Using the Pharmaceutical Collection Database to identify patient adherence to oral hypoglycaemic medicines
Mangesh D. Kharjul 1 4 , Claire Cameron 2 , Rhiannon Braund 31 School of Pharmacy, University of Otago, Dunedin, New Zealand
2 Dean’s Department, Dunedin School of Medicine, University of Otago, Dunedin, New Zealand
3 New Zealand Pharmacovigilance Centre, University of Otago, Dunedin, New Zealand
4 Corresponding author. Email: khama123@student.otago.ac.nz
Journal of Primary Health Care 11(3) 265-274 https://doi.org/10.1071/HC19017
Published: 30 September 2019
Journal Compilation © Royal New Zealand College of General Practitioners 2019 This is an open access article licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License
Abstract
INTRODUCTION: Poor adherence to oral hypoglycaemic medicines is a key contributor to therapy failure and sub-optimal glycaemic control among people with type 2 diabetes. It is unclear how commonly non-adherence to oral hypoglycaemics occurs in the general population. This information is essential to design and implement local adherence strategies.
AIM: This study aimed to determine levels of sub-optimal adherence and identify patient groups who may need additional adherence support.
METHODS: The dispensing data of 340,283 patients from one District Health Board was obtained from the Pharmaceutical Collection Database for the period 2008–15. Of these, 12,405 patients received oral hypoglycaemic therapy during the study period. The proportion of days covered (PDC) was calculated for patients with complete data and a PDC value of ≥80% was used to indicate sufficient adherence. Patient demographics (gender, ethnicity, age, socioeconomic status) and therapy type (mono- or combination) were described.
RESULTS: Overall, 54.5% of the patients were found to have a PDC of <80% and so were considered non-adherent. Non-adherence was significantly higher in patients receiving combination oral hypoglycaemic therapy than monotherapy; in male patients; in New Zealand Māori patients; and in patients with higher socioeconomic deprivation.
DISCUSSION: In the study region, non-adherence to oral hypoglycaemic medicines was significant and widespread. Identification of such patients is important so that strategies to enhance adherence can be implemented. Prescribers need to be encouraged to optimise monotherapy before the addition of another oral hypoglycaemic, and adherence support services should be offered not only to older patients.
KEYWORDS: Pharmaceutical Collection Database; oral hypoglycaemics; adherence
| WHAT GAP THIS FILLS |
| What is already known: Poor adherence to oral hypoglycaemic medicines is a key contributor to therapy failure and sub-optimal glycaemic control. It is unclear how common non-adherence to prescribed oral hypoglycaemics is in the general population. |
| What this study adds: By using regional level data, the degree of adherence to oral hypoglycaemic medications can be identified. This information can inform local strategies to be implemented that will target those patients who would benefit from adherence support services. |
Introduction
The high prevalence of type 2 diabetes continues to be of increasing international concern and it needs multiple strategies to achieve glycaemic control to avoid future complications.1,2 Type 2 diabetes is a truly global epidemic, from both a health outcome perspective and its associated health-care costs.3,4
Oral hypoglycaemic medications are the mainstay therapy for type 2 diabetes in conjunction with diet and exercise modifications.5–7 However, adherence to these medications continues to be sub-optimal, so health services are concerned with finding ways to improving patient adherence.8–10 Abnormally high levels of HbA1c (haemoglobin A1c) are a marker for hyperglycaemia. Type 2 diabetes treatment aims to achieve glycaemic control that can be reflected by change in HbA1c. Therapeutic outcomes of oral hypoglycaemics are measured by reductions in the level of HbA1c. One driver for increasing type 2 diabetes medication adherence is that there is clear evidence that increased adherence to these medications can decrease the HbA1c level over the time.11–13
Inadequate adherence to long-term medication is a recognised problem that must be addressed if benefits of medication are to be achieved.14–18 For this reason, adherence support services are increasing internationally, as individualised strategies may increase medication adherence.19–21 In 2007, the government-funded Medication Use Review service was introduced in New Zealand; however, uptake across District Health Boards was haphazard.22 A recent New Zealand study found that type 2 diabetic patients who received Medication Use Review adherence support improved their adherence scores and reduced their HbA1c levels.23 However, remaining unknown are adherence levels in local populations and the number of type 2 diabetes patients who would benefit from access to this service.
Representative samples of adequate size are needed to deliver valid findings from epidemiologic observational research that is generalizable to all populations.24,25 Administrative databases can fulfil data requirements for such research as they are the archives of health-care data obtained at a variety of occasions, including the prescription dispensing in community pharmacies, visits to physicians’ offices and admissions to hospitals.24,26 Health-care administrative data are also referred to as ‘claims data’, ‘administrative claims data’, ‘administrative healthcare billing records’ and ‘healthcare utilization data’.26 Their timely and systematic collection, wide coverage and large numbers are the main advantages qualifying these databases as a principal choice for epidemiological studies regarding drug utilisation.26–28
The proportion of days covered (PDC) is a surrogate marker for medication adherence, providing information about patients’ medication possession (number of days they had medication supply). Using the Pharmaceutical Collection Database, this study aimed to estimate the PDC in patients with type 2 diabetes receiving oral hypoglycaemics over an 8-year period (2008–15), and further identify characteristics such as age, gender, ethnicity and socioeconomic status. The intent was to determine the degree of adherence in one District Heath Board (DHB) and to estimate the number of patients who could derive benefit from adherence support services.
Methods
In New Zealand, each patient has a unique health identifier (the National Health Index (NHI) code). All medicines dispensed for individual patients under the government supply schedule are held in a central database, maintained by the Ministry of Health. In New Zealand, there are 20 DHBs that divide the country into 20 non-overlapping geographical areas, and each DHB has a degree of autonomy in providing health services. This study was set in one DHB and involves the population it covers in an area of 12,231 km2. Records for a total of 340,283 patients were accessed. This region was chosen as it is one of the few DHBs providing Medicines Use Review adherence support services to patients.
Data
We made a request to the Ministry of Health to access the Pharmaceutical Collection Database for the study region, for the period 2008–15 (inclusive). In the data we obtained, individual patients’ information was irreversibly de-identified, but individual level data were used as an encrypted NHI was provided. De-identification and encryption occurred before the data were sent to the research team so the data could not be linked back to identify individual patients. The study data included patients’ encrypted identity, age, gender, ethnicity, and medication dispensing dates, quantity dispensed, daily dose, total number of days’ supply, and chemical and therapeutic medication classification. All patients receiving oral hypoglycaemic medications within the study period were included. The Pharmaceutical Collection Database records only government-subsidised oral hypoglycaemic medications and has no information about non-subsidised ones.
Outcomes
There were two categories of information: (1) patient characteristics including gender, ethnicity, age group, age range and socioeconomic deprivation index; (2) type of therapy (monotherapy or combination of two or three medications).
The type of therapy consistently dispensed during the study period was included and categories of monotherapy or combination of therapy were based on counts of oral hypoglycaemic medications not in the therapeutic category of oral hypoglycaemic. A change or substitution of oral hypoglycaemic medication was not considered as long as the type of therapy stayed the same. Medication non-adherence in combination therapy was related to the count of oral hypoglycaemics and not individual drugs in the combination.
Adherence to the oral hypoglycaemics was the primary outcome of interest, assessed by calculating the PDC for each patient. The nature of medication refill is highlighted by this ratio, as it shows how often patients refill their medications.
The PDC in a year with oral hypoglycaemic medication was calculated using the following equation:29

In this equation, ‘D’ is days on which the patient has the medication available (total days’ supply including any overlapping supply from earlier fills); ‘–1’ is for a day of last dispensing. Most published literature observes the PDC of ≥80% as indicating adherence and patients with a PDC >90% achieve maximum benefit from their medication.14,16,29–33 A PDC of <50% indicates insufficient medication supply to achieve therapeutic benefits. Therefore, we categorised PDCs <50% as ‘extremely non-adherent’, PDCs 50–79.99% as ‘non-adherent’, PDCs 80–89.99% as ‘adherent’ and PDCs ≥90% as completely adherent.
As this study was over a defined period, the actual date of therapy initiation for each patient was unknown when it was before 1 January 2008. For consistency, we considered the first recorded dispensing of oral hypoglycaemic as index dispensing. Not all patients had their index dispensing on 1 January of every calendar year. Some patients had their index dispensing in the middle of the year and last dispensing extending over a period of years or months. To ensure that all analysis was consistent, the Pharmaceutical Collection data records were combined from 2008 to 2015 and days between different dispensings were added cumulatively. Dividing this cumulative total by 365 days produced the yearly analysis. Others have used different timeframes to measure adherence, and the most frequently used is 12 months.34 In the last study year, the cumulative days may be less than 365, as dispensing records may be available for only a few months. Incomplete data may lead to the calculation of false PDCs in the last year of study, and it may influence the average value of the PDCs for overall study years. Hence, the proportion of days that covered the analysis period was capped up to the last full year to avoid false medication adherence reports. A summary of the data filtering process is shown in Figure 1.

|
Data analysis
Microsoft Excel (Microsoft Corporation, Redmond, WA, USA) and IBM SPSS Statistics version 23 (SPSS Inc., Chicago, IL, USA) were used to collate and analyse the data. The test of the difference between two proportions was used for all comparisons. First, patients receiving and not receiving oral hypoglycaemics were compared, then further non-adherent (PDC <80%) and adherent (PDC ≥80%) patients were compared. Statistical significance was taken to be P < 0.05.
Results
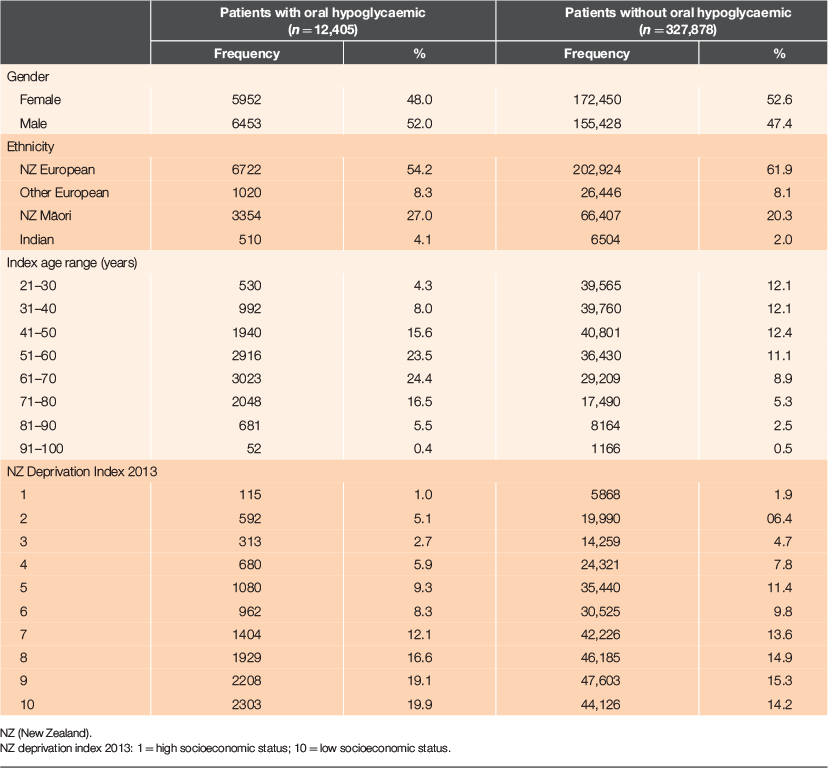
The demographics of patients receiving and not receiving oral hypoglycaemics is shown in Table 1. Use of oral hypoglycaemics was higher in male patients (52.0% vs. 47.4%, P < 0.001), patients of New Zealand Māori ethnicity (27% vs. 20.3%, P < 0.001), patients aged 41–80 years and in patients living in areas with higher socioeconomic deprivation (deprivation index 8–10, P < 0.001). There were 12,405 patients receiving oral hypoglycaemic therapy. Of these patients, 1775 (14.3%) were excluded from further analysis as they had only one dispensing recorded and no follow-up dispensing (to calculate PDCs there should be at least two dispensing records). There were 3492 patients excluded while capping the observation period to the last complete year. Finally, the data for 7138 patients were analysed (Fig. 1).
|
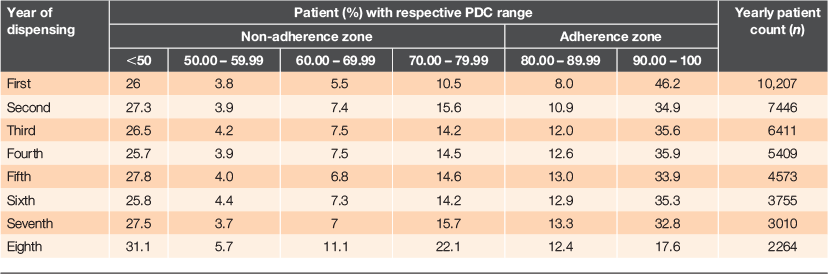
The range of PDCs for every dispensing year in patients receiving oral hypoglycaemics is summarised in Table 2. Extreme non-adherence (PDC <50%) was significantly higher in the eighth year than the first year (31.1% vs. 26.0%; P < 0.001), whereas complete adherence (PDC ≥90%) was significantly higher in the first year than the eighth year (46.2% vs. 17.6%; P < 0.001).
|
The range of PDCs in patients receiving oral hypoglycaemics (n = 7138) is summarised in Table 3. We found that 54.5% of patients dispensed oral hypoglycaemics were non-adherent (PDC <80%). Based on the type of therapy – monotherapy, combination of two and combination of three oral hypoglycaemics – non-adherence was 34.5%, 74.7% and 92.3% respectively. Extreme non-adherence (PDC <50%) was significantly (P < 0.001) higher in patients with combinations of three (41.9%) and two (19.4%) oral hypoglycaemics when compared to monotherapy (5.5%). Complete adherence (PDC ≥90%) was significantly (P < 0.001) higher in monotherapy (37.3%) when compared with combinations of three (8.6%) and two (1.0%) oral hypoglycaemics.

|
The demographics of the sub-sets of patients with PDC <80% and PDC ≥80% is shown in Table 4. Of the 3892 people in the sample who were non-adherent (PDC <80%), 54.0% were men and 33.3% were of New Zealand Māori ethnicity.

|
Discussion
We found that males, people aged 41–80 years, people of NZ Māori ethnicity and people living in socioeconomically deprived areas had higher use of oral hypoglycaemics than the general population of this DHB. Of the patients receiving oral hypoglycaemics, half were non-adherent (PDC <80%). In this group of patients, non-adherence was significantly higher in the eighth year than the first year, indicating a decrease in adherence level with time. Patients on monotherapy, combinations of two oral hypoglycaemics, and combinations of three oral hypoglycaemics, had successively higher levels of non-adherence, suggesting that increased medication burden may decrease adherence. Non-adherence was significantly higher in the NZ Māori ethnic group, in young and the middle aged (21–60 years) and in patients with low socioeconomic status, and correspondingly, adherence was significantly higher in the NZ European ethnic group and for people aged ≥61 years. This is an important finding as many adherence support services target patients aged >65 years when there may be a more pressing need for adherence support for people in the middle-aged bracket.
Previously reported research from the study evaluating the influence of a Medication Use Review service found that the type 2 diabetes patients who received adherence support improved their adherence scores and subsequently reduced their HbA1c levels.23 However, the number of other type 2 diabetes patients who would benefit from access to this service was unknown and neither did we previously know the overall oral hypoglycaemic medication adherence level in the local population. By using centrally held data and applying the PDC algorithm as a surrogate marker of medication adherence in this study, we were able to assess medication adherence in a real-world population.35–42
Continued treatment with oral hypoglycaemic medication is desirable for optimal outcomes of chronic type 2 diabetes treatment, and an important finding of this study is that the adherence rate was significantly higher in the first year but decreased over time. The significant rate of non-adherence in patients with low socioeconomic status may reflect a reluctance for low-income patients to refill their medications due to financial barriers. Primary healthcare accessibility is influenced by ethnicity and socioeconomic status in New Zealand.43,44 Reduced income may also affect dietary choices, particularly for low nutritional, low-cost food options, which may trigger high blood glucose levels.44 Non-adherence by patients aged 31–60 years is concerning, as these patients are of ‘actively working’ age. Poor glycaemic control will affect their working ability and productivity and may lead to hospitalisation and additional healthcare costs. We found that patients aged ≥60 years were reasonably adherent to oral hypoglycaemic therapy, as other studies have previously reported.45 Such patients may have become accustomed to their medications over a longer time, with established routines to remind them to take their medication.46 A meta-analysis has also shown that adherence can be better in older patients.45
Considering the chronic nature of type 2 diabetes, treatment intensification may demand an additional one or more drugs to the initial monotherapy. This additional oral hypoglycaemic medication may trigger treatment non-adherence, as the study demonstrated that the spread of non-adherence was higher in therapy with combinations of two and three medications than monotherapy. This non-adherence may complicate the disease’s progression, raise the chances of comorbidity, invite expensive health management and lead to death.33
Different tools to enhance medication adherence range from self-management to integrated care interventions.47–52 Pharmacist intervention has been well researched and shown to improve medication adherence and treatment outcomes (glycaemic control) in type 2 diabetes patients.23,53–59 Support services that provide structured patient education and counselling about type 2 diabetes, prescribed medications, proper dosage, possible side-effects and importance of medication adherence have been investigated.53,54 A common approach is to combine an educational with a behavioural strategy to optimise the use of oral hypoglycaemic medications.2,9,56,58 With 54.5% of type 2 diabetes patients in the current study not fully adhering to oral hypoglycaemic medication adherence, support services may be warranted in the study DHB.
This study has some limitations. The Pharmaceutical Collection data records medication dispensing funded by the Ministry of Health, but it does not collect data about medicines paid for privately. This study was conducted within a single DHB, and may not reflect the situation in other DHBs. A small number of patients will be receiving oral hypoglycaemic medication for conditions other than diabetes (e.g. metformin for polycystic ovary syndrome, and some type 1 diabetes patients receive oral hypoglycaemic in addition to insulin), but adherence is still important in those clinical conditions. The Pharmaceutical Collection data represents only information about the collection of medication. The fate of dispensed drugs is still unknown as there is no information about its administration; therefore, the results of this study may underestimate true non-adherence. This study could not account for patients receiving a prescription that was not presented for dispensing and patients who collected a single script, but did not persist with treatment for a full year. The accuracy of the data source is important for reliable results. Short-term analysis (<90 days) may result in bias and imprecise outcomes.59 People with type 2 diabetes may also have other chronic illnesses. Declining adherence with combination therapy could also indicate additional morbidities and addition of medicines other than oral hypoglycaemic for managing those conditions. The current study could not account for this as a confounding factor.
Not all patients can receive adherence support services, so use of a large dataset such as the Pharmaceutical Collection Database can provide insights into how widespread the adherence issue is and how many may benefit from adherence support. Knowing the characteristics of non-adherent patients is important so that strategies to enhance the adherence can be implemented. Prescribers could be encouraged to optimise monotherapy before the addition of another oral hypoglycaemic medication, and adherence support services should not only be offered to older patients.
Conclusion
This study showed how the degree of adherence to oral hypoglycaemic medications can be established using regional level data from the Pharmaceutical Collection Database. This information can inform local strategies to target patients who would benefit from adherence support services.
Competing interests
The authors declare that they have no conflicts of interest.
Funding
Mangesh D. Kharjul was supported by a School of Pharmacy PhD stipend.
Acknowledgements
Authors would like to thank the Ministry of Health, New Zealand, for providing Pharmaceutical Collection data and the School of Pharmacy, University of Otago, Dunedin, New Zealand for supporting this research. The authors also acknowledge the support of the University of Otago, by means of the University of Otago Postgraduate Publishing Bursary (Doctoral).
References
[1] World Health Organization. Global Report on Diabetes. Geneva: WHO; 2016. [Cited 2018 April 4]. Available from: http://apps.who.int/iris/bitstream/handle/10665/204871/9789241565257_eng.pdf?sequence=1.[2] Grant RW, Kirkman MS. Trends in the evidence level for the American Diabetes Association’s “Standards of Medical Care in Diabetes” from 2005 to 2014. Diabetes Care. 2015; 38 6–8.
| Trends in the evidence level for the American Diabetes Association’s “Standards of Medical Care in Diabetes” from 2005 to 2014.Crossref | GoogleScholarGoogle Scholar | 25538309PubMed |
[3] Zhang P, Gregg E. Global economic burden of diabetes and its implications. Lancet Diabetes Endocrinol. 2017; 5 404–5.
| Global economic burden of diabetes and its implications.Crossref | GoogleScholarGoogle Scholar | 28456417PubMed |
[4] Seuring T, Archangelidi O, Suhrcke M. The economic costs of type 2 diabetes: a global systematic review. Pharmacoeconomics. 2015; 33 811–31.
| The economic costs of type 2 diabetes: a global systematic review.Crossref | GoogleScholarGoogle Scholar | 25787932PubMed |
[5] Seib C, Parkinson J, McDonald N, et al. Lifestyle interventions for improving health and health behaviours in women with type 2 diabetes: a systematic review of the literature 2011–2017. Maturitas. 2018; 111 1–14.
| Lifestyle interventions for improving health and health behaviours in women with type 2 diabetes: a systematic review of the literature 2011–2017.Crossref | GoogleScholarGoogle Scholar | 29673826PubMed |
[6] Thent ZC, Das S, Henry LJ. Role of exercise in the management of diabetes mellitus: the global scenario. PLoS One. 2013; 8 e80436
| Role of exercise in the management of diabetes mellitus: the global scenario.Crossref | GoogleScholarGoogle Scholar | 24236181PubMed |
[7] Asif M. The prevention and control the type-2 diabetes by changing lifestyle and dietary pattern. J Educ Health Promot. 2014; 3 1–8.
| The prevention and control the type-2 diabetes by changing lifestyle and dietary pattern.Crossref | GoogleScholarGoogle Scholar | 24741641PubMed |
[8] Kirkman MS, Rowan-Martin MT, Levin R, et al. Determinants of adherence to diabetes medications: findings from a large pharmacy claims database. Diabetes Care. 2015; 38 604–9.
| 25573883PubMed |
[9] Krass I, Schieback P, Dhippayom T. Adherence to diabetes medication: a systematic review. Diabet Med. 2015; 32 725–37.
| Adherence to diabetes medication: a systematic review.Crossref | GoogleScholarGoogle Scholar | 25440507PubMed |
[10] Zullig LL, Gellad WF, Moaddeb J, et al. Improving diabetes medication adherence: successful, scalable interventions. Patient Prefer Adherence. 2015; 9 139–49.
| Improving diabetes medication adherence: successful, scalable interventions.Crossref | GoogleScholarGoogle Scholar | 25670885PubMed |
[11] Farmer AJ, Rodgers LR, Lonergan M, et al. Adherence to oral glucose-lowering therapies and associations with 1-Year HbA1c: a retrospective cohort analysis in a large primary care database. Diabetes Care. 2016; 39 258–63.
| 26681714PubMed |
[12] Butt M, Mhd Ali A, Bakry MM, Mustafa N. Impact of a pharmacist led diabetes mellitus intervention on HbA1c, medication adherence and quality of life: a randomised controlled study. Saudi Pharm J. 2016; 24 40–8.
| Impact of a pharmacist led diabetes mellitus intervention on HbA1c, medication adherence and quality of life: a randomised controlled study.Crossref | GoogleScholarGoogle Scholar | 26903767PubMed |
[13] Korcegez EI, Sancar M, Demirkan K. Effect of a pharmacist-led program on improving outcomes in patients with Type 2 diabetes Mellitus from Northern Cyprus: a randomized controlled trial. J Manag Care Spec Pharm. 2017; 23 573–82.
| Effect of a pharmacist-led program on improving outcomes in patients with Type 2 diabetes Mellitus from Northern Cyprus: a randomized controlled trial.Crossref | GoogleScholarGoogle Scholar | 28448779PubMed |
[14] Sabate E, editor. Adherence to long-term therapies: evidence for action. Geneva, Switzerland: World Health Organization; 2003. [cited 2018 November 16]. Available from: http://www.who.int/chp/knowledge/publications/adherence_report/en/.
[15] Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005; 353 487–97.
| Adherence to medication.Crossref | GoogleScholarGoogle Scholar | 16079372PubMed |
[16] Brown MT, Bussell JK. Medication adherence: WHO cares? Mayo Clin Proc. 2011; 86 304–14.
| Medication adherence: WHO cares?Crossref | GoogleScholarGoogle Scholar | 21389250PubMed |
[17] Robin DiMatteo M, Giordani PJ, Lepper HS, Croghan TW. Patient adherence and medical treatment outcomes: a meta-analysis. Med Care. 2002; 40 794–811.
| Patient adherence and medical treatment outcomes: a meta-analysis.Crossref | GoogleScholarGoogle Scholar |
[18] Kalogianni A. Factors affect in patient adherence to medication regimen. Health Sci J. 2011; 5 157–8.
[19] Ganguli A, Clewell J, Shillingto AC. The impact of patient support programs on adherence, clinical, humanistic, and economic patient outcomes: a targeted systematic review. Patient Prefer Adherence. 2016; 10 711–25.
| 27175071PubMed |
[20] Pharmaceutical Society of New Zealand Inc. New Zealand National Pharmacist Services Framework 2014. [cited 2017 November 02]. Available from: https://www.psnz.org.nz/Folder?Action=View%20File&Folder_id=86&File=PSNZPharmacistServices 11111Framework2014FINAL.pdf
[21] Tan EC, Stewart K, Elliott RA, George J. Pharmacist services provided in general practice clinics: a systematic review and meta-analysis. Res Social Adm Pharm. 2014; 10 608–22.
| Pharmacist services provided in general practice clinics: a systematic review and meta-analysis.Crossref | GoogleScholarGoogle Scholar | 24161491PubMed |
[22] Hatah E, Tordoff J, Duffull SB, et al. Retrospective examination of selected outcomes of Medicines Use Review (MUR) services in New Zealand. Int J Clin Pharm. 2014; 36 503–12.
| Retrospective examination of selected outcomes of Medicines Use Review (MUR) services in New Zealand.Crossref | GoogleScholarGoogle Scholar | 24626968PubMed |
[23] Kharjul M, Braund R, Green J. The influence of pharmacist-led adherence support on glycaemic control in people with type 2 diabetes. Int J Clin Pharm. 2018; 40 354–9.
| The influence of pharmacist-led adherence support on glycaemic control in people with type 2 diabetes.Crossref | GoogleScholarGoogle Scholar | 29468528PubMed |
[24] Gavrielov-Yusim N, Friger M. Use of administrative medical databases in population-based research. J Epidemiol Community Health. 2014; 68 283–7.
| Use of administrative medical databases in population-based research.Crossref | GoogleScholarGoogle Scholar | 24248997PubMed |
[25] Schneeweiss S, Avorn J. A review of uses of health care utilization databases for epidemiologic research on therapeutics. J Clin Epidemiol. 2005; 58 323–37.
| A review of uses of health care utilization databases for epidemiologic research on therapeutics.Crossref | GoogleScholarGoogle Scholar | 15862718PubMed |
[26] Cadarette SM, Wong L. An introduction to health care administrative data. Can J Hosp Pharm. 2015; 68 232–7.
| An introduction to health care administrative data.Crossref | GoogleScholarGoogle Scholar | 26157185PubMed |
[27] Rosella LC, Fitzpatrick T, Wodchis WP, et al. High-cost health care users in Ontario, Canada: demographic, socioeconomic, and health status characteristics. BMC Health Serv Res. 2014; 14 532
| High-cost health care users in Ontario, Canada: demographic, socioeconomic, and health status characteristics.Crossref | GoogleScholarGoogle Scholar | 25359294PubMed |
[28] Mazzali C, Paganoni AM, Ieva F, et al. Methodological issues on the use of administrative data in healthcare research: the case of heart failure hospitalizations in Lombardy region, 2000 to 2012. BMC Health Serv Res. 2016; 16 234
| Methodological issues on the use of administrative data in healthcare research: the case of heart failure hospitalizations in Lombardy region, 2000 to 2012.Crossref | GoogleScholarGoogle Scholar | 27391599PubMed |
[29] Lima-Dellamora EDC, Osorio-de-Castro CGS, Madruga LGDSL, Azeredo TB. Use of pharmacy records to measure treatment adherence: a critical review of the literature. Cad Saude Publica. 2017; 33 e00136216
| 28444026PubMed |
[30] Peterson AM, Nau DP, Cramer JA, et al. A checklist for medication compliance and persistence studies using retrospective databases. Value Health. 2007; 10 3–12.
| A checklist for medication compliance and persistence studies using retrospective databases.Crossref | GoogleScholarGoogle Scholar | 17261111PubMed |
[31] Hess LM, Raebel MA, Conner DA, Malone DC. Measurement of adherence in pharmacy administrative databases: a proposal for standard definitions and preferred measures. Ann Pharmacother. 2006; 40 1280–8.
| Measurement of adherence in pharmacy administrative databases: a proposal for standard definitions and preferred measures.Crossref | GoogleScholarGoogle Scholar | 16868217PubMed |
[32] Karve S, Cleves MA, Helm M, et al. Good and poor adherence: optimal cut-point for adherence measures using administrative claims data. Curr Med Res Opin. 2009; 25 2303–10.
| Good and poor adherence: optimal cut-point for adherence measures using administrative claims data.Crossref | GoogleScholarGoogle Scholar | 19635045PubMed |
[33] Chisholm-Burns MA, Spivey CA. The ‘cost’ of medication non-adherence: consequences we cannot afford to accept. J Am Pharm Assoc. 2012; 52 823–6.
| The ‘cost’ of medication non-adherence: consequences we cannot afford to accept.Crossref | GoogleScholarGoogle Scholar |
[34] Caetano PA, Lam JM, Morgan SG. Toward a standard definition and measurement of persistence with drug therapy: examples from research on statin and antihypertensive utilization. Clin Ther. 2006; 28 1411–24.
| Toward a standard definition and measurement of persistence with drug therapy: examples from research on statin and antihypertensive utilization.Crossref | GoogleScholarGoogle Scholar | 17062314PubMed |
[35] Karve S, Cleves MA, Helm M, et al. Prospective validation of eight different adherence measures for use with administrative claims data among patients with schizophrenia. Value Health. 2009; 12 989–95.
| Prospective validation of eight different adherence measures for use with administrative claims data among patients with schizophrenia.Crossref | GoogleScholarGoogle Scholar | 19402852PubMed |
[36] Vink NM, Klungel OH, Stolk RP, Denig P. Comparison of various measures for assessing medication refill adherence using prescription data. Pharmacoepidemiol Drug Saf. 2009; 18 159–65.
| Comparison of various measures for assessing medication refill adherence using prescription data.Crossref | GoogleScholarGoogle Scholar | 19109809PubMed |
[37] Kozma CM, Dickson M, Phillips AL, Meletiche DM. Medication possession ratio: implications of using fixed and variable observation periods in assessing adherence with disease-modifying drugs in patients with multiple sclerosis. Patient Prefer Adherence. 2013; 7 509–16.
| Medication possession ratio: implications of using fixed and variable observation periods in assessing adherence with disease-modifying drugs in patients with multiple sclerosis.Crossref | GoogleScholarGoogle Scholar | 23807840PubMed |
[38] Andrade SE, Kahler KH, Frech F, et al. Methods for evaluation of medication adherence and persistence using automated databases. Pharmacoepidemiol Drug Saf. 2006; 15 565–74.
| Methods for evaluation of medication adherence and persistence using automated databases.Crossref | GoogleScholarGoogle Scholar | 16514590PubMed |
[39] Karve S, Cleves MA, Helm M, et al. An empirical basis for standardizing adherence measures derived from administrative claims data among diabetic patients. Med Care. 2008; 46 1125–33.
| An empirical basis for standardizing adherence measures derived from administrative claims data among diabetic patients.Crossref | GoogleScholarGoogle Scholar | 18953222PubMed |
[40] Zhu VJ, Tu W, Rosenman MB, Overhage JM. A comparison of data driven-based measures of adherence to oral hypoglycemic agents in medicaid patients. AMIA Annu Symp Proc. 2014; 2014 1294–301.
| 25954441PubMed |
[41] Sattler ELP, Lee JS, Perri M. Medication (re)fill adherence measures derived from pharmacy claims data in older Americans: a review of the literature. Drugs Aging. 2013; 30 383–99.
| Medication (re)fill adherence measures derived from pharmacy claims data in older Americans: a review of the literature.Crossref | GoogleScholarGoogle Scholar |
[42] Training and Technical Assistance Support Center. Calculating medication adherence for antihypertensive and antidiabetic medications: an evaluation guide for grantees. National Center for Chronic Disease Prevention and Health Promotion (NCCDPHP); 2015. [cited 2018 May 24]. Available from: https://www.cdc.gov/dhdsp/docs/med-adherence-evaluation-tool.pdf.
[43] Jatrana S, Crampton P, Norris P. Ethnic differences in access to prescription medication because of cost in New Zealand. J Epidemiol Community Health. 2011; 65 454–60.
| Ethnic differences in access to prescription medication because of cost in New Zealand.Crossref | GoogleScholarGoogle Scholar | 20466707PubMed |
[44] Norris P, Tordoff J, McIntosh B, et al. Impact of prescription charges on people living in poverty: a qualitative study. Res Social Adm Pharm. 2016; 12 893–902.
| Impact of prescription charges on people living in poverty: a qualitative study.Crossref | GoogleScholarGoogle Scholar | 26681431PubMed |
[45] Assawasuwannakit P, Braund R, Duffull SB. A model-based meta-analysis of the influence of factors that impact adherence to medications. J Clin Pharm Ther. 2015; 40 24–31.
| A model-based meta-analysis of the influence of factors that impact adherence to medications.Crossref | GoogleScholarGoogle Scholar | 25328015PubMed |
[46] Bagge M, Tordoff J, Norris P, Heydon S. Older people’s attitudes towards their regular medicines. J Prim Health Care. 2013; 5 234–42.
| Older people’s attitudes towards their regular medicines.Crossref | GoogleScholarGoogle Scholar | 23998174PubMed |
[47] Vrijens B, De Geest S, Hughes DA, et al. A new taxonomy for describing and defining adherence to medications. Br J Clin Pharmacol. 2012; 73 691–705.
| A new taxonomy for describing and defining adherence to medications.Crossref | GoogleScholarGoogle Scholar | 22486599PubMed |
[48] Adler AJ, Martin N, Mariani J, et al. Mobile phone text messaging to improve medication adherence in secondary prevention of cardiovascular disease. Cochrane Database Syst Rev. 2017; 4 CD011851
| 28455948PubMed |
[49] Normansell R, Kew KM, Stovold E. Interventions to improve adherence to inhaled steroids for asthma. Cochrane Database Syst Rev. 2017; 4 CD012226
| 28417456PubMed |
[50] van Driel ML, Morledge MD, Ulep R, Shaffer JP, Davies P, Deichmann R. Interventions to improve adherence to lipid-lowering medication. Cochrane Database Syst Rev. 2016; 12 CD004371
| 28000212PubMed |
[51] Haynes RB, Ackloo E, Sahota N, et al. Interventions for enhancing medication adherence. Cochrane Database Syst Rev. 2008; CD000011
| 18425859PubMed |
[52] Nieuwlaat R, Wilczynski N, Navarro T, et al. Interventions for enhancing medication adherence. Cochrane Database Syst Rev. 2014; 11 CD000011
[53] Wishah RA, Al-Khawaldeh OA, Albsoul AM. Impact of pharmaceutical care interventions on glycemic control and other health-related clinical outcomes in patients with type 2 diabetes: randomized controlled trial. Diabetes Metab Syndr. 2015; 9 271–6.
| Impact of pharmaceutical care interventions on glycemic control and other health-related clinical outcomes in patients with type 2 diabetes: randomized controlled trial.Crossref | GoogleScholarGoogle Scholar | 25301007PubMed |
[54] Lim PC, Lim K, Embee ZC, et al. Study investigating the impact of pharmacist involvement on the outcomes of diabetes medication therapy adherence program Malaysia. Pak J Pharm Sci. 2016; 29 595–601.
| 27087103PubMed |
[55] Cassimatis M, Kavanagh DJ. Effects of type 2 diabetes behavioural telehealth interventions on glycaemic control and adherence: a systematic review. J Telemed Telecare. 2012; 18 447–50.
| Effects of type 2 diabetes behavioural telehealth interventions on glycaemic control and adherence: a systematic review.Crossref | GoogleScholarGoogle Scholar | 23209266PubMed |
[56] Omran D, Guirguis LM, Simpson SH. Systematic review of pharmacist interventions to improve adherence to oral antidiabetic medications in people with type 2 diabetes. Can J Diab. 2012; 36 292–9.
| Systematic review of pharmacist interventions to improve adherence to oral antidiabetic medications in people with type 2 diabetes.Crossref | GoogleScholarGoogle Scholar |
[57] Jarab AS, Alqudah SG, Mukattash TL. Randomized controlled trial of clinical pharmacy management of patients with type 2 diabetes in an outpatient diabetes clinic in Jordan. J Manag Care Pharm. 2012; 18 516–26.
| Randomized controlled trial of clinical pharmacy management of patients with type 2 diabetes in an outpatient diabetes clinic in Jordan.Crossref | GoogleScholarGoogle Scholar | 22971205PubMed |
[58] Odegard PS, Goo A, Hummel J, et al. Caring for poorly controlled diabetes mellitus: a randomized pharmacist intervention. Ann Pharmacother. 2005; 39 433–40.
| Caring for poorly controlled diabetes mellitus: a randomized pharmacist intervention.Crossref | GoogleScholarGoogle Scholar | 15701763PubMed |
[59] Jacobs M, Sherry PS, Taylor LM, et al. Pharmacist Assisted Medication Program Enhancing the Regulation of Diabetes (PAMPERED) study. J Am Pharm Assoc. 2012; 52 613–21.
| Pharmacist Assisted Medication Program Enhancing the Regulation of Diabetes (PAMPERED) study.Crossref | GoogleScholarGoogle Scholar |


