Timeliness of diagnosis and treatment of cutaneous melanoma with dermatology, general practice, plastics surgery collaboration – are we meeting standards?
Haein Na 1 * , Amanda Oakley
1 * , Amanda Oakley  2 3
2 3
1 Te Whatu Ora Waitematā, 124 Shakespeare Road, Takapuna, Auckland 0620, New Zealand.
2 Te Whatu Ora Waikato, 183 Pembroke Street, Hamilton 3204, New Zealand.
3 Faculty of Medicine and Health Sciences, The University of Auckland, Auckland, New Zealand.
Journal of Primary Health Care 15(3) 267-273 https://doi.org/10.1071/HC23013
Published: 26 April 2023
© 2023 The Author(s) (or their employer(s)). Published by CSIRO Publishing on behalf of The Royal New Zealand College of General Practitioners. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
Introduction: Melanoma is a serious type of skin cancer with a high burden in New Zealand. MelNet Quality Statements (2021) guide the timeliness of investigations and management for melanoma patients, who might experience long delays waiting for treatment.
Aim: To assess compliance of melanoma diagnosis and treatment timeliness with the MelNet Quality Statements at Waikato Hospital and in primary care for melanoma and melanoma in situ (MIS).
Methods: This is a retrospective clinical audit of patients referred via the Suspected Skin Cancer (SSC) teledermatology pathway between June 2020 and June 2022, and histologically confirmed as having melanoma or MIS. Time intervals between elements of service were analysed.
Results: For 43 melanomas and 105 MIS, compliance with MelNet Quality Statements across all melanoma services was poor, except for teledermatology response rates (100% compliance). From referral to first cancer treatment (Statement 2.1.1), compliance was 50% in general practice and 7.7% in Waikato Hospital. From teledermatologist response to biopsy (Statement 2.1.3), compliance was 65.2% in general practice and 7.7% in hospital plastics department. Histopathological reporting delays were also identified.
Discussion: Long delays for melanoma care in hospital likely reflect system failures (such as inadequate funding and human resources) and the increasing burden of skin cancer. In contrast, primary care provided quicker diagnostic biopsies and surgical treatments for melanoma.
Keywords: cancer treatment, clinical audit, cutaneous melanoma, dermatology, general practice, melanoma, New Zealand, primary care, skin cancer, telemedicine, treatment compliance.
| WHAT GAP THIS FILLS |
| What is already known: Long delays to treatment and poor compliance with timeliness standards for melanoma treatment have previously been reported in New Zealand. |
| What this study adds: A clinical audit of 43 patients with confirmed melanoma assessed timeliness against the recommendations of MelNet New Zealand Quality Statements (2021) and demonstrated: 100% compliance for first specialist assessment by dermatology (non-contact) within 14 days of referral; low compliance rates with the timeliness of diagnostic biopsy within 14 days of specialist assessment; and treatment within 62 days of referral. Diagnostic biopsies for the diagnosis of melanoma were undertaken more quickly in general practice than in a public hospital. |
Introduction
Aotearoa New Zealand (NZ) has the highest reported age-standardised incidence, cumulative risk, and mortality for melanoma worldwide.1
The Waikato health region of NZ serves a population of more than 425 000 people (approximately 9% of the country’s population) across 80 general practice clinics and at the main regional hospital, Waikato Hospital.2 In this district, general practitioners (GPs) can refer patients with lesions suspicious of skin cancer for advice via a Suspected Skin Cancer (SSC) pathway by completing a specific electronic template, including skin cancer risk factors and lesion details, and attaching regional, close-up and dermoscopic images.3 A dermatologist reviews the referral (non-contact First Specialist Appointment, ncFSA), and if suspecting melanoma or melanoma in situ (MIS), assigns an appropriate International Classification of Diseases (ICD)-10 code to their response and recommends excision. The patient’s GP may refer the patient to a suitably trained GP, a private plastic surgeon, or to the plastics service at the public hospital for the initial diagnostic excision or subsequent wide local excision (WLE) once melanoma is confirmed on histopathology.4 Funding has been available to GPs for diagnostic excision of dermatologist-confirmed melanoma and WLE of early melanoma since February 2020. Funding is excluded for lesions on the head/neck and relies on the self-assessed skills of the GP.
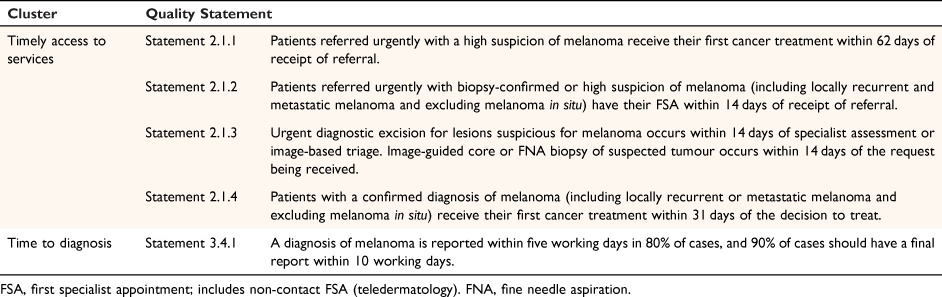
Skin cancer surgery waitlists in NZ public hospitals are long and may benefit from surgery undertaken in primary care. Melnet’s Quality Statements to guide melanoma diagnosis and treatment in New Zealand (November 2021)4 include Quality Statements for ‘Timely access to services’ and ‘Time to Diagnosis’ (Table 1).
|
The primary objective of this retrospective audit was to determine if the timeliness of diagnosis and treatment of confirmed primary melanoma between 1 June 2020 and 30 June 2022 met the recommendations of the MelNet Quality Statements. The secondary objective was to show that surgery undertaken in primary care for melanoma and MIS diagnosed through this pathway was quicker than in secondary care.
Methods
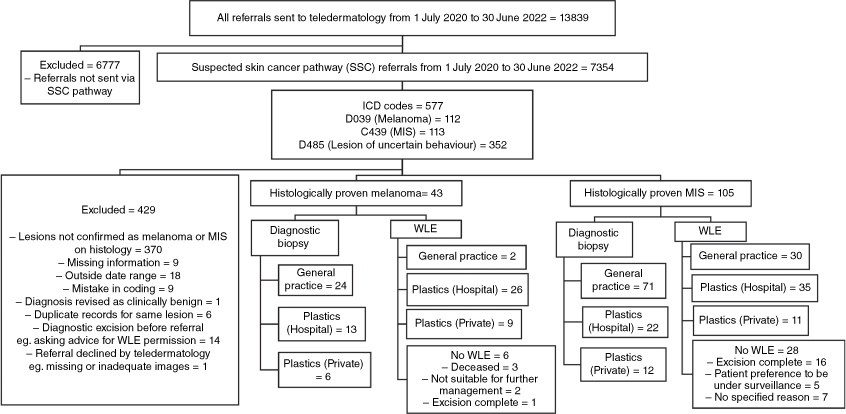
The dataset comprised patients referred from primary care via the SSC pathway and coded by the dermatologist as melanoma, MIS, or lesion of uncertain behaviour (ICD Codes D039, C439, and D485, respectively) from June 2020 to July 2022 (Fig. 1).
|
Demographic, lesion, skin cancer risk factors, and histological characteristics for each lesion, and the dates of different service elements outlined by the Melnet Quality Statements (Table 1),4 were collected as below.
Timeliness of first cancer treatment after referral (the first WLE, including those that were histologically incomplete).
Timeliness of FSA after referral (dermatology’s ncFSA response to the SSC referral).
Timeliness of diagnostic excision after the ncFSA response.
Timeliness of first cancer treatment from the decision to treat (date of the hospital outpatient clinic appointment at which the WLE was arranged). This criterion was not assessed in the general practice or private plastics settings.
Timeliness of generating initial and final histopathological reports after biopsy.
These were entered into an Excel (Microsoft Corporation, Redmond, WA, USA) spreadsheet. The univariable associations between the location of patient care and the duration (in days) between these services were examined using the Wilcoxon rank- sum test or the Kruskal–Wallis test. The Chi-squared or Fisher’s exact test was used to analyse compliance with Melnet’s timeliness of diagnosis and treatment. SAS software version 9.4 (SAS Institute Inc, Cary, NC, USA) was used for analyses. A two-tailored P < 0.05 was considered statistically significant.
Histologically confirmed melanoma and MIS were separately analysed, as the Quality Statements for timeliness do not apply to MIS.
This study was registered with the Clinical Audit Support Unit (June 2022). An ethics review is not required in New Zealand for a clinical audit.
Results
Patients with histologically confirmed melanoma
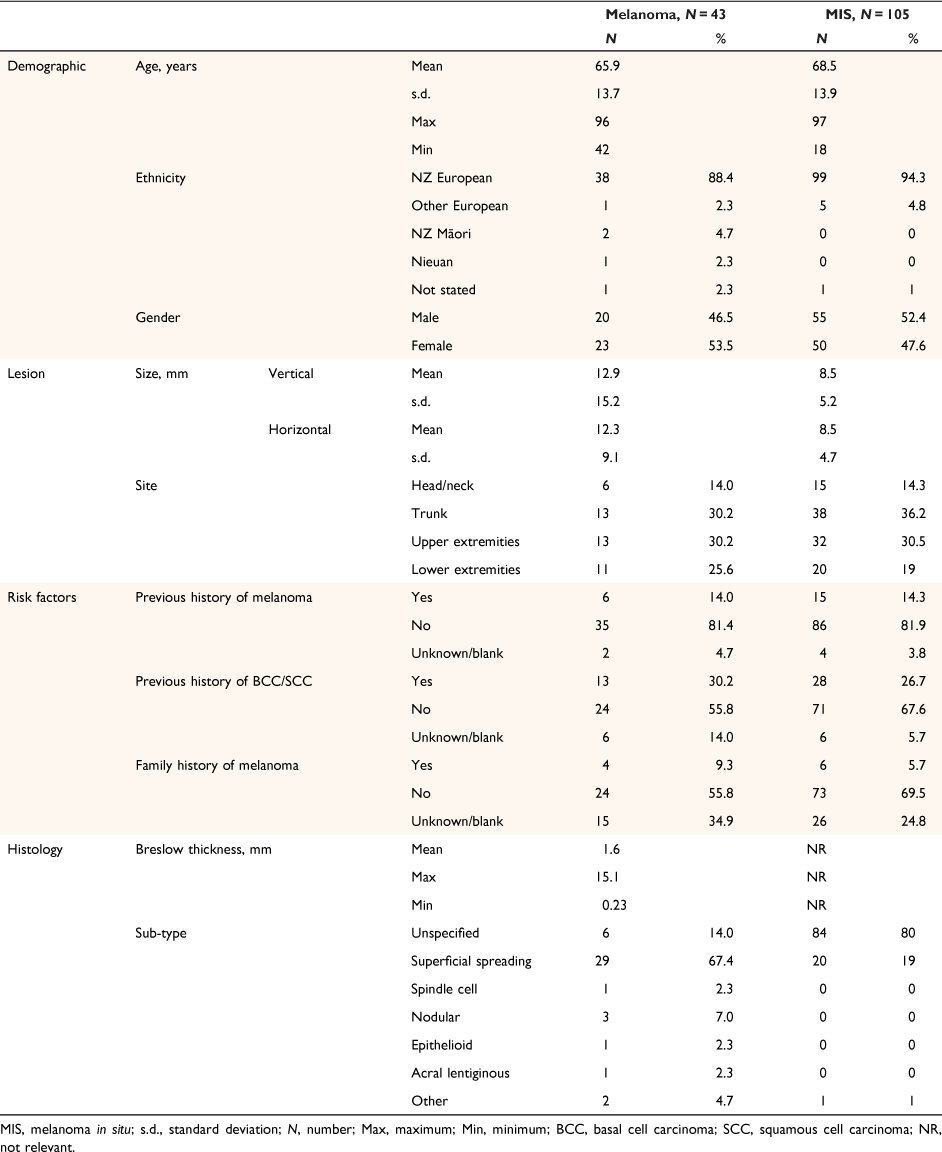
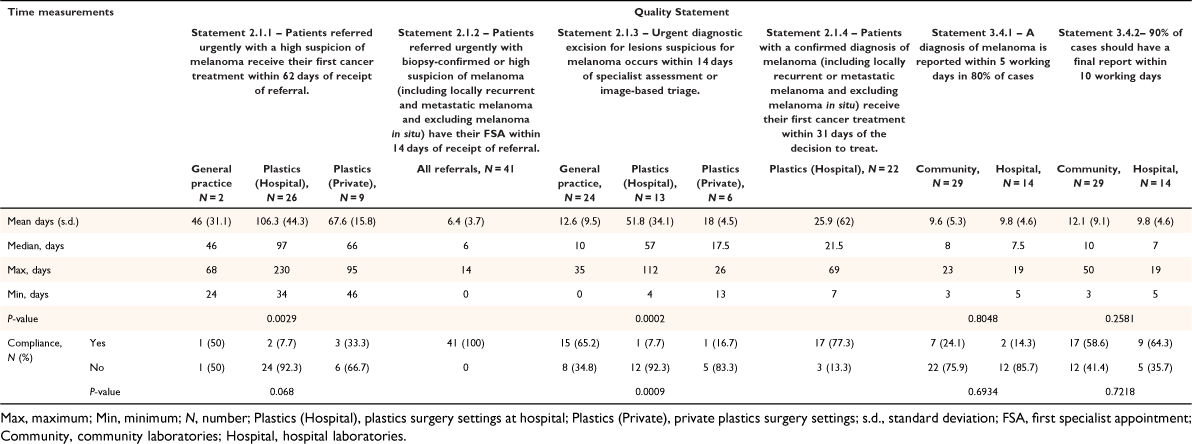
There were 43 patients with 43 histologically proven melanomas with an average Breslow thickness of 1.6 mm (Table 2). Time intervals between different elements of service and their compliance with the Quality Statements are shown in Table 3. Compliance to the 62-day rule for first cancer treatment after referral was only 7.7% in hospital, 33.3% in private plastics surgery settings, and 50% in general practice (n = 2). All teledermatology responses for the cohort were completed within 14 days. Compliance to the 14-day rule for time to diagnostic biopsy following dermatologist recommendation was 7.7% in hospital, 16.7% in private plastics surgery settings, and 65.2% in primary care. All were excisional biopsies except one incisional biopsy case in hospital on a patient’s foot.
|
|
Compliance to the 5- and 10-day standard for initial and final histology reports were 24.1 and 58.6% respectively in community laboratories, and 14.3 and 64.3% respectively in hospital laboratories.
Patients with histologically confirmed MIS
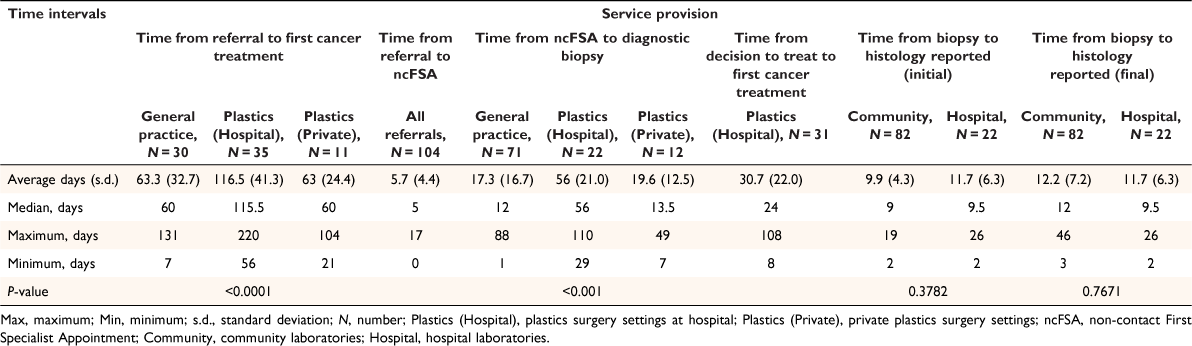
Demographic, lesion, and risk factor information for 102 patients with skin cancer and the histological characteristics of 105 histologically confirmed patients withs MIS are shown in Table 2. Time intervals between different elements of service are shown in Table 4.
|
Dermatology advice was given for confirmed MIS after an average of 5.7 days following referral. Biopsy was performed after an average of 56 days in a public hospital (n = 21), 19.6 days in private plastics surgery (n = 12) settings and 17.3 days in general practice (n = 71). All were excisional biopsies, except one punch biopsy of a lesion on a forearm and two incisional biopsies of the anterior thigh and chin, all in primary care.
Five MIS required re-excision due to insufficient margins on the first WLE (three had been done at hospital, and two in primary care). The average days to WLE following referral were 116.5 days in hospital, 63 days in private plastics surgery settings and 63.3 days in primary care.
The community laboratory produced initial and final reports of MIS an average of 9.9 days and 12.2 days after biopsy, respectively, whereas in hospital, the average duration was 11.7 days for both.
Discussion
The Ministry of Health proposed monitoring adherence to cancer standards nearly a decade ago, but treatment delays persist.5 Apart from teledermatology reporting rates, all other elements of melanoma care were poorly compliant with the Melnet Quality Statements. Demographic and skin cancer risk factors, characteristics of the lesion on histology, and provisional diagnoses did not influence the timeliness of interventions. There are significant delays to melanoma care in hospital. Disappointingly, these delays have scarcely improved since a 2016 audit,6 which showed an average melanoma WLE wait time of 138.7 days from referral and 0% compliance with the 62-day wait standard (Provisional NZ Melanoma Standards).7 In contrast, 90, 97.3 and 96.4% of skin cancer treatments occurred within 62 days in England, Wales and Scotland, respectively.8 Such ongoing delays in NZ public hospitals may reflect the lack of suitably trained surgical specialists; administrative and systems failures such as staff shortages, clinic space limitations, and inefficient triage; delays to histopathological reporting; on top of the recent impacts of the coronavirus disease 2019 (COVID-19) pandemic.9
Surgery for melanoma in primary care occurred quicker than in secondary care, particularly for MIS, where GPs undertook more procedures. A review of Waitematā GP skin cancer services identified 2705 lesion excisions by 13 specialist-trained GPs in 2016, with shorter treatment times than in secondary care.10 Although the impact of a long wait for WLE following biopsy on patient outcomes may be clinically minor,11,12 initial melanoma excision is required promptly to avoid tumour growth and metastatic spread. Delays may increase patient distress and reduce quality of life.13
An earlier review reported a 4-day median SSC teledermatology response time, compared to 26 days for an in-person dermatology assessment.3 Teledermatology can reduce unnecessary excisions of benign lesions, with a high positive predictive value (PPV) of melanoma and a reduction in the number of lesions that number needed to excise (NNE).14 Diagnostic biopsies of skin lesions are often undertaken in primary care without a dermatological diagnosis;10 unnecessary excisions of about 20 benign lesions (mainly melanocytic naevi and seborrhoeic keratosis) are estimated to detect a single melanoma.14 Local and international evidence shows that teledermatology is better, quicker, more convenient and cost-effective for diagnosing skin lesions than conventional outpatient clinics.3,15–17
With an ageing population and projected increasing burden of skin cancer in New Zealand,3 more skin cancer management undertaken in general practice can help provide treatment that is faster, more accessible, and cost- and resource-effective. Changes to national health policy funding distributions are warranted, as increased public funding for primary care treatment can prevent inequitable care between those that can and cannot afford self-funded procedures. Additionally, ongoing training, supervision, and auditing processes at an individual, clinic, and regional level will be essential to ensure safe treatment in primary care and improve compliance with the MelNet Quality Statements (2021).4 We also recommend the development of nationwide access to teledermatology, as established in the Waikato district, to prevent late melanoma diagnoses and numerous unnecessary procedures at enormous expense.
Limitations
We have not evaluated lesions managed independently by GPs, private surgeons, or direct referrals to plastics surgery settings. Melanoma cases not referred via the SSC pathway and incidental melanomas were not included. Low numbers and non-significant P-values may also reflect small sample sizes, particularly for melanoma WLEs in general practice. This is a single-centre study, and findings may not be generalisable to other NZ centres.
Conclusions
Teledermatology allows rapid assessment of lesions suspicious of melanoma, but diagnostic excision and treatment of confirmed melanoma are often delayed beyond acceptable standards. We have demonstrated shorter waits for surgery in primary care. We recommend increased public funding for skin cancer treatment in primary care, improved access to teledermatology, and nationwide monitoring to improve compliance with the MelNet Quality Statements (2021).4
Data availability
Anonymised data used to generate the results in the paper are available and can be provided upon request.
Conflicts of interest
The authors declare no conflicts of interest.
Declaration of funding
The research did not receive any specific funding. The primary investigator undertook the audit as a final-year medical student for a research elective project.
Acknowledgements
Many thanks to Dr Angela Fairweather (primary care liaison, Te Whatu Ora Waikato, elective supervisor), and Carol Chelimo for statistical analysis, and the Dermatology team at Waikato Hospital.
References
[1] Arnold M, Singh D, Laversanne M, et al. Global burden of cutaneous melanoma in 2020 and projections to 2040. JAMA Dermatol 2022; 158 495–503.| Global burden of cutaneous melanoma in 2020 and projections to 2040.Crossref | GoogleScholarGoogle Scholar |
[2] Te Whatu Ora. Snapshot of te whatu Ora Waikato. Waikato, New Zealand: Te Whatu Ora Health; 2022. Available at https://www.waikatodhb.health.nz/about-us/snapshot-of-waikato-dhb/#:~:text=Te%20Whatu%20Ora%20in%20the%20Waikato%20serves%20a%20population%20of,to%20Waihi%20on%20the%20east
[3] Jones L, Jameson M, Oakley A. Remote skin cancer diagnosis: adding images to electronic referrals is more efficient than waitlisting for a nurse-led Imaging Clinic. Cancers 2021; 13 5828
| Remote skin cancer diagnosis: adding images to electronic referrals is more efficient than waitlisting for a nurse-led Imaging Clinic.Crossref | GoogleScholarGoogle Scholar |
[4] MelNet. Quality Statements to Guide Melanoma Diagnosis and Treatment in New Zealand. Christchurch: Melanoma Network of New Zealand (MelNet); 2021.
[5] Ministry of Health. New Zealand Cancer Plan: Better, faster cancer care 2015–2018. Wellington: Ministry of Health; 2014.
[6] Brian T, Adams B, Jameson M. Cutaneous melanoma: an audit of management timeliness against New Zealand guidelines. N Z Med J 2017; 130 54–61.
[7] National Melanoma Tumour Standards. Working Group. Standards of Service Provision for Melanoma Patients in New Zealand - Provisional. Wellington: New Zealand Ministry of Health; 2013.
[8] Cancer Research UK. Melanoma skin cancer treatment statistics. Cancer Research UK; 2021. Available at https://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/melanoma-skin-cancer/diagnosis-and-treatment#heading-Zero [Accessed 3 August 2022].
[9] Kiely AL, Patel C, Ismail A. Achieving 62-day targets in the management of skin cancer: lessons learned and future directions for the post-COVID era. J Plast Reconstr Aesthetic Surg 2021; 74 1101–60.
| Achieving 62-day targets in the management of skin cancer: lessons learned and future directions for the post-COVID era.Crossref | GoogleScholarGoogle Scholar |
[10] Wen D, Gale K, Martin R. Quality assessment of a large primary GP skin cancer service in Auckland, New Zealand. N Z Med J 2020; 133 17–27. [PMID: 32027635]
[11] Mckenna DB, Lee RJ, Prescott RJ, et al. The time from diagnostic excision biopsy to wide local excision for primary cutaneous malignant melanoma may not affect patient survival. Br J Dermatol 2002; 147 48–54.
| The time from diagnostic excision biopsy to wide local excision for primary cutaneous malignant melanoma may not affect patient survival.Crossref | GoogleScholarGoogle Scholar |
[12] Conic RZ, Cabrera CI, Khorana AA, et al. Determination of the impact of melanoma surgical timing on survival using the National Cancer Database. J Am Acad Dermatol 2018; 78 40–6.e7.
| Determination of the impact of melanoma surgical timing on survival using the National Cancer Database.Crossref | GoogleScholarGoogle Scholar |
[13] Cornish D, Holterhues C, van de Poll-Franse LV, et al. A systematic review of health-related quality of life in cutaneous melanoma. Ann Oncol 2009; 20 vi51–8.
| A systematic review of health-related quality of life in cutaneous melanoma.Crossref | GoogleScholarGoogle Scholar |
[14] Barker S, Oakley A, Rademaker M. Retrospective review of primary melanomas excised at Waikato Hospital, New Zealand, 2002–2003. Australas J Dermatol 2007; 48 14–7.
| Retrospective review of primary melanomas excised at Waikato Hospital, New Zealand, 2002–2003.Crossref | GoogleScholarGoogle Scholar |
[15] Sunderland M, Teague R, Gale K, et al. E‐referrals and teledermatoscopy grading for melanoma: a successful model of care. Australas J Dermatol 2020; 61 147–51.
| E‐referrals and teledermatoscopy grading for melanoma: a successful model of care.Crossref | GoogleScholarGoogle Scholar |
[16] Lim D, Oakley AMM, Rademaker M. Better, sooner, more convenient: a successful teledermoscopy service. Australas J Dermatol 2011; 53 22–5.
| Better, sooner, more convenient: a successful teledermoscopy service.Crossref | GoogleScholarGoogle Scholar |
[17] Carter ZA, Goldman S, Anderson K, et al. Creation of an internal teledermatology store-and-forward system in an existing electronic health record. JAMA Dermatol 2017; 153 644–50.
| Creation of an internal teledermatology store-and-forward system in an existing electronic health record.Crossref | GoogleScholarGoogle Scholar |


