Body site locations of basal cell carcinoma, squamous cell carcinoma and actinic keratosis in patients referred to the Waikato District Health Board teledermoscopy clinic
Kyla Kim 1 * , Ji Won Kim
1 * , Ji Won Kim  1 , Isabella Santos
1 , Isabella Santos  1 , Amanda Oakley
1 , Amanda Oakley  2 3
2 3
1 University of Auckland, Department of Medicine, Auckland, New Zealand.
2 University of Auckland, Waikato Clinical Campus, New Zealand.
3 Waikato District Health Board, Department of Dermatology, Hamilton, New Zealand.
Journal of Primary Health Care 14(1) 80-86 https://doi.org/10.1071/HC21115
Published: 13 April 2022
© 2022 The Author(s) (or their employer(s)). Published by CSIRO Publishing on behalf of The Royal New Zealand College of General Practitioners. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
Introduction: Basal cell carcinoma (BCC), squamous cell carcinoma (SCC) and actinic keratosis (AK) are usually located on sun-exposed areas of the body.
Aims: Our main aims were to identify the common body site locations of BCC, SCC and AK in patients attending the Waikato District Health Board teledermoscopy clinic, also known as the Waikato Virtual Lesion Clinic, as well as to analyse whether the distribution of location changes with age and sex.
Methods: This is a retrospective study where the body site location of 3272 keratinocytic lesions was determined and analysed in 1864 patients attending the Waikato District Health Board teledermoscopy clinics between 2010 and 2021.
Results: All three types of lesion were most commonly located in the head and neck region (40.9% of BCCs, 38% of SCCs, 83.2% of AKs), followed by 26.8% on the trunk for BCC, 32.3% on the lower extremities for SCC, and 11.6% on the upper extremities for AK.
Discussion: Our findings of body site locations for keratinocytic lesions were consistent with other studies. Patients were commonly diagnosed with multiple keratinocytic lesions.
Keywords: actinic, basal cell, carcinoma, keratinocytes, keratosis, lesion, New Zealand, squamous cell.
Introduction
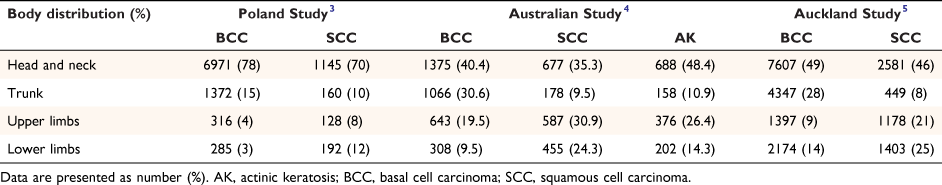
In 2005, it was estimated that all types of skin cancer together account for just over 80% of all new cancers diagnosed in New Zealand annually.1 In 2018, it was projected that over 90 400 people would be diagnosed with at least one in situ or invasive keratinocyte carcinoma in 2018.2 Keratinocyte cancer, also known as non-melanoma skin cancer, is commonly diagnosed and managed in primary care. The diagnosis can be made clinically or histologically with a biopsy. Solar ultraviolet (UV) radiation is the major risk factor for basal cell carcinoma (BCC), squamous cell carcinoma (SCC) and actinic keratosis (AK).3 Each type of lesion is reported to be most common on the head and neck, the body sites most exposed to the sun (Table 1).3–5
|
General practitioners (GPs) have referred patients with skin lesions of concern to the Waikato District Health Board (DHB) teledermoscopy service, also known as the Waikato Virtual Lesion Clinic, since January 2010. A trained melanographer records a standardised history assessing risk factors and captures regional, close-up and dermoscopic photographs of the referred lesions. A consultant dermatologist makes a diagnosis and management plan remotely, with a reported sensitivity of 100% and specificity of 90% for melanoma and keratinocyte cancer.6
This study addressed the following primary aims: to identify the common body site locations of BCC, SCC and AK in patients attending the Waikato DHB teledermoscopy clinic, and to analyse whether the distribution of location changes with age and sex. Secondarily, we also aimed to assess: the incidence of keratinocyte cancers in patients with a diagnosis of AK, whether patients with multiple lesions of the same tumour type have them on different body locations (both lesions coinciding within the same study period of January 2010–March 2021), and third, the body site location distribution of lesions specifically in our Māori population.
| WHAT GAP THIS FILLS |
| What is already known: Non-melanoma skin cancers are the commonest cancers diagnosed and managed in primary care. |
| What this study adds: This study examined the location of non-melanoma skin cancer and actinic keratosis in the Waikato population to determine whether lesion location can be a reliable factor to help make a clinical diagnosis. |
As this was a non-interventional data analysis, it was out of scope for Ethics review. All patients attending the teledermoscopy clinic had given consent for their data to be used for research.
Methods
The teledermoscopy database was queried for lesions diagnosed as BCC, SCC and AK, and the location of each lesion was determined. Where the database was ambiguous, the site was confirmed by reviewing images in the patient’s electronic medical record. Patient variables included age in years, sex (male or female), ethnicity, Fitzpatrick skin type, hair colour, eye colour and immunosuppression status.
The Ethnic Group Codes, as grouped by the Ministry of Health New Zealand, were used to classify patients’ ethnicities. We used Level 1 ethnic groups from the classification. This included Māori, Pacific people, Asian, combined Middle Eastern, Latin American and African (MELAA), other ethnicity, European and Residual Categories.7
To define the patients’ skin type, Fitzpatrick skin phototype was used. This is a method of classification according to one’s skin colour and extent of tanning and burning when exposed to the sun. Fitzpatrick skin phototype ranges from I to VI: skin type I typically always burns and does not tan, skin type II easily burns and tans with difficulty, skin type III burns moderately and tans moderately and uniformly, skin type IV burns minimally and tans moderately and easily, skin type V rarely burns and tans profusely, and skin type VI never burns and always tans profusely.8
Hair colour and eye colour were assessed subjectively. Hair colour included blonde, brown, black, red and unknown. Eye colour included blue, brown, hazel, green, grey and unknown. Patients who did not have their hair and eye colour assessed were categorised under ‘unknown’. Immunosuppression status of the patient was noted according to their past medical history.
Body sites were classified using the top two levels of a nine-level hierarchy of topography terms adopted by the World Health Organization (WHO) for the International Classification of Diseases (ICD), 11th revision. Level 1 consisted of five anatomical terms: head and neck, trunk, upper extremities, lower extremities and anogenital region. The 20 Level 2 locations were: head, neck, upper trunk, lower trunk, shoulder, axilla, upper arms, elbow, forearm, wrist, hand, buttocks, thigh, knee, lower leg, ankle, foot, genital, perigenital and perianal region.9
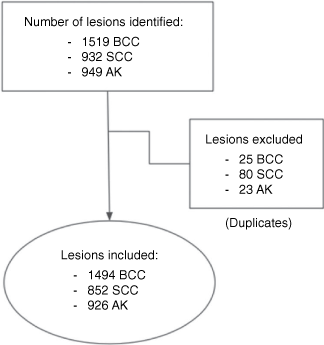
The inclusion criteria were patients attending the teledermoscopy clinic from January 2010 to March 2021 with at least one lesion diagnosed by a teledermatologist as BCC, SCC or AK. Clinical variants were included (keratoacanthoma, cutaneous horn, actinic cheilitis, keratotic lesion, basosquamous carcinoma). Duplicate lesions were excluded (Fig. 1).
|
Analytic approach
All collected data were initially summarised onto an Excel© (Microsoft Corporation) spreadsheet for review. The data were categorised according to the specific type of keratinocyte lesion (BCC, SCC and AK) on separate Excel© spreadsheets. Using Excel© functions, all data were stratified into body location following Level 2 of the nine-level hierarchy of topography terms. Further stratification into age and sex was performed to investigate any change in the lesion–location distribution. From the patients with a diagnosis of one or more AK, patients’ National Health Index (NHI) code was used to filter for patients with a previous, co-existing or later diagnosis of BCC or SCC within the same study period of 2010–21. Patients with multiple lesions were also identified using filter functions for duplicates of the patient NHI code. Stratification according to body site location of each lesion was carried out for each of these identified patients to assess the frequency of lesions in each body location of the individual.
Ethics
This study was out of scope of an Ethics application because it was a non-interventional review. All patients attending the teledermoscopy clinic agreed to their data being used for research.
Results
Table 1 shows the body site location of BCC, SCC and AK in this study and in research reports from Australia and Poland.
Patient demographics
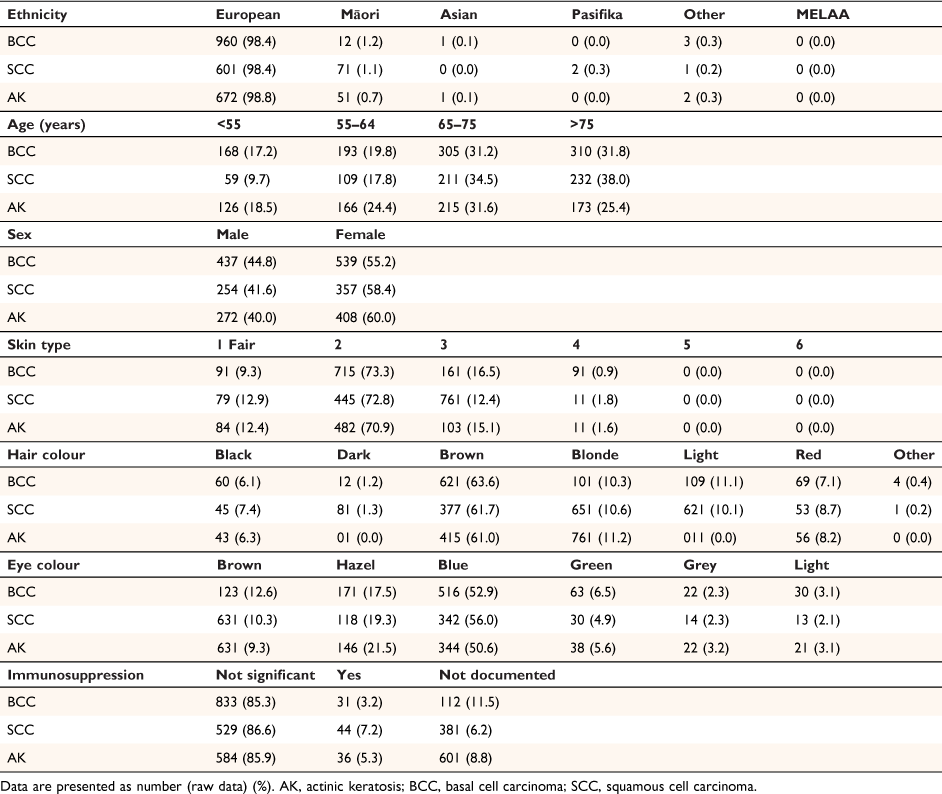
Table 2 shows the demographic details of study patients. The teledermoscopy clinic gave a diagnosis of keratinocytic lesions to 1864 patients during the study period of January 2010 to March 2021. Of these patients, the majority were women (42.3% men and 57.7% women), and the mean age was 68 years (range 24–100 years). The majority were aged 65–75 years (30.7%) or >75 years (30.2%). The most common ethnicity was European (98.4%), followed by Māori (1.2%). There were 976 patients with BCC, 611 patients with SCC, and 680 patients with AK; 5.0% of patients were diagnosed with BCC, SCC and AK; 13.5% with BCC and SCC; 8.7% with SCC and AK; and 8.9% of patients with BCC and AK.
|
Primary results
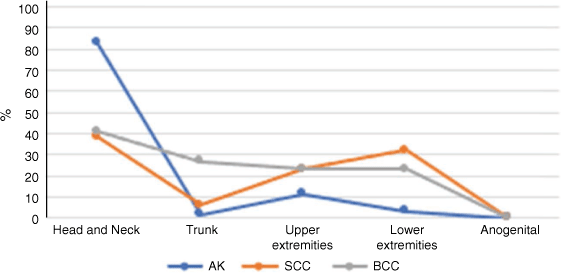
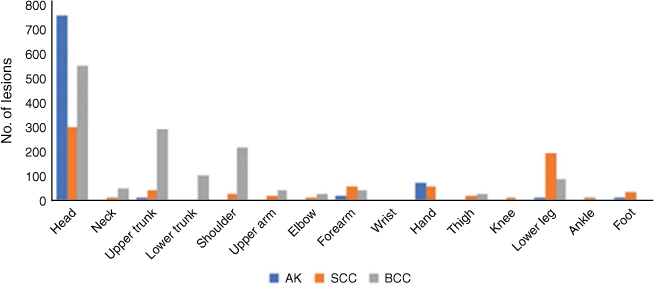
The database included a total of 3272 lesions from a total of 1864 patients. The most common Level 1 site of all keratotic lesions (BCC, SCC and AK) was head and neck. The next most common site was the trunk for BCC, lower extremities for SCC and upper extremities for AK (Fig. 2). No keratinocyte cancer was found in the anogenital region. The most common Level 2 site for all lesions was the head (Fig. 3).
|
|
Basal cell carcinoma body site location
The dataset included 1494 BCC. The most common Level 1 site of BCC was head and neck, followed by trunk, upper extremities and lower extremities. When subclassified under Level 2 locations, 37.2% of BCCs were on the head (3.7% on the neck), 19.8% of BCC were on the upper trunk (7.0% on the lower trunk), and 15.0% were on the shoulder. Location distribution did not change with any patient variable except for 44 lesions in immunosuppressed patients where there was an equal number on the trunk and on the head and neck.
Squamous cell carcinoma body site location
There were 852 SCC. The most common Level 1 site of SCC was the head and neck, followed by lower extremities, upper extremities and trunk (Fig. 4). When subclassified under Level 2 locations, 36.0% of SCC were on the head (2.0% on the neck), 23% were on the lower legs, and 7.2% were on the forearms. In patients aged >65 years, SCC were more evenly distributed across the head and neck and limbs. In females, 38% of SCC were on the upper limbs, and 25% were on the lower limbs. In males, 24% of SCC were on the lower limbs, and 21% were on the upper limbs. The distribution of location was unchanged for other variables.
Actinic keratosis body site location
There were 926 AK. The most common Level 1 site for AK was head and neck, followed by upper extremities, lower extremities and trunk. When subclassified under Level 2 locations, the majority (82.1%) were on the head (1.1% were found on the neck), 7.9% were on the hands, and 2.1% were on the forearms. Location distribution did not change with any patient variable.
Secondary results
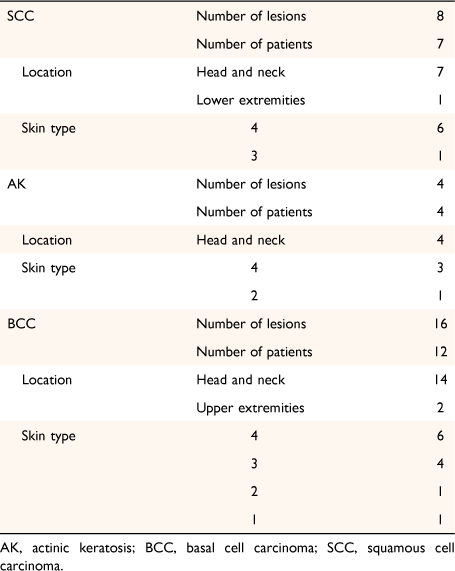
From our dataset, we found 85 patients (21.9%) with SCC and co-existing AK, and 87 patients (13.9%) with BCC and co-existing AK. In terms of multiple lesions of the same tumour type, we found that 265 patients were diagnosed with two or more BCCs; 171 (64.5%) were located in more than one region. Two or more SCCs were found in 151 patients; 80 (52.3%) were located in more than one region. Two or more AKs were found in 122 patients; 40 (32.8%) were located in more than one region. Lesions that were located in the same region were either recurrences of previous lesions (within the study period), or more than one lesion adjoined in the same region. Māori patients were only 0.86% of our study population (28 lesions in 23 Māori patients diagnosed by teledermoscopy). The lesions of Māori patients were found most commonly on the head and neck (Table 3).
|
Discussion
We examined the body site location of BCC, SCC and AK in patients diagnosed by the teledermoscopy service. We found that BCC, SCC and AK were most common in the head and neck area followed by the trunk for BCC, lower extremities for SCC, and upper extremities for AK, as reported in other studies (Table 1). In females, the most common body site location for SCC was the lower extremities, perhaps due to differences in hair styles and attire.
As expected, there were more BCC than SCC in our population. The proportion of SCC (36.3%) is greater than the 14.8% reported in a Poland study4 and the 26% in an Auckland study.6 We speculated that the teledermoscopy service was mainly used for diagnostic support. The higher proportion of SCC may be due to GPs finding BCC easier to diagnose than SCC, as was found in a study conducted in Korea.10 Alternatively, our local population may be at greater risk due to factors that are more strongly associated with SCC and therefore reflect a true trend. More females (57.7%) than males (42.3%) with keratinocyte cancer were referred to the teledermoscopy service, which is in contrast to a significantly higher prevalence of non-melanoma skin cancer reported in men.11
The local prevalence of Māori in our population is reported to be 24%.12 In reflection of the significant proportion of Māori in our population, we aimed to assess if the body site distribution of keratinocyte lesions differs in the Māori population in comparison to our total population. There is currently no study on the body site distribution of keratotic lesions in the Māori population. Few keratinocyte cancers in Māori are expected because fair skin is a significant risk factor for skin cancers and all of our Māori patients have skin type 4 (65.2%) or 3 (21.7%). Māori patients made up only 0.86% of our study population, with more than 98% of patients in this study being European. This indicates that the pattern of body site distribution of keratinocytic lesions found from our study is likely to be representative of the European population. On separate analysis of Level 1 body site location of lesions in our Māori population, we found that the head and neck was the most common location for all AK, SCC and BCC. This was followed by lower extremities for SCC and upper extremities for BCC (Table 3). This is similar to our findings for our total population, with head and neck being the most common location for all lesion types. One difference found is that the trunk was the second most common location for BCC in our total population, but upper extremities were the second most common in the Māori population. However, with data only from 28 lesions in 23 Māori patients, the pattern of body site distribution of lesions found from our data is not representative of the entire Māori population in New Zealand.
We found that a higher percentage of patients with SCC had coexisting AK than patients with BCC, consistent with the evolution of AK to SCC, and with other studies.13 However, the absolute numbers of patients with BCC and AK were about the same as patients with SCC and AK; this is due to the higher prevalence of patients with BCC than with SCC. Individuals often have multiple lesions in different body regions. The likely hypothesis is that the multiple lesions are due to a common cause such as chronic UV exposure, immunosuppression, human papillomavirus infection, or environmental factors including diet and arsenic exposure.14 The likelihood of multiple lesions stresses the importance of a whole-body skin check and monitoring for anyone with a history of keratinocyte cancer or AK.
Our findings reflect the epidemiological trend of non-melanoma skin cancer, and closely correlate with findings from other studies and literature. Some differences were noted regarding certain variables depending on the different type of keratinocytic lesion. Location is an essential factor to consider when making a clinical diagnosis. Thus, our findings can be applied in a primary care setting to help formulate a confident clinical diagnosis. To improve accuracy in diagnosis, the pattern of location and type of keratinocyte can be further explored, especially in the different ethnic groups.
The major strength of our study is the adequate sample size, consisting of over 3000 lesions in nearly 2000 patients. Moreover, a well-defined standard was used to define location. Limitations of our study include the use of retrospective data unconfirmed by histopathology and a population limited to lesions referred to the teledermoscopy service, which excluded those on the hair-bearing scalp and genitalia. We did not assess lesions in patients diagnosed in the community or the Waikato DHB hospital. Subtypes of each tumour were not recorded in the database.
In conclusion, AK, SCC and BCC referred to the Waikato DHB teledermoscopy service were most commonly found on the head and neck. The next most common site was the trunk for BCC, lower limb for SCC and upper limb for AK. The lower limb was the most common site in females with SCC compared to the head and neck in males. The dermatologists frequently diagnosed multiple keratinocytic lesions in the study patients.
Data availability
The data used to generate the results in the paper are available and can be provided upon request.
Conflicts of interests
Dr Oakley is paid a fee to diagnose for MoleMap New Zealand in the private sector. The other authors have no conflicts of interest to declare.
Declaration of funding
The research did not receive any specific funding.
Acknowledgements
The Waikato District Health Board teledermoscopy clinic, also known as the Waikato Virtual Lesion Clinic, is a private–public collaboration with MoleMap New Zealand, who collected data and images and supplied the database. Diagnoses were made by Waikato DHB dermatologists, Amanda Oakley, Marius Rademaker, Anthony Yung, Anita Eshraghi and Karen Koch.
References
[1] O’Dea D. The estimated costs - economic and human - of skin cancers to New Zealand. In: UV radiation and its effects: An update 2010: Report of the NIWA UV Workshop (NIWA Information Series No. 77). Wellington, New Zealand: National Institute of Water & Atmospheric Research; 2010, p. 79. Available at https://niwa.co.nz/sites/niwa.co.nz/files/estimated_cost_of_skin_cancers_to_nz.pdf [Accessed 2 May 2021][2] Sneyd MJ, Gray A. Expected non melanoma skin (Keratinocytic) cancer incidence in New Zealand for 2018. Wellington: Health Promotion Agency; 2018. Available at https://www.hpa.org.nz/sites/default/files/Expected%20Non%20Melanoma%20Skin%20KC%20incidence%20in%20NZ%20for%202018_FinalReport_777173.pdf [Accessed 10 May 2021]
[3] Ciążyńska M, Kamińska-Winciorek G, Lange D, et al. The incidence and clinical analysis of non-melanoma skin cancer. Sci Rep 2021; 11 4337
| The incidence and clinical analysis of non-melanoma skin cancer.Crossref | GoogleScholarGoogle Scholar | 33619293PubMed |
[4] Youl PH, Janda M, Aitken JF, et al. Body-site distribution of skin cancer, pre-malignant and common benign pigmented lesions excised in general practice. Br J Dermatol 2011; 165 35–43.
| Body-site distribution of skin cancer, pre-malignant and common benign pigmented lesions excised in general practice.Crossref | GoogleScholarGoogle Scholar | 21443534PubMed |
[5] Pondicherry A, Martin R, Meredith I, et al. The burden of non-melanoma skin cancers in Auckland, New Zealand. Australas J Dermatol 2018; 59 210–3.
| The burden of non-melanoma skin cancers in Auckland, New Zealand.Crossref | GoogleScholarGoogle Scholar | 29350397PubMed |
[6] Tan E, Yung A, Jameson M, et al. Successful triage of patients referred to a skin lesion clinic using teledermoscopy (IMAGE IT trial). Br J Dermatol 2010; 162 803–11.
| Successful triage of patients referred to a skin lesion clinic using teledermoscopy (IMAGE IT trial).Crossref | GoogleScholarGoogle Scholar | 20222920PubMed |
[7] Ministry of Health NZ. Ethnicity code tables [Internet]. 2010. Available at https://www.health.govt.nz/nz-health-statistics/data-references/code-tables/common-code-tables/ethnicity-code-tables [Accessed 21 January 2022]
[8] Sachdeva S. Fitzpatrick skin typing: applications in dermatology. Indian J Dermatol Venereol Leprol 2009; 75 93
| Fitzpatrick skin typing: applications in dermatology.Crossref | GoogleScholarGoogle Scholar | 19172048PubMed |
[9] Kenneweg KA, Halpern AC, Chalmers RJG, et al. Developing an international standard for the classification of surface anatomic location for use in clinical practice and epidemiologic research. J Am Acad Dermatol 2019; 80 1564–1584.
| Developing an international standard for the classification of surface anatomic location for use in clinical practice and epidemiologic research.Crossref | GoogleScholarGoogle Scholar | 31010690PubMed |
[10] Ryu TH, Kye H, Choi JE, et al. Features causing confusion between basal cell carcinoma and squamous cell carcinoma in clinical diagnosis. Ann Dermatol 2018; 30 64
| Features causing confusion between basal cell carcinoma and squamous cell carcinoma in clinical diagnosis.Crossref | GoogleScholarGoogle Scholar | 29386834PubMed |
[11] Kütting B, Drexler H. UV-induced skin cancer at workplace and evidence-based prevention. Int Arch Occup Environ Health 2010; 83 843–54.
| UV-induced skin cancer at workplace and evidence-based prevention.Crossref | GoogleScholarGoogle Scholar | 20414668PubMed |
[12] Ministry of Health NZ. Population of Waikato DHB. 2021. Available at https://www.health.govt.nz/new-zealand-health-system/my-dhb/waikato-dhb/population-waikato-dhb [Accessed 4 May 2021]
[13] Traianou A, Ulrich M, Apalla Z, et al. Risk factors for actinic keratosis in eight European centres: a case-control study. Br J Dermatol 2012; 167 36–42.
| Risk factors for actinic keratosis in eight European centres: a case-control study.Crossref | GoogleScholarGoogle Scholar | 22881586PubMed |
[14] Saini R, Sharma N, Pandey K, Puri KJPS. Multiple skin cancers in a single patient: multiple pigmented Bowen′s disease, giant basal cell carcinoma, squamous cell carcinoma. J Cancer Ther Res 2015; 11 669
| Multiple skin cancers in a single patient: multiple pigmented Bowen′s disease, giant basal cell carcinoma, squamous cell carcinoma.Crossref | GoogleScholarGoogle Scholar |



