‘The horror stories put me off!’: exploring women’s acceptability of the Levonorgestrel IntraUterine System (LNG-IUS) for endometrial protection
Claire Henry 1 4 , Alec Ekeroma 2 , Anthony Dowell 3 , Sara Filoche 1
1 4 , Alec Ekeroma 2 , Anthony Dowell 3 , Sara Filoche 1
1 Department of Obstetrics, Gynaecology and Women’s Health, University of Otago, Wellington, New Zealand
2 School of Medicine, National University of Samoa, Apia, Samoa
3 Department of Primary Health Care, University of Otago, Wellington, New Zealand
4 Corresponding author. Email: Claire.henry@otago.ac.nz
Journal of Primary Health Care 13(1) 55-62 https://doi.org/10.1071/HC20105
Published: 15 March 2021
Journal Compilation © Royal New Zealand College of General Practitioners 2021 This is an open access article licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License
Abstract
INTRODUCTION: There are few studies of user perceptions of the Levonorgestrel Intrauterine System (LNG-IUS; Mirena™), which now has the potential to play an important role in the treatment of women with hyperplasia or early stage endometrial cancer. There is limited evidence on how well the Mirena™ is perceived and accepted by women in this context.
AIM: To gain an understanding of New Zealand women’s views on the use of the Mirena™ contraceptive device to inform policies in endometrial cancer prevention.
METHODS: An online survey platform (Qualtrics™) was disseminated over social media sites such as Facebook once a week for 3 weeks. The survey used mixed methods (closed questions, multiple choice and open-ended questions) and covered topics relating to the knowledge and use of the Mirena™ for endometrial protection. Data were collected and explored using content and thematic analysis.
RESULTS: In total, 89 women responded to the survey. Half (42/89) of respondents had never used a Mirena™ in their life. Most women (79/89) did not know anyone who had had endometrial cancer. The frequency of negative comments about the Mirena™ was higher than positive comments (42 and 26 respectively), largely attributed to personal or reported poor experiences with other contraceptives (including the copper intrauterine device).
DISCUSSION: Although health-care providers may view the Mirena™ favourably, this view was not reciprocated in this community sample.
KEYwords: Intrauterine device; endometrial cancer; survey; acceptability; womens health; qualitative
| WHAT GAP THIS FILLS |
| What is already known: The LNG-IUS (Mirena™) contraceptive device has potential to play a role in the treatment of hyperplasia and early stage endometrial cancer. There is little information on how women perceive this. |
| What this study adds: Women’s ambivalence about the Mirena™ suggests the need for a sympathetic and respectful health literacy and promotion campaign if equitable access, and consequently benefit, is to be achieved in New Zealand and elsewhere. |
Introduction
Endometrial cancer is the most common gynaecological cancer, with approximately 400 women diagnosed in New Zealand (NZ) each year. Incidence rates are increasing, particularly in Pasifika women aged <50 years (2/100,000 in 1997 to 24/100,000 in 20121). Endometrial cancer incidence, morbidity and mortality rates in women who identify as Māori and Pasifika are much greater than among women who identify as European or Other ethnicities.2,3
Surgical resection, including hysterectomy, is the standard of care for women suspected to have endometrial cancer. However, it is becoming more common that other novel conservative therapeutic options are needed for women with higher risks of post-operative complications as a result of comorbidities (eg overweight or Body Mass Index >28) or who have not completed their families.4 The Levonorgestrel Intrauterine System (LNG-IUS), herein referred to as the Mirena™, delivers progestogen directly to the uterus to inhibit oestrogen-stimulated proliferation. At present, evidence points towards successful use of the Mirena™ to treat hyperplasia or early stage cancer. There are varied responses in patients with hyperplastia and endometrial cancer, with regression rates ranging from 75%5,6 to 94%.7 Notably, some research has shown successful conception following when the Mirena™ has been used as a fertility sparing technique.5,8 Furthermore, Mirena™ use may even decrease the risk of other malignancies, such as ovarian, pancreatic and lung cancer.9
There is limited research about user perceptions of the Mirena™, particularly in the context of heavy bleeding and for users aged >40 years. Some studies have investigated young women’s (from adolescents to age 30 years) attitudes relating to long-acting reversible contraceptives,10–12 finding that approximately half of women did not know about the Mirena™. In a prospective trial of 255 women with menorrhagia using the Mirena™, 98% reported a high degree of satisfaction.13
The Mirena™ has the potential to play an important role in treating women with hyperplasia or early stage endometrial cancer. Now that the Mirena is subsidised in NZ, this option may become more available to women, and may also be prescribed for its endometrial protection properties for at-risk women. Therefore, the aim of this study was to investigate, from a narrative perspective, the acceptability of the Mirena™ in the community.
Methods
This study received ethics approval from the University of Otago research committee (H19-096). An online survey was designed for use through the online platform, Qualtrics™. Women were provided with a brief summary infographic about the Mirena™ and endometrial cancer at the beginning of the survey. This included information on its use as a contraceptive and its potential as a preventive treatment for endometrial cancer.
The survey included close-ended multiple choice questions and open-ended free-text questions where participants could describe their experiences. Questions included demographic data (ethnicity, age, weight), information about the Mirena™ (whether participants had used it or knew anyone who had), information about endometrial cancer (whether they knew about it, or knew anyone who had received that diagnosis), and whether they would consider using the Mirena™ for abnormal bleeding or endometrial cancer.
The survey was distributed to six women’s community groups on Facebook™ and two on Reddit™, with approval from group administrators. The groups were NZ-based and women-focused (such as mothers’ groups, women’s discussion boards). The survey was re-posted to these groups three times. Women were invited to participate if they were aged ≥40 years. No identifying data were collected and participants remained anonymous.
Frequency and content analysis was used to analyse data from closed questions answers. Data from free-text responses of women’s views of the Mirena™ were analysed through inductive thematic coding, assisted by NVIVO™ software.
Results
Participant characteristics
Completed surveys were received from 89 women across NZ. Respondents were mainly aged 40–49 years (66/89) and self identified their ethnicity as NZ European (65/89). The respondents were evenly split between women who had and had not previously had a Mirena™ at any time during their life (47/89 and 42/89 respectively). Seven women had not heard of the Mirena™ before participating in the survey. Characteristics of the study group are presented in Table 1. Most women aged 40–60 years had known a friend or family member who had the Mirena™ during their life (40/89, Supplementary material Table S1, available at the journal’s website). Women had heard about the Mirena™ from their doctor or general practitioner (GP) (51/107 answers) or from family and friends (26/107) or the Internet (16/107).

|
Perceptions of the Mirena™ and its use for endometrial protection
Half of the participants (51/89) would consider using the Mirena™ for abnormal or heavy uterine bleeding, but only 42/89 would consider it for protection against endometrial cancer (Table 2). Nearly all British women in the sample would consider the Mirena™ for abnormal uterine bleeding and endometrial cancer (9/10 and 8/10 respectively).
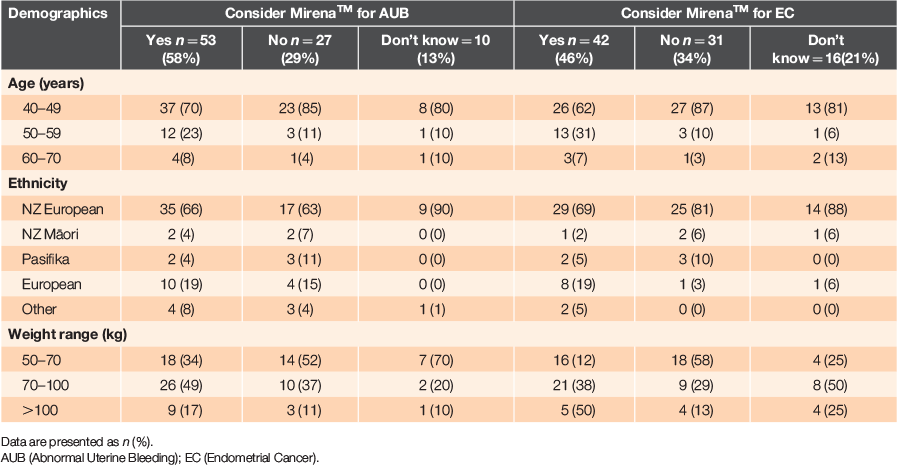
|
The frequency of negative comments, views and experiences about the Mirena™ was higher than that for positive comments (42 and 26 respectively; Supplementary Table S2). Previous experience with contraceptives, including the Mirena™, Copper intrauterine device (IUD) and pill had the greatest impact on participants considering the Mirena™ for abnormal uterine bleeding or endometrial cancer. Overall, the frequency of previous negative experiences with contraceptives was higher than positive previous experiences (Table S2).
Women were invited to describe in detail their own views on the Mirena™ and reasons for or against considering the Mirena™ for protection against abnormal uterine bleeding and endometrial cancer. Themes were inductively coded and analysed separately for respondents favouring and against the Mirena™.
Support for the Mirena™
Five themes were identified from the group of women who would consider Mirena™ for abnormal uterine bleeding or endometrial cancer prevention (Figure 1). Previous positive experiences were the main reason why women would consider the Mirena™. Many comments were based on the effect of the Mirena™ on heavy periods:
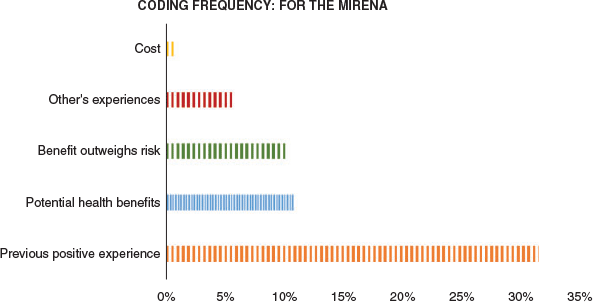
|
‘I suffered very heavy ongoing bleeding and having the Mirena™ slowed things down greatly ... it's been the best decision ever.’
One woman described her experience with seeking care for heavy bleeding, detailing dismissive and fat-shaming by GPs. Her story speaks to the continued barriers women face for accessing care for menstrual and uterine conditions:
‘The Mirena™ was a lifesaver for me. I had incredibly heavy periods where I would be regularly passing clots that were half the size of my palm. Nobody cared that much due to a combination of how poorly women’s issues are regarded by medical professionals as well as my weight: fat women, in my experience, find it very difficult to get appropriate medical care and no matter what we approach our doctor with it’s blamed on our fat and therefore considered ‘our fault’... and so either not treated or poorly treated. So to be offered the Mirena™ was just amazing. I had no side effects other than a lack of bleeding. Finally I could work, run my family household, without interruption. Quite frankly I would have done almost anything to get the bleeding to stop. I just wanted my life back.’
Oppose Mirena™
Multiple themes were identified from the group of women who would not consider Mirena™ for abnormal uterine bleeding or endometrial cancer (Figure 2). Many expressed the need to have more information before considering this type of treatment:
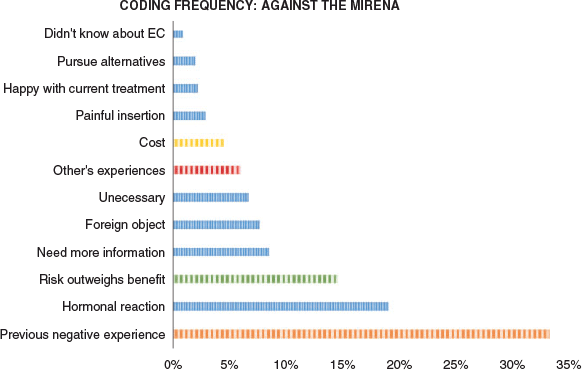
|
‘I had a friend that took the same pill as me and had no side effects. I took it for less than two months and I got a huge clot. I would want to know everything under the sun about it [the Mirena™] before I would even entertain the idea of getting one.’
Many women were worried about hormonal effects of the Mirena™, considering their bad experiences in the past with other forms of contraceptives:
‘My concerns are based on my personal reaction to hormonal contraceptives. They’ve messed with my body a lot so I’m reluctant to try.’
Women were also concerned about the effect of hormones on their mental health:
‘When I was on the progesterone pill I had irregular bleeding and terrible mood swings and depression.’
‘I have it in at the moment and getting removed today as I’ve had bad anxiety. Vaginal thrush. And mood swings.’
Personal experience or stories of painful and stressful previous experiences with the copper IUD meant that some women were reluctant to consider the Mirena™:
‘I had the copper coil inserted after a recent termination (40 years old). The coil expelled after 6 months, during which time I experienced the worst periods cramps of my life. I’ve heard of other women having similar experiences with these devices. That, coupled with my own experience, is enough to put me off ever using one again.’
‘I get heavy bleeding when using the copper IUD and I don’t want to risk the same with Mirena so I [would] choose an alternative form of contraception.’
The comparison of the copper IUD, which is known to cause heavy and painful bleeding, to the Mirena™ suggests that informed discussion around the use of these different methods had not been undertaken.
One woman described her frustration with different treatments for her abnormal uterine bleeding, caused by fibroids, adenomyosis, endometriosis and adhesions:
‘I’m very much sick of crappy, stopgap solutions... Even my specialist admits that it will just mask the symptoms until it doesn’t work then my conditions will probably be worse. I am an educated, supposedly advantaged person who was badly served by treatments advised by medical professionals which mask symptoms while the condition grows worse. In addition, I’ve had some very negative side effects to most treatments offered so far so don’t believe the hype.’
Several women were concerned with the placement of a foreign object in their body and the ‘uncomfortable part of getting it put in’. In this context, the risk of complications seems to outweigh any potential health benefits.
‘Would much rather pursue alternative ways to fix problems rather than putting foreign man made things into my body.’
‘Seems unnatural to have this inserted.’
‘I’m not comfortable with the thought of an IUD inside my body. I’m not convinced of the certainty of the benefits.’
‘I don’t like to introduce medical devices or medication if not necessary as I worry there may be consequences which are unknown now i.e. cancer even if research says otherwise.’
One woman took a pragmatic view point, and discussed her feelings around the impact of preventative therapy on her whanau (family):
‘My other whanau member had one form of cancer, went into remission, and then died of another type of cancer. And then another whanau member nearly died in a car accident, while a 4th younger whanau member was killed in one. I guess the point I’m making, is that we can try to do all manner of things to try and prolong our lives, but we can’t really control when we might die. And I’d hate to rest all my hopes in something like that, to find that I might be the atypical one, or outlier. That’s a burden I don’t want my whanau, myself or my health professional having to bear.’
Discussion
This study explored women’s perspective towards the use of the Mirena™ in the contexts of abnormal uterine bleeding and endometrial cancer. From the small sample, our findings show that more women would consider the Mirena™ for abnormal uterine bleeding and fewer for endometrial protection against cancer. It appears that older women (aged 50–70 years) were more accepting of the Mirena™ than women aged <50 years. It was not possible to compare the responses of Europeans and Māori or Pasifika due to the lower number of participants from the latter ethnicities; this should be the aim of a further study.
The study is one of few to obtain women’s narratives about the acceptability of Mirena™, most of which were negative. The negative attitudes were based on either previous experiences of an implant contraceptive device, negative experiences based on other women’s stories and needing more certainty around the efficacy of the Mirena™ to treat early endometrial cancer in order for them to accept it as a treatment option. These comments were often based on the copper IUD, which has been known to cause cramping and heavy periods.14
Given the median age of survey participants, use of the copper IUD was one of the more popular contraceptive choices during participants’ earlier reproductive years. Therefore, it is understandable that women in this cohort related copper IUD experiences with the Mirena™, even though it was explained that the Mirena™ may not have the same adverse effects.
Some women were concerned about the effects of hormones on their mental health. The current literature around this is inconsistent. Some large cohort studies and systematic reviews show no association between poor mental health and contraceptive use, including intra uterine devices,15–17 whereas others show the opposite.18,19 In our survey, women indicated that even though these symptoms were experienced, appropriate care, or discussion around other options was not received. There is little research about the degree to which mental health is considered when prescribing contraceptives, and mental health considerations are not included in clinical guidelines.20 Therefore, we recommend that further research is needed in this area to support an update of clinical practice with a view to improving outcomes for women.
In the United States, IUDs are reported to be underused for long-term contraception, representing ~5.5% of women who use contraception (2006–08 data);21 even when barriers to access such as cost were eliminated, uptake of the Mirena™ was only 55%.22 The reasons for low uptake were not known and internationally, there is little research in this area. Studies of the acceptability of the Mirena™ as a method of contraception have been based retrospectively on the number of women who continue to use the Mirena™ after a certain amount of time, and satisfaction has been assessed using quality of life surveys.23 Satisfaction with the Mirena™ as a contraceptive varies, with some studies estimating 70–90% satisfaction,24,25 and others indicating that many women have the device removed because they were unsatisfied with it.26 Satisfaction with the Mirena™ for the treatment for menstrual symptoms also varies from ~40%23 to 70%.27
The uptake (and satisfaction) with the Mirena™ does not appear to match medical expectation; that is, uptake is still relatively low when the safety and efficacy of the Mirena™ is considered as a long-term contraceptive option and for the treatment of menstrual disorders.28 The use of progestins has potential to improve long-term female health significantly, but this message has not been received well in the community. Although this study was based on a small convenience sample, the views of women align with anecdotal evidence from GPs and sexual health providers around low uptake. Furthermore, the qualitative data captured from free-text have enabled us to carry out a rich analysis of women’s viewpoints, which strengthen the findings obtained from the quantitative survey.
This study was limited by the recruitment of women to the survey using social media platforms. This may cause bias in the sample. Because of COVID-19 restrictions during the data collection period, women could not be recruited face-to-face, as designed. However, social media-based recruitment has been shown to yield high response rates to web-based surveys compared to in-clinic recruitment.29 It would be important to extend the survey into focus groups or interview approaches to include women with limited internet access. Second, we chose to only include in the survey the Mirena™, rather than other hormonal IUDs. This was for simplicity in communicating with participants, popularity of the Mirena™ over the Jaydess in NZ (which releases less LNG (13.5 mg)) and the fact that the Mirena™ has been the most investigated IUD for the treatment of hyperplasia or endometrial cancer.
In the modern world, the nature of menstruation is changing and hormonal therapy has now been described as a ‘pregnancy substitute’ to be embraced rather than feared, and with the potential to improve long-term women’s health substantially.30 The reported ambivalence women hold towards the device suggests the need for a sympathetic and respectful health literacy and promotion campaign about the Mirena™ for endometrial protection if equitable access and benefit is to be achieved. This is important as the Mirena™ is rapidly becoming the standard of care for treating heavy or abnormal bleeding, and holds potential as treatment for atypia or early stage endometrial cancer when surgical intervention is not an option.
Competing interests
The authors declare no competing interests.
Acknowledgements
This research was supported by a University of Otago Research Grant to CH.
References
[1] Scott OW, Tin Tin S, Bigby SM, et al. Rapid increase in endometrial cancer incidence and ethnic differences in New Zealand. Cancer Causes Control. 2019; 30 121–7.| Rapid increase in endometrial cancer incidence and ethnic differences in New Zealand.Crossref | GoogleScholarGoogle Scholar | 30671687PubMed |
[2] Soeberg M, Blakely T, Sarfati D. Trends in ethnic and socioeconomic inequalities in cancer survival, New Zealand, 1991–2004. Cancer Epidemiol. 2015; 39 860–2.
| Trends in ethnic and socioeconomic inequalities in cancer survival, New Zealand, 1991–2004.Crossref | GoogleScholarGoogle Scholar | 26651447PubMed |
[3] Firestone RT, Ellison-Loschmann L, Shelling AN, et al. Ethnic differences in disease presentation of uterine cancer in New Zealand women. J Fam Plann Reprod Health Care. 2012; 38 239–45.
| Ethnic differences in disease presentation of uterine cancer in New Zealand women.Crossref | GoogleScholarGoogle Scholar | 22241766PubMed |
[4] Staples JN, Rauh L, Peach MS, et al. Endometrial cancer in an increasingly obese population: exploring alternative options when surgery may not cut it. Gynecol Oncol Rep. 2018; 25 30–4.
| Endometrial cancer in an increasingly obese population: exploring alternative options when surgery may not cut it.Crossref | GoogleScholarGoogle Scholar | 29977988PubMed |
[5] Pronin SM, Novikova OV, Andreeva JY, et al. Fertility-sparing treatment of early endometrial cancer and complex atypical hyperplasia in young women of childbearing potential. Int J Gynecol Cancer. 2015; 25 1010–4.
| Fertility-sparing treatment of early endometrial cancer and complex atypical hyperplasia in young women of childbearing potential.Crossref | GoogleScholarGoogle Scholar | 25950126PubMed |
[6] Pal N, Broaddus RR, Urbauer DL, et al. Treatment of low-risk endometrial cancer and complex atypical hyperplasia with the levonorgestrel-releasing intrauterine device. Obstet Gynecol. 2018; 131 109–16.
| Treatment of low-risk endometrial cancer and complex atypical hyperplasia with the levonorgestrel-releasing intrauterine device.Crossref | GoogleScholarGoogle Scholar | 29215513PubMed |
[7] Gallos ID, Krishan P, Shehmar M, et al. LNG-IUS versus oral progestogen treatment for endometrial hyperplasia: a long-term comparative cohort study. Hum Reprod. 2013; 28 2966–71.
| LNG-IUS versus oral progestogen treatment for endometrial hyperplasia: a long-term comparative cohort study.Crossref | GoogleScholarGoogle Scholar | 23975691PubMed |
[8] Gallos ID, Yap J, Rajkhowa M, et al. Regression, relapse, and live birth rates with fertility-sparing therapy for endometrial cancer and atypical complex endometrial hyperplasia: a systematic review and metaanalysis. Am J Obstet Gynecol. 2012; 207 266.e1–266.e12.
| Regression, relapse, and live birth rates with fertility-sparing therapy for endometrial cancer and atypical complex endometrial hyperplasia: a systematic review and metaanalysis.Crossref | GoogleScholarGoogle Scholar |
[9] Soini T, Hurskainen R, Grénman S, et al. Cancer risk in women using the levonorgestrel-releasing intrauterine system in Finland. Obstet Gynecol. 2014; 124 292–9.
| Cancer risk in women using the levonorgestrel-releasing intrauterine system in Finland.Crossref | GoogleScholarGoogle Scholar | 25004338PubMed |
[10] Kavanaugh ML, Frohwirth L, Jerman J, et al. Long-acting reversible contraception for adolescents and young adults: patient and provider perspectives. J Pediatr Adolesc Gynecol. 2013; 26 86–95.
| Long-acting reversible contraception for adolescents and young adults: patient and provider perspectives.Crossref | GoogleScholarGoogle Scholar | 23287602PubMed |
[11] Spies EL, Askelson NM, Gelman E, et al. Young women’s knowledge, attitudes, and behaviors related to long-acting reversible contraceptives. Women’s Health Issues. 2010; 20 394–9.
| Young women’s knowledge, attitudes, and behaviors related to long-acting reversible contraceptives.Crossref | GoogleScholarGoogle Scholar | 21050998PubMed |
[12] Higgins JA, Kramer RD, Ryder KM. Provider bias in long-acting reversible contraception (LARC) promotion and removal: perceptions of young adult women. Am J Public Health. 2016; 106 1932–7.
| Provider bias in long-acting reversible contraception (LARC) promotion and removal: perceptions of young adult women.Crossref | GoogleScholarGoogle Scholar | 27631741PubMed |
[13] Lete I, Obispo C, Izaguirre F, et al. The levonorgestrel intrauterine system (Mirena) for treatment of idiopathic menorrhagia. Assessment of quality of life and satisfaction. Eur J Contracept Reprod Health Care. 2008; 13 231–7.
| The levonorgestrel intrauterine system (Mirena) for treatment of idiopathic menorrhagia. Assessment of quality of life and satisfaction.Crossref | GoogleScholarGoogle Scholar | 18609346PubMed |
[14] Hubacher D, Chen P-L, Park S. Side effects from the copper IUD: do they decrease over time? Contraception. 2009; 79 356–62.
| Side effects from the copper IUD: do they decrease over time?Crossref | GoogleScholarGoogle Scholar | 19341847PubMed |
[15] Robakis T, Williams KE, Nutkiewicz L, et al. Hormonal contraceptives and mood: review of the literature and implications for future research. Curr Psychiatry Rep. 2019; 21 57
| Hormonal contraceptives and mood: review of the literature and implications for future research.Crossref | GoogleScholarGoogle Scholar | 31172309PubMed |
[16] Toffol E, Heikinheimo O, Koponen P, et al. Hormonal contraception and mental health: results of a population-based study. Hum Reprod. 2011; 26 3085–93.
| Hormonal contraception and mental health: results of a population-based study.Crossref | GoogleScholarGoogle Scholar | 21840911PubMed |
[17] Toffol E, Heikinheimo O, Koponen P, et al. Further evidence for lack of negative associations between hormonal contraception and mental health. Contraception. 2012; 86 470–80.
| Further evidence for lack of negative associations between hormonal contraception and mental health.Crossref | GoogleScholarGoogle Scholar | 22465115PubMed |
[18] Nygaard Andersen M, Bech P, Csillag C. Development and remission of depressive symptoms and treatment with hormonal contraceptives. Oxf Med Case Reports. 2014; 2014 63–4.
| Development and remission of depressive symptoms and treatment with hormonal contraceptives.Crossref | GoogleScholarGoogle Scholar | 25988030PubMed |
[19] de Wit AE, Booij SH, Giltay EJ, et al. Association of use of oral contraceptives with depressive symptoms among adolescents and young women. JAMA Psychiatr. 2020; 77 52–9.
| Association of use of oral contraceptives with depressive symptoms among adolescents and young women.Crossref | GoogleScholarGoogle Scholar |
[20] Faculty of Sexual & Reproductive Healthcare Clinical Effectiveness Unit. Combined Hormonal Contraception Guideline. January 2019. London: Royal College of the Obstetricians and Gynaecologists; 2019.
[21] Kavanaugh ML, Jerman J, Hubacher D, et al. Characteristics of women in the United States who use long-acting reversible contraceptive methods. Obstet Gynecol. 2011; 117 1349–57.
| Characteristics of women in the United States who use long-acting reversible contraceptive methods.Crossref | GoogleScholarGoogle Scholar | 21606745PubMed |
[22] ACOG Committee Opinion no. 450: Increasing use of contraceptive implants and intrauterine devices to reduce unintended pregnancy Obstet Gynecol. 2009; 114 1434–38.
| 20134301PubMed |
[23] Lete I, del Carme Cuesta M, Marín J, et al. Acceptability of the levonorgestrel intrauterine system in the long-term treatment of heavy menstrual bleeding: how many women choose to use a second device? Eur J Obstet Gynecol Reprod Biol. 2011; 154 67–70.
| Acceptability of the levonorgestrel intrauterine system in the long-term treatment of heavy menstrual bleeding: how many women choose to use a second device?Crossref | GoogleScholarGoogle Scholar | 20728261PubMed |
[24] Baldaszti E, Wimmer-Puchinger B, Löschke K. Acceptability of the long-term contraceptive levonorgestrel-releasing intrauterine system (Mirena®): a 3-year follow-up study. Contraception. 2003; 67 87–91.
| Acceptability of the long-term contraceptive levonorgestrel-releasing intrauterine system (Mirena®): a 3-year follow-up study.Crossref | GoogleScholarGoogle Scholar | 12586318PubMed |
[25] Diaz J, Bahamondes L, Monteiro I, et al. Acceptability and performance of the levonorgestrel-releasing intrauterine system (Mirena®) in Campinas, Brazil. Contraception. 2000; 62 59–61.
| Acceptability and performance of the levonorgestrel-releasing intrauterine system (Mirena®) in Campinas, Brazil.Crossref | GoogleScholarGoogle Scholar | 11102588PubMed |
[26] Moreau C, Cleland K, Trussell J. Contraceptive discontinuation attributed to method dissatisfaction in the United States. Contraception. 2007; 76 267–72.
| Contraceptive discontinuation attributed to method dissatisfaction in the United States.Crossref | GoogleScholarGoogle Scholar | 17900435PubMed |
[27] Robinson R, China S, Bunkheila A, et al. Mirena® intrauterine system in the treatment of menstrual disorders: a survey of UK patients’ experience, acceptability and satisfaction. J Obstet Gynaecol. 2008; 28 728–31.
| Mirena® intrauterine system in the treatment of menstrual disorders: a survey of UK patients’ experience, acceptability and satisfaction.Crossref | GoogleScholarGoogle Scholar | 19065370PubMed |
[28] Wildemeersch D. Why perimenopausal women should consider to use a levonorgestrel intrauterine system. Gynecol Endocrinol. 2016; 32 659–61.
| Why perimenopausal women should consider to use a levonorgestrel intrauterine system.Crossref | GoogleScholarGoogle Scholar | 26930021PubMed |
[29] Admon L, Haefner JK, Kolenic GE, et al. Recruiting pregnant patients for survey research: a head to head comparison of social media-based versus clinic-based approaches. J Med Internet Res. 2016; 18 e326
| Recruiting pregnant patients for survey research: a head to head comparison of social media-based versus clinic-based approaches.Crossref | GoogleScholarGoogle Scholar | 28003174PubMed |
[30] Goldstuck ND. Modern menstruation: is it abnormal and unhealthy? Med Hypotheses. 2020; 144 109955
| Modern menstruation: is it abnormal and unhealthy?Crossref | GoogleScholarGoogle Scholar | 32526510PubMed |


