, Zhao Z, Osuagwu UL, et al. Ethnic differences in mortality and hospital admission rates between Māori, Pacific, and European New Zealanders with type 2 diabetes between 1994 and 2018: a retrospective, population-based, longitudinal cohort study. Lancet Glob Health 2021; 9 e209–e217.
| Ethnic differences in mortality and hospital admission rates between Māori, Pacific, and European New Zealanders with type 2 diabetes between 1994 and 2018: a retrospective, population-based, longitudinal cohort study.Crossref | GoogleScholarGoogle Scholar | 33069275PubMed |
[67] New Zealand Ministry of Health. Mortality and demographic data 2006. 2019. Available at https://www.health.govt.nz/publication/mortality-and-demographic-data-2006 [cited 9 September 2021]
[68] New Zealand Pharmaceutical Management Agency. Achieving medicine access equity in Aotearoa New Zealand: towards a theory of change. 2019. Available at https://pharmac.govt.nz/assets/achieving-medicine-access-equity-in-aotearoa-new-zealand-towards-a-theory-of-change.pdf [cited 8 September 2021]
[69] New Zealand Pharmaceutical Management Agency. Proposal to fund two new medicines for type 2 diabetes. 2020. Available at https://pharmac.govt.nz/news-and-resources/consultations-and-decisions/proposal-to-fund-two-new-medicines-for-type-2-diabetes/
[70] New Zealand Pharmaceutical Management Agency. Te rautaki o te whaioranga. Māori responsiveness strategy. 2020. Available at https://pharmac.govt.nz/assets/Te-Whaioranga-August-2020.pdf [cited 8 September 2021]
[71] New Zealand Pharmaceutical Management Agency. The consultation feedback on a proposal to fund two new treatments for type 2 diabetes and amend the contractual terms for one currently funded diabetes treatment through provisional agreements with three suppliers. 2020. Available at https://pharmac.govt.nz/assets/2020-diabetes-RFP-combined-consultation-responses-for-release-under-OIA.pdf [cited 8 September 2021]
[72] New Zealand Pharmaceutical Management Agency. Decision to fund rosuvastatin for people with high cholesterol. 2021. Available at https://pharmac.govt.nz/news-and-resources/consultations-and-decisions/2021-08-17-decision-rosuvastatin/ [cited 8 September 2021]
[73] Morgan SG, Leopold C, Wagner AK. Drivers of expenditure on primary care prescription drugs in 10 high-income countries with universal health coverage. CMAJ 2017; 189 e794–e799.
| Drivers of expenditure on primary care prescription drugs in 10 high-income countries with universal health coverage.Crossref | GoogleScholarGoogle Scholar | 28606975PubMed |
[74] Cumming J, Mays N, Daubé J. How New Zealand has contained expenditure on drugs. Br Med J 2010; 340 1224–1227.
| How New Zealand has contained expenditure on drugs.Crossref | GoogleScholarGoogle Scholar |


