Chronic pain: good management of practical pain control strategies is associated with being older, more health activated and having better mental health
Claire Budge 1 4 , Melanie Taylor 1 , Materoa Mar 2 , Chiquita Hansen 1 , Folole Fai 31 THINK Hauora, 200 Broadway Avenue, Palmerston North 4410, New Zealand
2 Te Tihi o Ruahine Whānau Ora Alliance, 200 Broadway Avenue, Palmerston North 4410, New Zealand
3 MidCentral DHB, PO Box 2075, Palmerston North 4410, New Zealand
4 Corresponding author. Email: clairebudge@gmail.com
Journal of Primary Health Care 12(3) 225-234 https://doi.org/10.1071/HC19066
Published: 29 September 2020
Journal Compilation © Royal New Zealand College of General Practitioners 2020 This is an open access article licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License
Abstract
INTRODUCTION: Chronic pain affects a large proportion of the adult population and people in pain need to learn how to manage it in order to maintain quality of life.
AIM: This study aimed to examine how well people with long-term conditions make use of self-management strategies to control their pain, and to identify personal attributes associated with a higher degree of success.
METHODS: People with chronic pain who participated in the first phase of a longitudinal long-term conditions study in the MidCentral region made up the study sample (N = 326, response rate 21%). They completed a questionnaire by mail or online, which included items on pain management, general health, patient activation, experiences with general practice and demographics.
RESULTS: Pain control strategies were managed fairly well overall. Taking pain medication and avoiding caffeine, alcohol, heavy meals and exercise before bed were managed best, whereas sleep, relaxation breathing and remaining socially active were managed least well. A multiple regression analysis found higher scores on patient activation, self-rated overall pain management at home, mental health and older age (≥75 years) to be associated with better management.
DISCUSSION: This study identified pain control strategies that are managed well, and less well, outside of a specific intervention. Results highlight topics for discussion in consultations and identify areas where general practice could provide better self-management support, such as sleep and exercise. Better overall pain control strategy management was most strongly associated with patient activation; that is, a combination of knowledge, skills and confidence to manage health and health care that is amenable to intervention. Improving the level of activation in people with long-term conditions may enhance their use of pain control strategies.
KEYwords: Chronic pain; self-management; primary health care
| WHAT GAP THIS FILLS |
| What is already known: Chronic pain commonly accompanies other long-term conditions and affects ~20% of the adult population. Self-management is a recognised treatment approach for people living with chronic pain. |
| What this study adds: This non-intervention study explored self-management strategies people engage in to manage their pain. People who were older, had better mental health and were more highly activated were found to manage pain control strategies best. |
Introduction
Long-term conditions are ongoing or recurring and can have a significant effect on people’s lives.1 A common, but sometimes overlooked long-term condition is pain. One study of over 2000 people with chronic pain found that one in five people had not sought help from a physician.2 Chronic pain affects approximately one in six adult New Zealanders3 and its prevalence increases with age.4 Data from the New Zealand Health Surveys reveal a growing problem, as chronic pain prevalence rose from 17% in 2007 to 20% in 2016.5 Pain is complex to manage due to its psychological and functional effect6 and can be accompanied by acute episodes or flare-ups, causing deterioration in physical and mental functioning.7 It is also rarely the sole long-term condition people experience, but is a common comorbidity to others, including diabetes, respiratory and mental health conditions.8
The scarcity of specialist pain management services in this country9,10 and the sheer number of adults living with ongoing pain mean that most chronic pain management is provided through primary care, if at all. It is not just a New Zealand (NZ) problem, as it has been reported that although 20% of the adult population in the United Kingdom (UK) experience chronic pain, fewer than 2% ever attend a pain clinic.11 Medication can be integral to the treatment plan, but is often an incomplete solution, meaning that people need to adopt a range of approaches to pain management. Many people are doing so even if they are unaware of it.12 Self-management has become a key treatment modality for people with long-term conditions in general, as well as for people living with chronic pain.13 The history of the chronic illness self-management movement has been comprehensively described;14,15 the main premise being that increases in numbers of affected people, complexity and comorbidities experienced have led to a need for a more planned, collaborative and holistic approach to care than was traditionally provided through the medical acute care model.
There is a body of research considering the outcomes of specific pain intervention programmes, and a recent review of 33 such studies16 concluded that motivation to continue with suggested management techniques may wane over time in the absence of ongoing support. For people with chronic pain, self-management is an ongoing requirement involving ‘behaviors, strategies and activities that may help to control the destructive effects of pain on their quality of life’ (p. 46).17 Lorig and Holman describe three main aspects of pain self-management as medical management (medications, diet); adapting behaviours; and roles to keep functioning and dealing with emotional responses to having a long-term condition.18 A systematic review identified 13 studies, none based in NZ, and concluded that medication and physical strategies were the most common self-management options.19 Lewis and O’Sullivan propose that pain management should be aligned with management of other long-term conditions and include exercise, sleep hygiene, diet, stress management and lifestyle changes. They too note that ongoing self-management is essential.20
Less is known about the pain management strategies people successfully engage with in the absence of an intervention. Consequently, our interest was in the types of strategies people engage in at home on a day-to-day basis that do not require enrolment in a programme or attendance at a class. A set of practical pain control strategies was identified21 and the first aim of the current study was to examine how well people with long-term conditions use them in their daily lives.
Various factors can affect the success with which people engage in self-management behaviours. For example, pain is often accompanied by anxiety or depression, adding to functional impairment22–24 and potentially limiting successful self-management.25 A review of pain and depression comorbidity studies found 5% to 46% of primary care patients with pain also experience depression,22 and a NZ study found 32% of chronic pain participants to have depression and 33% to have anxiety.26 Other barriers to self-management include poor communication with doctors, fatigue, financial constraints and lack of family support.27 Enablers of self-management include level of health activation and engagement28 and support received from health practitioners.29 The second aim of the current study was to identify which of a range of patient health, health activation, general practice experience and demographic characteristics are associated with better management of pain control strategies to identify who manages best.
Methods
Participants
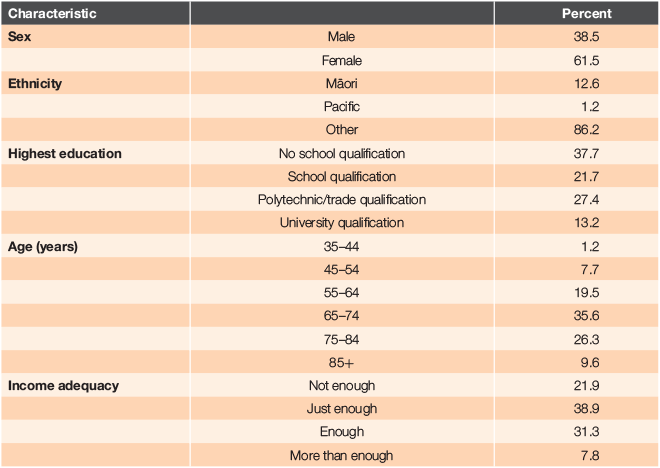
Study data were drawn from the ‘Talking about Health’ long-term conditions study being conducted within the MidCentral region. Invitation letters were sent to all people aged ≥18 years in the DHB region who had been enrolled in the Enhanced Care+ programme or who received a package of care from a Community Clinical Nurse specialising in long-term conditions management (CCN:LTC) during the previous three (Māori and Pasifika) or two (other ethnicities) years (N = 2730). The extended time period for Māori and Pacific people was implemented to achieve a better response rate for people of these ethnicities. After interested individuals returned consent forms, questionnaires were sent out and made available online. Questionnaires were returned by 569 people, 57% of whom indicated they had chronic pain. This latter group (N = 326) constitutes the sample for the present analyses and their demographic characteristics are summarised in Table 1.
|
More women than men participated; most were not of Māori or Pasifika ethnicity and most were aged 65–84 years. Just over one-third had no school qualifications and 61% had insufficient or only just enough income to meet their daily needs. The number of long-term conditions experienced ranged from 1 to 11 (M = 3.9, s.d. = 1.5). Pain as a sole condition was reported by 3.7%. Additional conditions included hypertension (56.7%), diabetes (47.2%), a respiratory condition (41.4%), anxiety/depression associated with having a long-term condition (26.0%), heart failure (11.0%) and chronic kidney disease (7.1%).
Materials
The questionnaire contained questions on general health, long-term conditions, pain, pain control strategies, patient activation, experiences with doctors and nurses in general practice and demographics.
Pain: A set of 16 pain control strategies (Box 1) was developed from a pain strategy wheel.21 Participants were asked how well they managed these strategies to help control their pain levels. Response options were: 1 = ‘not well managed’; 2 = ‘fairly well managed’; and 3 = ‘well managed’. A total pain management score was calculated by averaging the scores on all items. The total score was calculated enabling two items to be missing, thus retaining 94% of participants for analysis. An overall (0–10) rating of how well people felt they were managing their pain at home was added. The level of pain experienced in the last week was measured by the Patient-Reported Outcomes Measurement Information System (PROMIS).30
| Box 1. Pain control strategy questions |
Question stem: How well do you manage these things to help control your pain levels? |
| Response options: Not well managed (1); Fairly well managed (2); Well managed (3) |
Items:
|
General health and long-term conditions: The general health question plus the overall mental and physical health scales from the PROMIS global short form (SF)30 were used to measure health. A list of long-term conditions was provided on the consent form, with a space for others to be added. The list included anxiety or depression as a result of having one or more long-term conditions.
Patient activation: Participants’ knowledge, skill, and confidence in managing their health were assessed using the 13-item Patient Activation Measure (PAM).31 Respondents indicated their level of agreement with 13 statements using a four-point scale ranging from ‘disagree strongly’ to ‘agree strongly’ scored as 1 to 4, or ‘not applicable’. Following the PAM guidelines, activation scores were calculated with a potential range of 0 to 100. PAM scores can then be classified into four levels: level 1 (<47.0), level 2 (47.1–55.1), level 3 (55.2–67.0) and level 4 (>67.0).28 People at lower levels tend to have less understanding of their conditions and are more passive recipients of care, viewing self-management as compliance, whereas people at higher levels work more actively in partnership with health professionals.28
General practice experiences: These were assessed in relation to doctors and nurses separately, using nine questions from the NZ version of the General Practice Assessment Questionnaire.32 Minor wording changes were made and five additional questions were developed by the study team. The item stem was ‘when you see the doctor (nurse) at your practice, how good are they at …’ and items covered various aspects of the consultation including listening, spending enough time, being patient and knowing you as an individual. Response options ranged from excellent (6) to very poor (1). Two of the 14 questions – relating to involvement of family or whānau in decision-making and learning about social support needs – were considered not applicable by several respondents, and the General Practitioner and Nurse scales were calculated allowing two missing responses to accommodate this.
Demographics: Ethnicity was measured as in the New Zealand Health Study,33 and participants were allowed to select more than one option. Anyone identifying as Māori or Māori and one or more ethnicities was counted as Māori and ethnicity was coded as Māori or non-Māori for analyses. Age and income adequacy were measured, as reported in Table 1, and age was collapsed into three groups (≤64, 65–74 and ≥75 years).
This project received ethics approval from the Health and Disability Ethics Committee (ref. 16/NTA/32). Data were analysed using SPSS Statistics 20 (SPSS Inc., Chicago, IL, USA).
Results
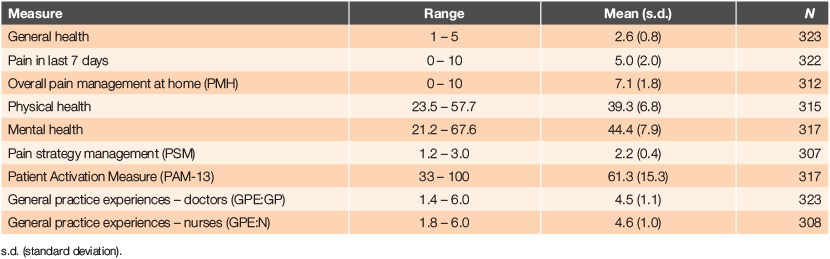
Descriptive statistics for the main variables are shown in Table 2. The varying number of responses to measures reflects missing data.
|
Both GP and nurse experience scores were relatively high, with interactions with nurses rated slightly higher than interactions with GPs. Regular pain medication was taken by 70.4% of respondents. Anxiety/depression associated with having a long-term condition was reported by 26.1%.
Patient activation scores ranged from 33 to 100, with 90% providing scores of 35–95. When the scores were converted into levels, 16.1% of respondents were at Level 1, 18.0% at Level 2, 45.1% at Level 3 and 20.8% at Level 4. The patient activation measure had an acceptable α of .89.
Pain management
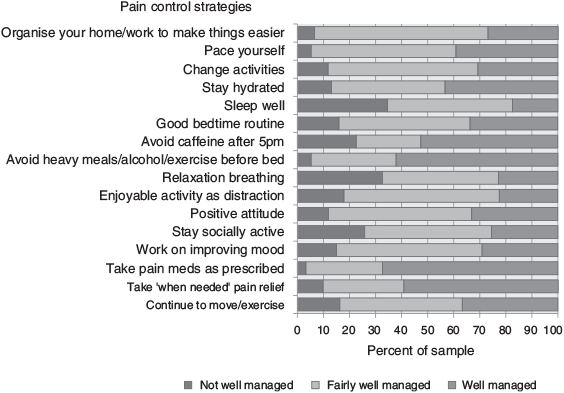
Responses to the pain control strategies are presented in Figure 1. Taking pain medication and avoiding caffeine, alcohol, heavy meals and exercise before bed were managed best, whereas sleep, relaxation breathing and remaining socially active were managed least well. Other strategies generally managed fairly well, but indicating scope for improvement, were organisation of the home environment and changing activities to avoid repeated movement. Total pain management scores ranged from 1.2 to 3.0 (M = 2.2, s.d. = 0.4) and the pain management scale had an acceptable level of internal consistency (α = .82). The number of responses to items was variable; the lowest relating to the use of additional pain medication as needed.
|
Correlates of pain management
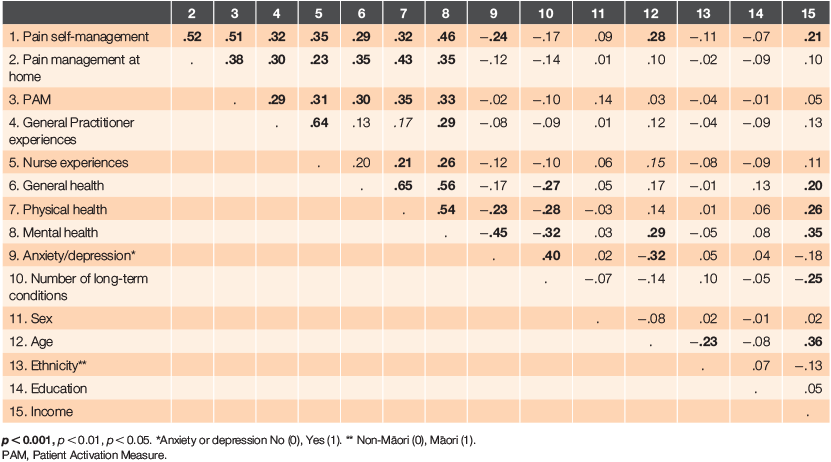
To explore characteristics related to better engagement in pain self-management behaviours, correlations were first used to examine associations between all the variables of interest (Table 3). Pearson’s correlations were used for the interval level and dichotomous variables and Spearman’s rho for age, education and income.
|
The bivariate results demonstrate weak-to-moderate correlations between pain strategy management and a set of nine variables (age, health, patient activation, overall pain management at home, anxiety/depression and general practice experiences). Table 3 shows these variables were correlated with each other and with some demographic variables, so a standard regression analysis was conducted to control for confounding.
Variables were included in the regression model without transformation, except for age, education and income where dummy variables were created. The lowest levels were used as the reference groups. Assumptions for multiple regression were adequately met with residuals relatively normally distributed, predictors having linear and homoscedastic relationships and no apparent outliers.
The regression results are provided in Table 4 and suggest that, in order of contribution, patient activation, pain management at home, mental health and being aged ≥75 years are significantly associated with pain strategy management. Controlling for the correlations between variables resulted in general health, physical health and general practice experiences no longer being significantly associated with pain strategy management.
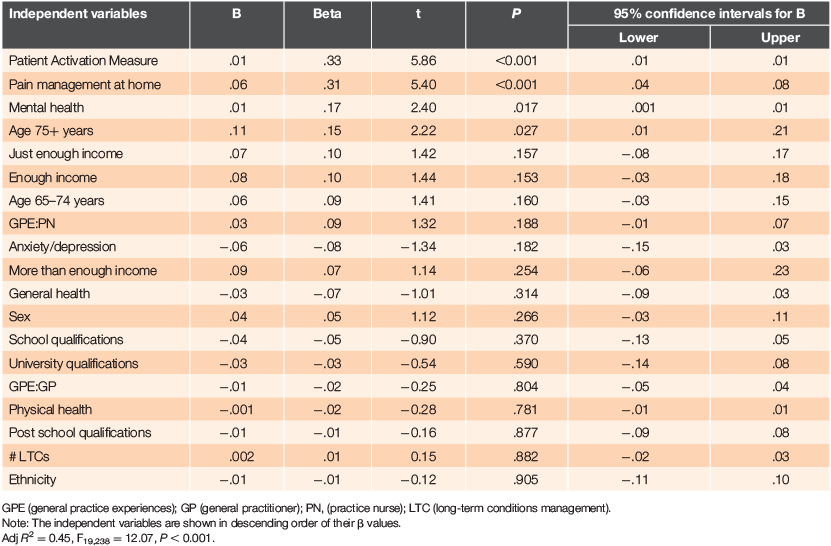
|
Discussion
The first aim of the present study was to explore the pain control strategies used by people with chronic pain. No definitive list of self-management strategies was identified, and the UK’s National Institute for Health and Care Excellence34 is currently developing a chronic pain assessment and management guideline. However, the types of recommended activities include physical activity, diet, sleep and psychological processes, all included in the practical strategies used for this study.
One of the strategies managed best was use of pain medication, although one-third of the respondents indicated their prescribed pain medication was not well managed. Pain medication is often not taken as prescribed, possibly because it is considered less important than medication for other conditions.35 It is possible that there is poor understanding about the need for pain medication to be taken regularly to be maximally effective, or perhaps pain is more often considered ‘normal’ for older adults.35,36
Although, on average, people felt they were managing sleep hygiene well with respect to avoiding caffeine, heavy meals, alcohol and exercise before bed, good sleep was not well managed by over one-third of participants. Sleep disturbances affect 50–80% of people with chronic pain, yet assessment of sleep in patients with pain,37 or patients in general,38 is often overlooked in clinical encounters. There is evidence to suggest that the association between pain and sleep is reciprocal, but that although sleep management can significantly improve pain perception and function, better pain control does not always benefit insomnia.39
Continuing to move and exercise was managed less well by approximately two-thirds of the sample. This is not surprising as there is likely to be reluctance to engage in activities that increase pain. Some people fear doing more musculoskeletal damage if they exercise,40,41 which can also lead to activity avoidance. However, exercise can be beneficial for controlling pain,42 with painful therapeutic exercise potentially having advantages over non-painful exercise.43 A guide for approaching discussions, aiding understanding and allaying fears is provided by Smith et al.43
In considering the characteristics of patients who were good pain strategy managers, the regression analysis primarily identified them to be more health activated. There is a growing body of evidence to suggest that more activated individuals take more control of their own management,28 activation levels are amenable to intervention44 and that activation levels are linked to health outcomes, costs and experiences of care.45,46 Other characteristics of good managers were that they perceived themselves to be managing their pain better at home, to have better mental health and were older than people managing less well.
The connection between depression and pain is well established, and in this sample, 26% reported experiencing long-term condition-related anxiety or depression. Although self-reported anxiety or depression did not contribute significantly to the regression model, mental health was significantly associated with better management of pain control strategies. Feeling more positive may enable people to engage more actively with helpful pain management activities which in turn may engender further positivity. Conversely, feeling anxious or depressed may act as a de-motivator, making it difficult to engage socially or invest effort into achieving goals in the presence of pain. From a clinical perspective, Bair et al.22 describe a reciprocal relationship between depression and pain, stating ‘depression complicates the management of patients with pain and is associated with poorer outcomes. In patients with pain, depression is associated with more pain complaints, greater pain intensity, longer duration of pain, and greater likelihood of nonrecovery’ (p. 2442). Effort invested into either side of this equation may therefore assist patients to manage both their pain levels and the anxiety/depression associated with living in pain.
People aged 75+ years were better able to engage in pain self-management strategies supporting the finding that people report learning from experience about their own bodies and how to manage their pain.12 Older people may also have fewer work and family commitments and are consequently freed up to focus on their own health needs.
A surprising finding was the absence of a relationship between strategy management and general practice experiences as providing self-management support should be an integral part of general practice care. However, it has been suggested that the New Zealand health system tends to encourage passivity and a sense of failure rather than supporting the development of ‘expert’ patients.47 The time limitations of general practice appointments may act as a barrier to in-depth discussion or support for self-management, meaning that better self-managers are obtaining support from other sources or are more highly activated and independent in relation to their own health.
Limitations for this study include its cross-sectional design, meaning that the identified relationships cannot be interpreted as causal. For example, it is unclear whether people who are more highly activated engage in better self-management activities or whether better engagement leads to higher levels of activation, or both.48 Additionally, the study relied on self-report data, which are prone to biases, but was necessary to capture beliefs and experiences. The low response rate could also be considered a limitation, but the overall number of respondents was sufficient for the analyses undertaken.
To support people with chronic pain, clinicians must first be aware of who has it. Are patients specifically and routinely asked if they have pain? It is likely that many patients with chronic pain have felt they just had to put up with it or prioritised another concern during consultations. The review by Devan et al. identified a therapeutic alliance with clinicians enabled ongoing pain self-management.16 Using a pre-consultation sheet49,50 enables people to identify their health needs and may be of use.
The study responses suggest interventions could be conducted by primary care practitioners with respect to the common combination of pain, sleep disruption and anxiety/depression. One-third of participants indicated that their use of pain medication was not well managed; some of these medications will be targeted at improving sleep. The link between pain and poor sleep may not be openly discussed, but each can affect the other. Remaining socially active was one of the strategies managed least well; promoting the benefits of social engagement would be helpful, especially if social activities could be combined with exercise. The results suggest that encouraging active engagement with pain management strategies, particularly in younger pain sufferers and people with poorer mental health, may be beneficial. Greatest effect may come from identifying how health care can provide effective self-management support to individuals and family or whānau with ongoing pain, as this is likely to lead to increased patient activation. In our region, work is underway to build an improved pathway for people with chronic pain. The Chronic Pain Self Management Programme51 is now being offered in primary care and the use of tools and resources for both patients and health practitioners are promoted through the NZ website, Health Navigator. Although this is a good start, the next steps need to include patient identification, discussion during consultations and the development of active self-management support by primary care clinicians.
Competing interests
The authors declare no competing interests.
Acknowledgements
Funding for this study was provided by the Central Primary Health Organisation (THINK Hauora).
References
[1] NHC. Meeting the needs of people with chronic conditions. Wellington: National Health Committee; 2007. [cited 2020 June 24]. Available from: https://www.health.govt.nz/system/files/documents/publications/meeting-needs-chronic-conditions-feb07.pdf[2] Watkins E, Wollan PC, Melton LJ, et al. Silent pain sufferers. Mayo Clin Proc. 2006; 81 167–71.
| Silent pain sufferers.Crossref | GoogleScholarGoogle Scholar | 16471069PubMed |
[3] Health Navigator. Chronic pain. 2018. [cited 2019 June 12]. Available from: https://www.healthnavigator.org.nz/health-a-z/c/chronic-pain/
[4] Dominick C, Blyth F, Nicholas M. Patterns of chronic pain in the New Zealand population. N Z Med J. 2011; 124 63–76.
| 21946879PubMed |
[5] Ministry of Health. Annual data explorer 2016/17: New Zealand Health Survey [data file]. [cited 2019 June 30]. Available from: https://www.health.govt.nz/publication/annual-update-key-results-2016-17-new-zealand-health-survey
[6] Hariharan J, Lamb GC, Neuner JM. Long-term opioid contract use for chronic pain management in primary care practice: a five year experience. J Gen Intern Med. 2007; 22 485–90.
| Long-term opioid contract use for chronic pain management in primary care practice: a five year experience.Crossref | GoogleScholarGoogle Scholar | 17372797PubMed |
[7] McGhie J, Grady K. Where now for UK chronic pain management services? Br J Anaesth. 2016; 116 159–62.
| Where now for UK chronic pain management services?Crossref | GoogleScholarGoogle Scholar | 26787786PubMed |
[8] Davis JA, Robinson RL, Le TK, et al. Incidence and impact of pain conditions and comorbid illnesses. J Pain Res. 2011; 4 331–45.
| Incidence and impact of pain conditions and comorbid illnesses.Crossref | GoogleScholarGoogle Scholar | 22090802PubMed |
[9] Shipton EA. Recognition of the vocational practice of the scope of Pain Medicine in New Zealand. N Z Med J. 2012; 126 5–8.
| 23385829PubMed |
[10] Swain N, Parr-Brownlie LC, Thompson BL, et al. Six things you need to know about pain. N Z Med J. 2018; 131 5–8.
| 30496161PubMed |
[11] Smith BH, Torrance N. Management of chronic pain in primary care. Curr Opin Support Palliat Care. 2011; 5 137–42.
| Management of chronic pain in primary care.Crossref | GoogleScholarGoogle Scholar | 21415754PubMed |
[12] MacKichan F, Paterson C, Britten N. GP support for self-care: the views of people experiencing long-term back pain. Fam Pract. 2013; 30 212–8.
| GP support for self-care: the views of people experiencing long-term back pain.Crossref | GoogleScholarGoogle Scholar | 23042439PubMed |
[13] Lukewich J, Mann E, WanDenKerkhof E, et al. Self-management support for chronic pain in primary care: a cross-sectional study of patient experiences and nursing roles. J Adv Nurs. 2015; 71 2551–62.
| Self-management support for chronic pain in primary care: a cross-sectional study of patient experiences and nursing roles.Crossref | GoogleScholarGoogle Scholar | 26118587PubMed |
[14] Cramm JM, Nieboer AP. Disease management: the need for a focus on broader self-management abilities and quality of life. Popul Health Manag. 2015; 18 246–55.
| Disease management: the need for a focus on broader self-management abilities and quality of life.Crossref | GoogleScholarGoogle Scholar | 25607246PubMed |
[15] Grady PA, Gough LL. Self-management: a comprehensive approach to management of chronic conditions. Am J Public Health. 2014; 104 e25–31.
| Self-management: a comprehensive approach to management of chronic conditions.Crossref | GoogleScholarGoogle Scholar | 24922170PubMed |
[16] Devan H, Hale L, Hempel D, et al. What works and does not work in a self-management intervention for people with chronic pain? Qualitative Systematic Review and Meta-Synthesis. Phys Ther. 2018; 98 381–97.
| What works and does not work in a self-management intervention for people with chronic pain? Qualitative Systematic Review and Meta-Synthesis.Crossref | GoogleScholarGoogle Scholar | 29669089PubMed |
[17] Turner BJ, Ogbeide S. Self-management of chronic pain: a plan for primary care. Pract Pain Manag. 2018; 18 45–50.
[18] Lorig KR, Holman HR. Self-management education: history, definition, outcomes and mechanisms. Ann Behav Med. 2003; 26 1–7.
| Self-management education: history, definition, outcomes and mechanisms.Crossref | GoogleScholarGoogle Scholar | 12867348PubMed |
[19] Bemis L, Harper B, Molla-Hosseini S. Self-management strategies for chronic pain reported in population-based surveys: a systematic review. Final Report. The University of Arizona; 2017. [cited 2019 July 31]. Available from: https://repository.arizona.edu/handle/10150/624027
[20] Lewis J, O’Sullivan P. Is it time to reframe how we care for people with non-traumatic musculoskeletal pain? Br J Sports Med. 2018; 52 1543–4.
| Is it time to reframe how we care for people with non-traumatic musculoskeletal pain?Crossref | GoogleScholarGoogle Scholar | 29941618PubMed |
[21] Wanlass R, Fishman D. Strategy wheel. [cited 2019 July 20]. Available from: http://www.ucdmc.ucdavis.edu/nursing/Research/INQRI_Grant/strategy_wheel/index.html
[22] Bair MJ, Robinson RL, Katon W, et al. Depression and pain comorbidity: a literature review. Arch Intern Med. 2003; 163 2433–45.
| Depression and pain comorbidity: a literature review.Crossref | GoogleScholarGoogle Scholar | 14609780PubMed |
[23] Gambassi G. Pain and depression: the egg and the chicken story revisited. Arch Gerontol Geriatr. 2009; 49 103–12.
| Pain and depression: the egg and the chicken story revisited.Crossref | GoogleScholarGoogle Scholar | 19836622PubMed |
[24] Gordon K, Rice H, Allcock N, et al. Barriers to self-management of pain in primary care: a qualitative focus group study. Br J Gen Pract. 2017; 67 e209–17.
| Barriers to self-management of pain in primary care: a qualitative focus group study.Crossref | GoogleScholarGoogle Scholar | 27993899PubMed |
[25] Bair MJ, Matthias MS, Nyland KA, et al. Barriers and facilitators to chronic pain self-management: a qualitative study of primary care patients with comorbid musculoskeletal pain and depression. Pain Med. 2009; 10 1280–90.
| Barriers and facilitators to chronic pain self-management: a qualitative study of primary care patients with comorbid musculoskeletal pain and depression.Crossref | GoogleScholarGoogle Scholar | 19818038PubMed |
[26] Swain N, Johnson M. Chronic pain in New Zealand: a community sample. N Z Med J. 2014; 127 21–30.
| 24481383PubMed |
[27] Jerant AF, von Friederichs-Fitzwater MM, Moore M. Patients’ perceived barriers to self-management of chronic conditions. Patient Educ Couns. 2005; 57 300–7.
| Patients’ perceived barriers to self-management of chronic conditions.Crossref | GoogleScholarGoogle Scholar | 15893212PubMed |
[28] Dixon A, Hibbard J, Tussler M. How to people with different levels of activation self-manage their chronic conditions? Patient. 2009; 2 257–68.
| How to people with different levels of activation self-manage their chronic conditions?Crossref | GoogleScholarGoogle Scholar | 22273246PubMed |
[29] Health Foundation. Helping people helping themselves: a review of the evidence considering whether it is worthwhile to support self management. London: Health Foundation; 2011. [cited 2019 August 1]. Available from: https://www.health.org.uk/sites/default/files/HelpingPeopleHelpThemselves.pdf
[30] Hays RD, Bjorner J, Revicki RA, et al. Development of physical and mental health summary scores from the Patient Reported Outcomes Measurement Information System (PROMIS) global items. Qual Life Res. 2009; 18 873–80.
| Development of physical and mental health summary scores from the Patient Reported Outcomes Measurement Information System (PROMIS) global items.Crossref | GoogleScholarGoogle Scholar | 19543809PubMed |
[31] Hibbard JH, Mahoney ER, Stockard J, et al. Development and testing of a short form of the Patient Activation Measure. Health Serv Res. 2005; 40 1918–30.
| Development and testing of a short form of the Patient Activation Measure.Crossref | GoogleScholarGoogle Scholar | 16336556PubMed |
[32] Zwier G. A standardized and validated patient survey in primary care: introducing the New Zealand General Practice Assessment Questionnaire. N Z Med J. 2013; 126 47–54.
| 23793177PubMed |
[33] Ministry of Health. Content Guide 2013/14: New Zealand Health Survey. Wellington: Ministry of Health; 2014. [cited 2019 August 1]. Available from: https://www.health.govt.nz/system/files/documents/publications/content-guide-2013-14-nzhs-dec14-v3.pdf
[34] National Institute for Health and Care Excellence. Guideline Scope. Chronic pain: assessment and management. London, England; 2020. [cited 2020 June 24]. Available from: https://www.nice.org.uk/guidance/gid-ng10069/documents/final-scope
[35] Budge C, Carryer J, Boddy J. Learning from people with chronic pain: messages for primary care practitioners. J Prim Health Care. 2012; 4 306–12.
| Learning from people with chronic pain: messages for primary care practitioners.Crossref | GoogleScholarGoogle Scholar | 23205380PubMed |
[36] Molton I, Jensen MP, Ehde DM, et al. Coping with chronic pain amongst younger, middle-aged and older adults living with neurological injury and disease. J Aging Health. 2008; 20 972–96.
| Coping with chronic pain amongst younger, middle-aged and older adults living with neurological injury and disease.Crossref | GoogleScholarGoogle Scholar | 18791184PubMed |
[37] Cheatle MD, Foster S, Pinkett A, et al. Assessing and managing sleep disturbance in patients with chronic pain. Anesthesiol Clin. 2016; 34 379–93.
| Assessing and managing sleep disturbance in patients with chronic pain.Crossref | GoogleScholarGoogle Scholar | 27208716PubMed |
[38] Jank R, Gallee A, Boeckle M, et al. Chronic pain and sleep disorders in primary care. Pain Res Treat. 2017; 2017 9081802
| Chronic pain and sleep disorders in primary care.Crossref | GoogleScholarGoogle Scholar | 29410915PubMed |
[39] Koffel E, Kroenke K, Bair MJ, et al. The bidirectional relationship between sleep complaints and pain: analysis of data from a randomized trial. Health Psychol. 2016; 35 41–9.
| The bidirectional relationship between sleep complaints and pain: analysis of data from a randomized trial.Crossref | GoogleScholarGoogle Scholar | 26076002PubMed |
[40] Booth J, Moseley GL, Schiltenwolf M, et al. Exercise for chronic musculoskeletal pain: a biopsychosocial approach. Musculoskeletal Care. 2017; 15 413–21.
| Exercise for chronic musculoskeletal pain: a biopsychosocial approach.Crossref | GoogleScholarGoogle Scholar | 28371175PubMed |
[41] Bee P, McBeth J, MacFarlane GJ, et al. Managing chronic widespread pain in primary care: a qualitative study of patient perspectives and implications for treatment delivery. BMC Musculoskelet Disord. 2016; 17 354
| Managing chronic widespread pain in primary care: a qualitative study of patient perspectives and implications for treatment delivery.Crossref | GoogleScholarGoogle Scholar | 27549811PubMed |
[42] Daenen L, Varkey E, Kellmann M, et al. Exercise, not to exercise, or how to exercise in patients with chronic pain? Applying Science to Practice. Clin J Pain. 2015; 31 108–14.
| Exercise, not to exercise, or how to exercise in patients with chronic pain? Applying Science to Practice.Crossref | GoogleScholarGoogle Scholar | 24662498PubMed |
[43] Smith BE, Hendrick P, Bateman M, et al. Musculoskeletal pain and exercise—challenging existing paradigms and introducing new. Br J Sports Med. 2019; 53 907–12.
| Musculoskeletal pain and exercise—challenging existing paradigms and introducing new.Crossref | GoogleScholarGoogle Scholar | 29925503PubMed |
[44] Solomon M, Wagner SL, Goes J. Effects of a web-based intervention for adults with chronic conditions on patient activation: online randomized controlled trial. J Med Internet Res. 2012; 14 e32
| Effects of a web-based intervention for adults with chronic conditions on patient activation: online randomized controlled trial.Crossref | GoogleScholarGoogle Scholar | 22353433PubMed |
[45] Hibbard JH, Greene J. What the evidence shows about patient activation: better health outcomes and care experiences; fewer data on costs. Health Aff. 2013; 32 207–14.
| What the evidence shows about patient activation: better health outcomes and care experiences; fewer data on costs.Crossref | GoogleScholarGoogle Scholar |
[46] Greene J, Hibbard JH, Sacks R, et al. When patient activation levels change, health outcomes and costs change too. Health Aff (Millwood). 2015; 34 431–7.
| When patient activation levels change, health outcomes and costs change too.Crossref | GoogleScholarGoogle Scholar | 25732493PubMed |
[47] Francis H, Carryer J, Wilkinson J. Patient expertise: contested territory in the realm of long term condition care. Chronic Illn. 2019; 15 197–209.
| Patient expertise: contested territory in the realm of long term condition care.Crossref | GoogleScholarGoogle Scholar | 29466874PubMed |
[48] Mosen DM, Schmittdiel J, Hibbard J, et al. Is patient activation associated with outcomes of care for adults with chronic conditions? J Ambul Care Manage. 2007; 30 21–9.
| Is patient activation associated with outcomes of care for adults with chronic conditions?Crossref | GoogleScholarGoogle Scholar | 17170635PubMed |
[49] Lee YK, Ng CJ, Low WY. Addressing unmet needs of patients with chronic diseases: impact of the VISIT website during consultations. J Eval Clin Pract. 2017; 23 1281–8.
| Addressing unmet needs of patients with chronic diseases: impact of the VISIT website during consultations.Crossref | GoogleScholarGoogle Scholar | 28585242PubMed |
[50] Zanini C, Maino P, Moller JC, et al. Enhancing clinical decisions about care through a pre-consultation sheet that captures patients’ views on their health conditions and treatments: a qualitative study in the field of chronic pain. Patient Educ Couns. 2016; 99 747–53.
| Enhancing clinical decisions about care through a pre-consultation sheet that captures patients’ views on their health conditions and treatments: a qualitative study in the field of chronic pain.Crossref | GoogleScholarGoogle Scholar | 26673108PubMed |
[51] Self-Management Resource Center. Chronic Pain Self-Management Program. Aptos, California. [cited 2020 July 21]. Available from: https://www.selfmanagementresource.com/programs/small-group/chronic-pain-self-management/


