Barriers and facilitators to prescribing medicinal cannabis in New Zealand
Vinuli Withanarachchie 1 * , Marta Rychert 1 , Chris Wilkins 1
1 * , Marta Rychert 1 , Chris Wilkins 1
1 Shore & Whāriki Research Centre, College of Health, Massey University, Auckland, 1010, New Zealand.
Journal of Primary Health Care 15(2) 135-146 https://doi.org/10.1071/HC22122
Published: 2 March 2023
© 2023 The Author(s) (or their employer(s)). Published by CSIRO Publishing on behalf of The Royal New Zealand College of General Practitioners. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
Introduction: The New Zealand Medicinal Cannabis Scheme (NZMCS) was established in April 2020 with the aim of expanding access to quality controlled medicinal cannabis products and developing a domestic medicinal cannabis industry. Yet, two years later, many patients report challenges in utilising the NZMCS, including physicians’ reluctance to provide prescriptions for products.
Aim: To explore the barriers and facilitators to prescribing medicinal cannabis in New Zealand.
Methods: We conducted semi-structured interviews with 31 New Zealand physicians (general practitioners, specialists, and cannabis clinicians) who had discussed medicinal cannabis with patients in the last 6 months.
Results: Physicians reported the principal barrier to prescribing medicinal cannabis was the limited clinical evidence to support cannabis therapy. Further barriers included: a perceived lack of knowledge of medicinal cannabis; concerns over professional reputation; social stigma; and the price of products. Conversely, the factors that facilitated cannabis prescribing included patients’ and physicians’ knowledge of medicinal cannabis; some physicians’ desire to avoid patients having to engage with private cannabis clinics; and the timing of prescription requests (ie considering medicinal cannabis after other treatments had been exhausted).
Discussion: Further clinical research of medicinal cannabis medications, education and training, and information would support physicians to deliver more informed advice to patients and enhance professional confidence with cannabis therapies.
Keywords: access to medicines, cannabinol, CBD, general practice, medical marijuana, medicinal cannabis, physicians, primary health care.
| WHAT GAP THIS FILLS |
| What is already known: The New Zealand Medicinal Cannabis Scheme was established in April 2020 with the intention of widening access to quality controlled cannabis products and developing a domestic cannabis industry. However, access to legal cannabis products remains a challenge for a significant portion of patients, partly due to physicians’ hesitance to prescribe. |
| What this study adds: The barriers and facilitators to prescribing medicinal cannabis in New Zealand are explored through the perspectives of general practitioners, specialists, and cannabis clinicians. The limited evidence of efficacy and clinical trials to support medicinal cannabis was a major concern. Further, drug and reputational risks, perceived lack of knowledge, and costly products underpin clinicians’ hesitance to recommend cannabis therapy to their patients. Patients’ and physicians’ knowledge of medicinal cannabis, physicians’ desire to avoid the patients engaging with private cannabis clinics, and exploring medicinal cannabis as a last resort treatment option, acted as facilitators. |
Introduction
Global demand for medicinal cannabis (MC) has grown in recent decades and a growing number of countries have responded with policies that enable patients to legally access cannabis-based products for medicinal use. Australia, New Zealand (NZ), Canada, UK, many US states, and parts of Europe have adopted prescription-based or medical ‘recommendation’ schemes that require a qualified physician to certify access to cannabis-based products.1,2 Though these schemes are relatively heterogeneous across jurisdictions, they all create a central role for health professionals in the operation of the medicinal cannabis regime. In NZ, any registered physician is able to prescribe ‘approved’ medicinal cannabis products that have been assessed as meeting quality standards under the NZ Medicinal Cannabis Scheme (NZMCS).3
In both the NZMCS and more established regimes overseas, wider adoption of MC into clinical practice has been hindered by physicians’ reluctance to prescribe. This reticence reportedly centres on the limited scientific clinical trial evidence for cannabis therapies, including for which indications there is efficacy and any potential side effects.4–11 Based on the available studies, moderate-level evidence shows cannabinoids reduce spasticity, pain and bladder dysfunction for patients with multiple sclerosis, and there is reasonable evidence to support the use of cannabinoids in chemotherapy-induced nausea and vomiting.12–14 Cannabidiol (CBD) has shown to be well-tolerated and effective in reducing seizures in some childhood epilepsy syndromes (ie Dravet and Lennox–Gastaut syndrome, for patients aged ≥2 years).15,16 The evidence for the effectiveness of cannabis to treat affective disorders, anxiety, and post-traumatic stress disorder is scarce.17 There are isolated cases to support the treatment of schizophrenia, social anxiety, and ADHD symptoms; however, cannabis-based interventions for psychiatric disorders are ‘premature’, and larger studies are required to determine its use in mental health applications.18 A large-scale scoping review of systematic reviews (N = 72) found adverse side-effects were reported by patients in 83% of studies, including psychotic symptoms, severe dysphoric reactions, and seizures, as well as minor adverse physical symptoms (ie drowsiness, dizziness, dry mouth, and nausea).19
Although the strongest empirical evidence for the use of cannabinoid-based products exists only for certain conditions with relatively low patient numbers, a recent narrative review of major reviews and ‘real-world evidence’ studies found improvements in patient-reported outcomes in a range of other symptoms and conditions, including neuropathic pain and cancer-related pain.20 In the case of chronic pain, a 2022 case series of UK patients with a diagnosed chronic pain condition (N = 190) found improvement in pain-related symptoms and other health-related factors (ie anxiety, depression, discomfort, and sleep), in the 1–6 months following cannabis treatment.21 However, since 2018, The Australian and New Zealand College of Anaesthetists and Faculty of Pain Medicine (ANZFPM) have advised against prescribing MC for chronic, non-cancer pain outside of clinical trials due to an insufficient evidence base.22,23 In 2021, the International Association For The Study of Pain’s comprehensive review concluded there was limited evidence to use cannabinoids in the treatment of pain.24 In response, the UK’s Faculty of Pain Medicine of the Royal College of Anaesthetists issued a formal stance similar to that of the ANZFPM.25
Few studies have examined MC prescribing in New Zealand. Rychert et al. found 47% of the 3,634 surveyed MC users had discussed their MC use with their health provider, and only one-third of MC prescription requests were successful.26 Another recent study of 76 NZ general practitioners (GPs) found nearly 80% were reluctant to prescribe MC, citing no evidence for certain conditions, and had limited awareness of regulatory processes in the NZMCS.10 In the Manoharan et al. (2022) study (N = 14), physicians were concerned entering into MC prescribing would open them up to an influx of inappropriate MC requests from patients, thereby damaging their professional reputation.11 Indeed, prior to the NZMCS, Sheridan and Butler (2011) found NZ community pharmacists and GPs (N = 33) were concerned about detecting and responding to potential drug-seeking behaviour, particularly for requests for opioids and benzodiazepines.27 Due to cannabis’ previous prohibited status and stigma, fostering trust in the patient–physician relationship mitigated concerns of drug abuse and facilitated MC prescriptions.11 Current media reports suggest the gap in MC prescribing left by physicians has been met by cannabis clinicians (ie GPs and specialists who position themselves as experienced prescribers of MC), who offer patients consultations in private clinics.28
The aim of this study is to explore, in depth, the factors that create barriers and alternatively facilitate the adoption of MC into clinical practice in New Zealand.
Methods
Participants and recruitment
In an effort to recruit a diverse participant group, the research advertisement was circulated widely to NZ GP practices, hospitals and cannabis clinics via professional communication channels and through the authors’ professional networks. In total, 31 participants who had discussed MC as a treatment option with their patients in the last 6 months provided written consent to participate in interviews between July and December 2021. Participants included 16 GPs (primary care) and 15 specialist clinicians (including seven pain specialists, five working in palliative care, one psychiatrist, one anaesthesiologist, and one gynaecologist). Seven of the physicians self-identified as ‘cannabis clinicians’ (six GPs and one specialist); that is, worked in private cannabis clinics and/or promoted themselves as a prescriber of cannabis-based products. GPs and specialists not working in cannabis clinics will be interchangeably referred to as ‘non-cannabis clinicians’. Ten of the interviewed clinicians practised in privately owned clinics and 21 in GP clinics or hospitals, which are supported by mixed government and private funding. 21 respondents worked in the Auckland area (the biggest city in NZ), seven in the Northland region, and three in the South Island of NZ. Interviewees had practiced medicine from between 5 and 55 years, with a mean of 27.5 years. Twenty physicians in the sample prescribed medicinal cannabis in the past 6 months (all cannabis clinicians, nine GPs and four specialists).
Data collection
Face-to-face semi-structured interviews were designed with the goal of generating higher levels of trust, rapport and disclosure in the interview discussions. Due to coronavirus disease 2019 (COVID-19) lockdown restrictions, the face-to-face interview method was interrupted, resulting in four in-person face-to-face interviews and 27 online interviews conducted via Zoom video conference. Although arguably online face-to-face interviewing may impact the capacity to develop rapport compared to in-person interviewing, online interviewing also provided benefits such as more flexible scheduling, no travel expenses, and access to participants in harder-to-reach areas.29 Interview times ranged from 21 to 94 min, with a mean duration of 53 min. The 36-item, semi-structured interview guide included questions about practitioners’ professional background; clinical experience with MC; views, knowledge and beliefs about MC; understanding of the scientific evidence for cannabis therapy; and discussions with patients about MC. The interview guide was informed by a review of the literature and earlier stages of this project, which involved a survey of MC users26,30 and interviews with patients who discussed their use of cannabis for medicinal reasons with their doctor. The interviews were conducted by the first author, audio-recorded and transcribed ad verbatim.
Data analysis
This study used the inductive approach within thematic analysis to uncover patterns and underlying meanings across the participant data.31 Our analysis explored salient and shared views across participant responses to form a network of core themes. The first and second authors initially read the transcripts to familiarise themselves with the data. The first author then used Nvivo qualitative software (QSR International) to code the data by grouping together views on perceived knowledge, beliefs, concerns, and patient discussions related to prescribing MC. Next, codes were grouped into two metathemes (facilitators and barriers to prescribing MC), with a number of lower-order themes under each (Table 1). To establish investigator triangulation, the first and second authors performed cross-coding comparisons over several meetings to agree on the lower-order themes. The second and third authors provided detailed feedback and cross-checked interpretations over several rounds of discussions to ensure the trustworthiness of the results. The results were reported via the qualitative description method, with participant responses used to add richness of detail to the key themes from the analysis.32
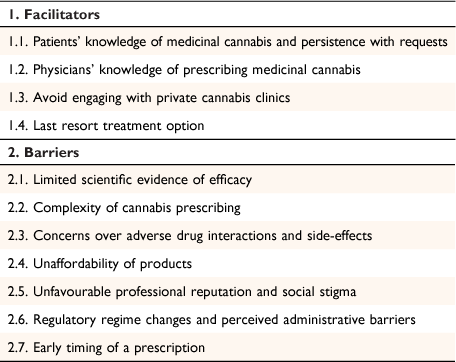
|
Ethics approval
The Massey University Human Ethics Committee reviewed the study protocol and provided ethics approval (SOA 18/85). The study was performed in accordance with ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. Informed consent was gained from participants before the interviews.
Results
Facilitators of cannabis prescribing
Patients’ knowledge of medicinal cannabis and persistence with requests
The most consistent facilitator of prescribing MC product was as a result of a patient request, and in particular, patients’ persistence in making requests. Cannabis conversations were largely patient-led rather than initiated or recommended by physicians, even when physicians had previously prescribed MC products to other patients. Most of the non-cannabis GPs and five specialists had previously prescribed or exhibited an open mind about trialling MC at the request of a patient:
They [patients] will bring it up in the conversation. They’ll say, ‘what do you think of this to try’ and bring it into the conversation. I kind of encourage to discuss it because it’s interesting to know their views and thoughts…’ (Specialist 6)
One non-cannabis specialist stressed the importance of meaningfully engaging with patients during MC discussions despite personal reservations, to avoid patients otherwise purchasing untested cannabis products from the illegal market. This sentiment suggests some physicians learn to deal with the uncertainty of cannabis prescribing through a risk management approach (ie balancing patient safety and interest with the limited clinical information on MC available to them):
If you’re [the patient] motivated and you’re seeking it out, if we weren’t to prescribe it, they might try sourcing it from somewhere else of unknown quantity or quality. We won’t know necessarily what they’re taking because we’ve disengaged with them in their conversation. (Specialist 1)
A cannabis clinician described how witnessing their patients’ experiences with cannabis therapy during their years of general practice engendered confidence in the treatment. It is important to note that the observations in the quote below are based on patients’ self-reports to the doctor, as there is still limited evidence of clinical efficacy to support cannabis therapy for most conditions. There is also the added complexity of the financial profit gained by prescribing unsubsidised cannabis products via private consultations in cannabis clinics:
For almost 40 years as a GP I’ve asked patients, do they drink, do they smoke cigarettes, and do they take drugs, and in almost 40 years, patients have said that smoking cannabis is the only thing that gives them relief from pain, anxiety, depression, and sleep problems. I read the research and those things do respond to cannabis and I’m there for the patient and willing to prescribe. (Cannabis clinician 1)
Several cannabis clinicians attested to having patients who were well-informed about MC, and therefore conversations were often patient-guided. Commonly, these patients had either been recommended MC by a friend/family member and wanted to consult a cannabis clinician for a prescription, or had previously been using cannabis from the illegal market for medical purposes and wanted to explore a legal pathway for accessing it. One cannabis clinician expressed routinely encouraging patients to learn about MC use and guide their own treatment plans, as illustrated in the quote below:
You know a lot of my patients are educating me… because medicinal cannabis is something where the patient really does have to steer the whole process, so we want patients that are awake and understanding of what the pros and cons of medicinal cannabis might be for them. (Cannabis clinician 2)
Both GPs and cannabis clinicians in this study explained that some of their patients had used cannabis illegally for many years to treat their medical conditions, and many reported they found relief. These patients mostly consumed home-grown cannabis, with unregulated levels of cannabidiol (CBD) and tetrahydrocannabinol (THC) content, to treat pain, disturbed sleep, and mental distress. One GP commented that they were willing to prescribe cannabis for medicinal purposes if the patient had experienced safe and effective treatment from using it illegally:
If someone said, ‘hey, I’ve been using dry herb in my heat not burn vaporiser and it works really well for me and I have about this much and that’s my dose and it gets me to sleep’, I’d be happy prescribing that if requested. (GP 7)
Patients’ knowledge of MC was a significant facilitator of MC prescriptions for cannabis clinicians, who encouraged patients to self-educate and lead their treatment. As one cannabis clinician commented, ‘if a person wants to do something… they’re actually already decided that this is a path that they want to follow and so you just provide them with the tool or the medicine’ (Cannabis clinician 2). In contrast, non-cannabis physicians were more conservative about patients self-educating due to fear of online misinformation creating unrealistic expectations of cannabis therapy.
Physicians’ knowledge of prescribing medicinal cannabis
Most non-cannabis GPs (9/10) and the few specialists (4/14) who had prescribed MC were more comfortable continuing a prescription previously initiated by another GP, specialist or cannabis clinician than starting one themselves. One GP described how, through self-education and taking on the practice of prescribing, over time, they overcame their initial discomfort with repeating CBD prescriptions for patients that had been initiated by other physicians. This suggests that after initially hesitating over writing the first prescription, greater confidence can be built with more positive experiences and accumulated knowledge of prescribing:
The bigger concern I had was with the repeating of prescription and I was like ‘oh do I know enough about this to be doing this?’ and decided I was okay with it, that was really my biggest hesitation. The prescriptions I have done for CBD only I sort of, I felt like I was getting confidence after a couple… (GP 10)
Other GPs overcame their initial hesitation to prescribe by consulting cannabis clinics for information on dosage and how to monitor MC treatment, which helped facilitate discussions with their patients and enabled them to prescribe more confidently:
I would say, ‘Look, there is this specialist who runs a clinic and if you would like to go and see them, these are their details,’ and I’d probably provide their name and details of their clinic. I am happy to refer people in an area where I don’t see that there’s enough answer. If they want to further discuss that with someone who’s better informed. (Specialist 4)
Avoid engaging with private cannabis clinics
This lower-order theme was specific to a couple of non-cannabis GPs and specialists who commented they would rather administer a prescription for cannabis-based products themselves than refer their patients to specialised private cannabis clinics, noting three main reasons. First, one GP felt that in general practice, there would likely be a colleague they could rely on for advice on cannabis prescribing, rather than engaging an external provider:
Literally, it can take an hour to learn how to prescribe cannabis products and if there’s one GP that doesn’t know, then surely, there’s another GP in the clinic that the patient can see, rather than going to a different clinic. (GP 4)
Second, many participants highlighted the cost of consultations with private cannabis clinicians as a financial burden on their patients. Several GPs and specialists felt cannabis clinics created further barriers to patients accessing MC due to the high consultation fees. This motivated one GP, in particular, to upskill in cannabis prescribing to avoid patients paying higher than necessary fees to see a cannabis clinician:
I actually think it should be accessible to all and you’re putting a lot of barriers to people in terms of the costs and the kind of clientele they’re serving with those clinics, so that’s why I would rather work within the system that’s already existing, be able to use it rather than making it a separate thing that people have to pay high costs for it. (GP 5)
The third reason GPs and specialists gave for upskilling on MC in order to inform their prescribing was to avoid engaging additional providers in a patient’s treatment plan. The reasons given for avoiding cannabis clinics, particularly by palliative care specialists, were the risks of compartmentalising care, creating conflicts in the treatment plan, and the emotional stress on the patient through having to see and disclose their condition to several different physicians:
I think one of the biggest complaints I hear about is from my patients’ group, is that they all hate talking to more than one person. They just want one person to deal with their issue and I think that’s why I would kind of try – I’d rather upskill everyone to use a single drug. (Specialist 6)
In contrast, one non-cannabis GP commented that they would be unwilling to repeat a MC prescription that had been initiated by a cannabis clinician without assessing the patients’ eligibility for the prescription themselves first:
A young woman who went and saw cannabis doctors as it were and got prescriptions for anxiety. I didn’t have a clue that she had anxiety, she had never seen me about it ever and I’d known this patient for about 10 years. She hasn’t asked for a repeat prescription. In that situation I probably would decline and say how about you come in and we’ll actually assess your anxiety and see if we can use traditional things first. (GP 1)
Last resort treatment option
The timing of the patient's request for a MC prescription during the consultation was reported to be a key factor influencing non-cannabis physicians’ responses. In situations where other treatment options had been discussed and already exhausted, a request for a prescription was more likely to be favourably received by physicians:
I would prescribe it if I was asked to prescribe it but I probably wouldn’t offer it fresh off the bat. For example, I’d say, ‘hey, you’ve tried a tricyclic, I think that the cannabinoids are a third line that they do before things like opiates, and I would say to start with either a CBD or a THC CBD combo orally. (GP 7)
Some palliative care specialists, however, indicated they were more likely than other physicians to trial MC with patients at any stage if it provided them with some relief and they could afford it:
So if I have a patient who’s in excruciating agony in palliative care and they want to try it, then I would definitely say yeah, sure, let’s give it a try, we don’t have much to lose here. (Specialist 13)
Barriers to cannabis prescribing
Limited scientific evidence of efficacy
The lack of clinical trial evidence demonstrating the therapeutic efficacy of cannabis products (conducted by accredited institutions) was described as the greatest barrier to prescribing by non-cannabis GPs and specialists. Several physicians were highly concerned about prescribing MC to patients without a strong evidence base to justify the safety and efficacy of products. Two participants questioned how MC products have entered the clinical field without undergoing the rigorous, scientific testing phases that other medications are subjected to. For example:
Phase one, phase two, phase three, phase four and post-marketing surveillance. It needs to go through that and medicinal cannabis should be no different to medicinal opioids, medicinal antibiotics, medicinal anything… everything we prescribe goes through that pathway… (Specialist 9)
There was a near consensus among non-cannabis GPs and specialists that their interpretation of the scientific evidence available for MC did not result in them preferring it over other mainstream medicines that were already in use. As one specialist (6) describes it, ‘it’s just that I haven’t seen anything convincing that it’s [medicinal cannabis] particularly better than any other treatment that we’ve got at that moment.’ The reasons given were that mainstream treatment options often had stronger proven efficacy, clear indications, and a known risk profile, all of which supported physicians to prescribe these drugs confidently. In contrast, though cannabis clinicians also agreed that overall MC required a greater scientific evidence base, it was less of a barrier to their prescribing, as they felt that there was reasonable evidence to support the use of cannabis treatment for a range of medical conditions, and placed the emphasis on the patient’s experiences and preferences when prescribing.
Concerns over the efficacy of MC were particularly evident in responses by most of the pain specialists in the sample, who felt cannabis is not an effective tool in pain management and there are better evidence-based options available. One pain specialist commented that given the NZMCS preceded an established evidence base for MC products, they did not support further progress of the regime until more clinical data became available:
The problem is the regime has moved ahead in front of the evidence already. I would not want the regime to move ahead at all. I want the regime to step back a bit and wait for the evidence to catch up. (Specialist 11)
Some participants disclosed to patients early into consultations that they were unwilling to prescribe cannabis at all, explaining that there was poor evidence to support cannabis therapy and that they should consider other options. A few participants applied this sentiment to any new medications that were not strongly evidence-based:
I can see a lot of practitioners who would refrain from using it [medicinal cannabis] simply because there is not enough evidence. And this is like any other facet of medicine… what you don’t know, you tend to avoid. (GP 8)
Complexity of cannabis prescribing
The complexity of cannabis prescribing was a key concern expressed by non-cannabis physicians and specialists. Much of these concerns were rooted in the lack of clinical research available on the properties of MC and the chemical variability across products. Participants commented on the dearth of information available to support physicians to make informed prescribing decisions related to MC (ie for which indications cannabis treatment would be appropriate, and how to develop dosing regimens for products). Without a means of reconciling concerns about MC and being tasked with the responsibility to prescribe, physicians were left in a vulnerable position to judge the suitability of MC prescriptions for patients while still grappling with fundamental concerns:
You’re writing a prescription for a substance that you don’t really know how it works and you don’t really know what’s in it. You don’t really know the evidence for it but you just write it on a script pad and then the pharmacy just sells it to the person. (Specialist 13)
When asked about the properties of MC, few of these physicians were able to demonstrate in-depth knowledge, and after further probing, most were unsure about the clinical purpose of CBD and THC, and the effects of prescribing a combined product:
I suppose my discomfort is just my inexperience with it, because I haven’t prescribed it very many times. I am still not that familiar with the dosing, the different types, you know THC, CBD, you know that’s a little bit confusing and the side effects. (GP 1)
Many physicians not working in cannabis clinics believed a CBD–THC combination is more effective in pain management than a CBD product alone. However, some chose to prescribe CBD only due to their fear of the unknown risks, side effects, and psychoactive nature of THC:
I’m generally fairly sceptical about it, I think there is a lot more research that needs to be done to work out just what works for what, it seems that you really need the combination of CBD and THC to perhaps get a pain effect, but the THC adds a whole new layer of complexity when it comes to prescribing. (Specialist 10)
Though all the cannabis clinicians interviewed believed a CBD–THC combined product is safe, natural, and useful for pain, one cannabis clinician had decided to prescribe CBD only in their clinical practice due to the complex impacts THC is perceived to have on individuals under the age of 30 years:
There are issues with THC, excess THC, THC in a developing brain is not healthy so there’s a risk with that yes. But remember all I’m involved in is CBD oil I’m not involved in THC, so I’m involved in the safest of the safe. (Cannabis clinician 5)
Concerns over adverse drug interactions and side-effects
The risk profile and possible side effects of consuming MC products alongside other medications was also considered a barrier to prescribing. Many non-cannabis physicians were concerned they did not understand how cannabis interacts with other medicines they were more familiar with and how this would affect the patient's health:
I don’t prescribe it, [it] is just because it’s not a prescription medication [in my opinion], not everything they say is a prescription, if a medication doesn’t have a side-effect profile, monitoring profile that is set, regardless of what this medication is, even if it’s a medication that is available overseas but not here in New Zealand, I’m not for prescribing it. (GP 9)
Most participants felt there was a lack of guidance available on cannabis dosing and titration, and as a result, they were forced to prescribe based on clinical judgement and advice from their colleagues, which deterred some doctors from prescribing it at all. Most frequently, the ambiguity surrounding dosing MC resulted in non-cannabis physicians in this study choosing to prescribe mainstream medicines over MC:
I actually know what they’re getting when I prescribe them medicines. I can titrate their medicines. I can switch medicines depending on the side effects they have. I can switch medicines based on that. I feel like cannabis does not – because it’s not regulated really, in the way that we need it to be for a pharmaceutical. (GP 7)
In some situations, hesitant physicians empathised with patients and agreed to trial MC at a low dose, with slow titration, and close monitoring. Several physicians used this cautious approach to create a safe environment for their patients to explore MC as a treatment option; however, they expressed unease about not having access to the resources that could support them in monitoring the prescription:
It’s just the uncertainty because as a doctor, any drug that we prescribe, we sort of like to know the mechanism of action. The side effects, the drug interactions and long-term consequences. All of that sort of stuff and I just don’t feel comfortable until I’ve got that knowledge. (GP 3)
Participants also discussed accessing MC information from trusted sources as supporting their decision to prescribe. Most participants drew their knowledge from literature in peer-reviewed journals, and resources provided by the Best Practice Advocacy Centre, Ministry of Health (MOH), Medsafe, and the Goodfellow Unit, which delivers continuing professional development for primary healthcare professionals. Many commented, however, that these resources were scarce and at times outdated; therefore, they sought resources from Australia and Canada, despite these sometimes having less relevance to the New Zealand environment:
It really doesn’t make very easy reading from my perspective or understanding and then they’re horribly out of date and not very much depth to what they’ve written and I use a lot of international websites for information but then of course it’s not directly relevant to here in terms of what we can prescribe. (GP 5)
Unaffordability of products
Most non-cannabis GPs and specialists in this study commented that the high price of approved cannabis-based products was a significant barrier to recommending and prescribing them. Participants considered the average cost of $300–$400 for a cannabis product (eg as estimated by Cannabis clinician 6 at the time of interviewing) to be unaffordable for patients. This sentiment was particularly evident among those practising in poorer areas such as South Auckland and Northland. This issue was compounded by the concern that the medication might not be effective in relieving symptoms for the patient:
It’s just a cruel and unusual punishment to give a patient a chemical as a trial that they’ll never be able to afford long term. (Specialist 12)
Some participants commented that CBD-only products are safer than other pain medications; however, they felt that as the latter are subsidised by Pharmac (the government drug-buying agency that decides which medicines are publicly funded), an unsuccessful trial would not cause unnecessary financial costs to the patient in the same way as an unsuccessful CBD-only trial. One specialist argued that if cannabis-based products were subsidised, the low cost of trialling them for individual patients would motivate physicians to consider them as a treatment option, like other affordable medications:
You’re not thinking about a non-subsidised medication the person can’t pay for out of pocket. You’re thinking okay what can I prescribe from Pharmac that they can afford so you have $5 co-pay. I’m not going to prescribe something that I think is going to cost them $350 a month you know because they’re not going to be able to pay it so it wouldn’t even enter my mind. So if doctors knew that this stuff can be subsidised then that’s different and then that becomes more and more things you can prescribe. (Specialist 7)
A few respondents commented that the high cost of approved MC products has further contributed to inequitable access to health care in NZ, as only patients who can afford it, can consider medicinal cannabis as a treatment option. One GP described their view of the main patient demographic for MC:
Who has access to it is people that can afford it. It’s not the average patient for me. It’s the working, probably my age, 50ish working woman, who’s kids have left home, who has a little bit of anxiety and wants it now and again. Or the elderly affluent that want it for their pain. (GP 2)
Notably, two GPs suggested they were more inclined for patients to continue consuming homegrown cannabis if they had prior, positive experiences with it, even though it is non-prescribed and illegal in NZ. This was justified in terms of their patients’ inability to afford prescribed MC products. As more domestic legal cannabis products become available under the MCS and the price of products lowers, physicians may be less inclined in the future to overlook patients accessing cannabis illegally for medicinal use and encourage utilisation of the MCS.
Unfavourable professional reputation and social stigma
During interviews, some non-cannabis physicians expressed hesitation to prescribe MC, as they felt it would attract negative comments from their peers or negatively impact patients and public perceptions of their professional reputation. For example, one GP working in a rural clinic stated they were only one of two doctors willing to prescribe MC in their practice, but they did not actively communicate this to patients to avoid being personally sought out for a prescription:
I wouldn’t have it as part of my bio on the website and definitely don’t want to be kind of known as a “cannabis doctor”, I only really do it if a patient brings it up with me, rather than the other way round. My nervousness around is if I become then the cannabis doctor of [city name] because we are a small place you know and I don’t want to have every patient coming at me left right and centre expecting that. (GP 10)
Some physicians indicated that their peers’ views of adopting MC into clinical practice shaped their own prescribing behaviours. They preferred to align their prescribing practices with that of their colleagues, and thus delayed recommending MC to patients if their peers within the clinic were slow to adopt it:
I don’t recommend it [medicinal cannabis] as my first option because we have other options that are kind of recommended I suppose and so you tend to lean towards that to ensure that your own personal practice is reflective of your peers essentially. That’s a driver. (Specialist 1)
One pain specialist commented that the Faculty of Pain Medicine, with which they are affiliated, strongly considers prescribing MC to be ‘unethical’ and unsupported by sufficient evidence for the treatment of persistent pain or chronic pain. Though this may be a significant disincentive for other health professionals to prescribe, they and their colleagues had collectively taken a non-confirmative stance and decided to prescribe it based on their clinical judgement. They articulated how this is communicated to patients:
I would be very clear that this is controversial, very controversial, very open that the body that I’m affiliated with, the Pain Faculty, says it’s unethical. So, I put that on the table. And I say I have had some people who find it useful, especially for sleep, and also that even though the evidence suggests it’s not useful, there’s a group of people that I see in a clinic, so anecdotally there’s a group of people that do tend to find it useful. (Specialist 15)
Regulatory regime changes and perceived administrative burden
Evident across the interviews was frustration at the lack of communication physicians had received about the staggered implementation process of the NZMCS. As one specialist (15) put it, ‘there’s these gradual changes that are creeping in without a clear pathway or guidance around it.’ In response, physicians have had to allocate their already limited time to stay informed on the NZMCS, and as one specialist (2) described it, ‘sifting through all these websites to try and find what had happened and you know dig out all this information out ourselves.’
Many physicians stated that they were not informed of changes as they happened through the phased implementation of the NZMCS, and voiced scenarios in which they were unable to confidently guide MC discussions with their patients. One specialist described a vulnerable interaction in which their patient corrected their knowledge of the NZMCS:
I had someone asking me for a script… and I said ‘well you know you don’t kind of fit the criteria’ and then actually something else had changed and they could access it… I felt like it’s kind of embarrassing being behind the eight ball and not knowing what’s happening. (Specialist 2)
It was also frequently expressed in our study that regulatory regime changes deterred some physicians from exploring MC products as a treatment option because they struggled to stay informed about which products had been approved and at what financial cost. These physicians were already overworked and inundated with ongoing changes to other more commonly used medications; therefore, MC was only explored when necessary:
We’re overwhelmed by multiple things on a multiple basis and it may not be until you’re faced with a choice that you’re having to prescribe it [medicinal cannabis] that you’ll go and seek out those things. (Specialist 4)
When asked about their knowledge of the NZMCS and its products, many GPs could not confidently state which products had been approved before and after the interim MCS scheme, which ended in September 2021, and they were confused by the approved/unapproved terminology used to describe product status:
They [GPs] also believe that you are putting yourself at risk for prescribing something that’s so called unapproved. So, the category actually is a disincentive. You know that it’s been classified as unapproved. So, they don’t feel that they have the backing of the medical profession to prescribe it. (Cannabis clinician 2)
It is also important to note that at the time of the interviews, none of the non-cannabis clinicians knew that vaping MC was permitted under the NZMCS, illustrating a lack of knowledge about the details of the regime. The quote below reflected the views of many of the GPs and specialists interviewed:
I was not aware that the new legislation had shifted that much. I have real problems with that… It’s a way of people legally and without being prosecuted being able to utilise smoked cannabis. (Specialist 8)
Early timing of a prescription
When receiving frequent patient requests for MC too early into consultations, or when other established treatment pathways had not yet been tried, many non-cannabis physicians would openly communicate their opposition to prescribing medicinal cannabis to such patients. This was particularly evident among specialists. One specialist described the stance taken by their practice regarding making it clear to patients that they did not support immediate use of cannabis therapy, even at the patients’ request:
We actually made a conscious decision to turn down those referrals. What we said is, ‘We’re happy to see you and we’re also happy to discuss medicinal cannabinoids as one aspect of managing your chronic pain, but we’re not going to recommend medicinal cannabinoids because actually the evidence is not good for it.’ (Specialist 8)
Discussion
The aim of this study was to explore the barriers and facilitators to prescribing MC in New Zealand. Our findings resemble international studies4–11 that identified a limited evidence base for MC as the principal barrier to prescribing. Most physicians in this study self-educated by sourcing cannabis-specific medical literature, as well as studies suggested by their colleagues and patients, to inform their prescribing decisions. This finding is dichotomous with the study by Manoharan et al. in which NZ physicians were reluctant to self-educate due to pre-determined views about MC.11 Recent studies have also argued that the limited formal MC training, combined with access to various unverified cannabis information on the Internet, may challenge GPs’ abilities to effectively counsel patients in cannabis treatment.33–36 In accordance with the findings of other studies, our participants agreed that MC education and training are imperative to improve understanding and preparedness to discuss MC in clinical settings.37,38 The MC information sheet developed by the Best Practice Advocacy Centre New Zealand may support health professionals to facilitate MC discussions and prescribe confidently.39 Research suggests experienced and educated cannabis prescribers may be better placed to evaluate the benefits and harms of prescribing cannabis, and discuss the trade-offs with patients.8 Reflected in our study, physicians’ ability to make clinical decisions related to MC improved with knowledge gain and experience.
Participants also reported a number of novel factors that facilitated MC prescribing, including exploring MC after exhausting other treatment options, previous positive experiences of prescribing cannabis, and patients’ existing use of illegally procured cannabis for medical reasons and reporting positive outcomes. Most physicians in this study trusted patients that had previously used illegal cannabis for medical purposes and agreed to provide them with a prescribed alternative, unlike other studies that have observed fear of drug abuse.11 This approach may reflect a patient-guided shared decision-making process, which emphasises trust and considers individual medical history.40 Trust in the patient–physician relationship is associated with greater treatment adherence, patient satisfaction, health outcomes, and disclosure rates.41–43 Considering cannabis’ close ties with the illicit unregulated market and previous studies reporting patients’ discomfort discussing MC with health providers,26 fostering trust in the patient–physician relationship may help alleviate stigma in MC discussions.11
The desire to avoid outsourcing prescribing to private cannabis clinics, which many non-cannabis clinicians considered too expensive and not offering comprehensive care, is a new factor we identified that facilitates non-cannabis physicians' prescribing. This may be specific to NZ, where cannabis clinics do not have an established role in the health system yet as they do in some jurisdictions overseas. In Canada, for example, studies report some GPs refer patients to cannabis clinics, though not legally required to, thereby the patient ‘hand off’ facilitates a smooth transfer of patient care between clinics.44–46 NZ cannabis clinics do not require a GP or specialist referral; therefore, there is a greater risk of dissonance between medical providers when treating patients.28 This challenge may require close attention as the number of cannabis clinics continues to increase in NZ.
Our research study identified other unique barriers to prescribing cannabis in NZ, including the changing regulations under the MCS, and reputational risks related to MC prescribing with clinical peers, patients, and the wider community within smaller rural practices. One NZ study (N = 14) demonstrated that garnering a negative reputation for being a MC prescriber by peers was a significant concern for NZ physicians.11 A survey of 11 958 clinicians in Pennsylvania (US) found peer networks exert significant influence over physicians’ prescribing practices, particularly when patient care is shared among practitioners in the clinic.47 An Israeli study of 272 physicians identified that the views of professional peers and associated practice were a greater predictor of intent to recommend MC than perceived knowledge of the treatment.48 Indeed, reputation was an important factor in our study that influenced physicians’ decision/or not to prescribe MC. This finding lends itself to a wider discussion on the need for early medical training on MC to address preconceived construals about cannabis prescribing.
More broadly, barriers and facilitators to prescribing MC parallel the adoption of new therapies into clinical practice in general. Examples of factors that affect day-to-day practices around prescribing new medicines include peer-reviewed journals as sources of information,49,50 early experiences prescribing specific drugs,51 professional peer influences,52,53 and geographical location (urban doctors are more likely to adopt new drugs than rural physicians).54,55 Other indicators centre on attitudes and influences of patients and management (ie patients’ past medical history (poor response to current treatments and previous experiences with drugs),56 ambiguous drug guidelines and risk profiles,57 and financial cost to the patient).58 One large-scale review reported, however, that financial cost is less of a determining factor in prescribing new drugs by GPs than safety and efficacy.47 Patients’ interest and requests for new drugs are also influencing factors in physicians’ prescribing decisions.59 For example, one survey showed 88 out of 107 GPs interviewed identified patients’ requests for new medicines as an important factor, and commented that prescription requests would be obliged to maintain the physician–patient relationship, avoid confrontation, and when current treatments failed.60
NZ GPs have indicated they would be more likely to prescribe MC if it was funded by Pharmac and supported by scientific evidence for specific conditions.10 Subsidising MC products would likely require a significant budget commitment by Pharmac, which may be difficult to justify due to insufficient evidence to support its use and competing funding demands. Going forward, investment in well-designed randomised controlled trials (RCT) on MC must be prioritised to evaluate the safety and efficacy of products and inform clinical decision-making.61 Current high-quality RCT evidence for MC is limited,62 and barriers to attaining more clinical data (ie high costs, regulatory restrictions, and lack of sustainable funding) persist.20,63 Real-world evidence (ie public health registries, electronic medical records) from prescribed MC users may be used in conjunction with findings from RCTs to aid regulatory decision-making and to develop the evidence base.64,65
Limitations
The recent establishment of New Zealand’s MCS mean physicians in this study may have been less informed on and experienced with cannabis prescribing compared to medical practitioners overseas, where schemes have been in place longer. The purposive sampling in this research enabled in-depth interviews with physicians in New Zealand who had discussed medicinal cannabis with their patients recently; however, it does not represent the views of all practitioners.
Conclusion
In conclusion, a number of the identified factors that are barriers to prescribing MC products in New Zealand are similar to those affecting medicinal cannabis schemes overseas. These include a limited scientific evidence base, previous prohibited status and stigma, the regulatory complexity of medicinal cannabis regimes, the illegal market supply of cannabis, and patient demand placing clinicians under pressure to prescribe. More clinical trial evidence evaluating the safety and efficacy of MC products will ultimately facilitate prescribing of cannabis therapies. Issuing suppliers a single permit to undertake the manufacturing of MC products, as Australia’s Therapeutic Goods Administration is considering, would also reduce the current regulatory burden. A focus on providing continuing medical education on MC, in particular resources on dosing, side effects, and interactions with other mainstream drugs, could also reduce physicians’ concerns about prescribing. We have also proposed in the past that New Zealand may consider reclassifying MC products as over-the-counter alternative therapies without requiring a prescription.28,66 This would remove physicians as the gatekeepers to some types of cannabis-based medicines, which will eliminate current access barriers to MC products caused by physicians’ hesitance to prescribe. Other immediate initiatives to consider are clear communication of regulatory changes to the NZMCS as they occur, and training on MC from trusted sources to improve confidence in prescribing. These initiatives may mitigate some of the personal and professional barriers reported by physicians when considering MC as a treatment option and contribute to more informed clinical decision-making and patient advice.
Data availability
The larger dataset generated and analysed during the current study is not publicly available due to confidentiality reasons (ie contains information that could compromise research participant privacy/consent), but may be available from the second author on reasonable request (m.rychert@massey.ac.nz).
Conflicts of interest
The authors declare no competing interests.
Declaration of funding
The research was funded by the New Zealand Health Research Council grant (19/647).
References
[1] Aguilar S, Gutiérrez V, Sánchez L, et al. Medicinal cannabis policies and practices around the world. Briefing paper; International Drug Policy Consortium, London, The United Kingdom; 2018. pp. 1–32.[2] Abuhasira R, Shbiro L, Landschaft Y. Medical use of cannabis and cannabinoids containing products – Regulations in Europe and North America. Eur J Intern Med 2018; 49 2–6.
| Medical use of cannabis and cannabinoids containing products – Regulations in Europe and North America.Crossref | GoogleScholarGoogle Scholar |
[3] Withanarachchie V, Rychert M, Wilkins C. Assessing progress with the implementation of the New Zealand Medicinal Cannabis Scheme. N Z Med J 2022; 135 7–12.
[4] Nutt D, Bazire S, Phillips LD, et al. So near yet so far: why won’t the UK prescribe medical cannabis? BMJ Open 2020; 10 e038687
| So near yet so far: why won’t the UK prescribe medical cannabis?Crossref | GoogleScholarGoogle Scholar |
[5] Philpot LM, Ebbert JO, Hurt RT. A survey of the attitudes, beliefs and knowledge about medical cannabis among primary care providers. BMC Fam Pract 2019; 20 17
| A survey of the attitudes, beliefs and knowledge about medical cannabis among primary care providers.Crossref | GoogleScholarGoogle Scholar |
[6] Zolotov Y, Vulfsons S, Zarhin D, et al. Medical cannabis: an oxymoron? Physicians’ perceptions of medical cannabis. Int J Drug Policy 2018; 57 4–10.
| Medical cannabis: an oxymoron? Physicians’ perceptions of medical cannabis.Crossref | GoogleScholarGoogle Scholar |
[7] Carlini BH, Garrett SB, Carter GT. Medicinal cannabis: a survey among health care providers in Washington State. Am J Hosp Palliative Care 2017; 34 85–91.
| Medicinal cannabis: a survey among health care providers in Washington State.Crossref | GoogleScholarGoogle Scholar |
[8] Rønne ST, Rosenbæk F, Pedersen LB, et al. Physicians’ experiences, attitudes, and beliefs towards medical cannabis: a systematic literature review. BMC Fam Pract 2021; 22 212
| Physicians’ experiences, attitudes, and beliefs towards medical cannabis: a systematic literature review.Crossref | GoogleScholarGoogle Scholar |
[9] Hachem Y, Abdallah SJ, Rueda S, et al. Healthcare practitioner perceptions on barriers impacting cannabis prescribing practices. BMC Complement Med Ther 2022; 22 237
| Healthcare practitioner perceptions on barriers impacting cannabis prescribing practices.Crossref | GoogleScholarGoogle Scholar |
[10] Oldfield K, Braithwaite I, Beasley R, et al. Medical cannabis: knowledge and expectations in a cohort of North Island New Zealand general practitioners. N Z Med J 2020; 133 12–28.
[11] Manoharan R, Kemper J, Young J. Exploring the medical cannabis prescribing behaviours of New Zealand physicians. Drug Alcohol Rev 2022; 41 1355–66.
| Exploring the medical cannabis prescribing behaviours of New Zealand physicians.Crossref | GoogleScholarGoogle Scholar |
[12] Bowling AC. Multiple Sclerosis and Cannabis – Benefits, Risks, and Special Considerations. In: Finn K, editor. Cannabis in Medicine – An Evidence-based Approach. Switzerland: Springer Nature Publishing; 2020. pp. 246–9.
| Crossref |
[13] Nielsen S, Germanos R, Weier M, et al. The use of cannabis and cannabinoids in treating symptoms of multiple sclerosis: a systematic review of reviews. Curr Neurol Neurosci Rep 2018; 18 8
| The use of cannabis and cannabinoids in treating symptoms of multiple sclerosis: a systematic review of reviews.Crossref | GoogleScholarGoogle Scholar |
[14] Allan GM, Finley CR, Ton J, et al. Systematic review of systematic reviews for medical cannabinoids: pain, nausea and vomiting, spasticity, and harms. Can Fam Physician 2018; 64 e78–94.
[15] Tzadok M, Uliel-Siboni S, Linder I, et al. CBD-enriched medical cannabis for intractable pediatric epilepsy: the current Israeli experience. Seizure 2016; 35 41–4.
| CBD-enriched medical cannabis for intractable pediatric epilepsy: the current Israeli experience.Crossref | GoogleScholarGoogle Scholar |
[16] Chen JW, Borgelt LM, Blackmer AB. Cannabidiol: a new hope for patients with Dravet or Lennox-Gastaut syndromes. Ann Pharmacother 2019; 53 603–11.
| Cannabidiol: a new hope for patients with Dravet or Lennox-Gastaut syndromes.Crossref | GoogleScholarGoogle Scholar |
[17] Stanciu CN, Brunette MF, Teja N, et al. Evidence for use of cannabinoids in mood disorders, anxiety disorders, and PTSD: a systematic review. Psychiatr Serv 2021; 72 429–36.
| Evidence for use of cannabinoids in mood disorders, anxiety disorders, and PTSD: a systematic review.Crossref | GoogleScholarGoogle Scholar |
[18] Sarris J, Sinclair J, Karamacoska D, et al. Medicinal cannabis for psychiatric disorders: a clinically-focused systematic review. BMC Psychiatry 2020; 20 24
| Medicinal cannabis for psychiatric disorders: a clinically-focused systematic review.Crossref | GoogleScholarGoogle Scholar |
[19] Pratt M, Stevens A, Thuku M, et al. Benefits and harms of medical cannabis: a scoping review of systematic reviews. Syst Rev 2019; 8 320
| Benefits and harms of medical cannabis: a scoping review of systematic reviews.Crossref | GoogleScholarGoogle Scholar |
[20] Schlag AK, O’Sullivan SE, Zafar RR, et al. Current controversies in medical cannabis: recent developments in human clinical applications and potential therapeutics. Neuropharmacology 2021; 191 108586
| Current controversies in medical cannabis: recent developments in human clinical applications and potential therapeutics.Crossref | GoogleScholarGoogle Scholar |
[21] Harris M, Erridge S, Ergisi M, et al. UK Medical Cannabis registry: an analysis of clinical outcomes of medicinal cannabis therapy for chronic pain conditions. Expert Rev Clin Pharmacol 2022; 15 473–85.
| UK Medical Cannabis registry: an analysis of clinical outcomes of medicinal cannabis therapy for chronic pain conditions.Crossref | GoogleScholarGoogle Scholar |
[22] Australia New Zealand College of Anaesthetists. Never too young… Embracing National Anaesthesia Day. ANZCA Bulletin, December 2018. Sect. NAD 18, opioids and anaphylaxis hot topics for media coverage. p. 8. Available at https://www.anzca.edu.au/getattachment/34dcc7f7-8d0d-445b-8f9a-bb9fc2bd6bc2/ANZCA-Bulletin-December-2018 [Accessed 6 November 2022].
[23] Newton-Howes G, McBride S. Cannabis in New Zealand: smoking gun or medicalised smokescreen? N Z Med J 2016; 129 13–6.
[24] IASP Position Statement on the Use of Cannabinoids to Treat Pain. Washington, DC, USA: International Association For The Study of Pain; 2021. Available at https://www.iasp-pain.org/publications/iasp-news/iasp-position-statement-on-the-use-of-cannabinoids-to-treat-pain/?ItemNumber=11145&navItemNumber=643 [Accessed 6 November 2022].
[25] Update: Faculty position statement on the medicinal use of Cannabinoids in Pain Medicine. London, UK: Faculty of Pain of the Royal College of Anaesthetists; 2021. Available at https://fpm.ac.uk/update-faculty-position-statement-medicinal-use-cannabinoids-pain-medicine [Accessed 6 November 2022].
[26] Rychert M, Wilkins C, Parker K, et al. Exploring medicinal use of cannabis in a time of policy change in New Zealand. N Z Med J 2020; 133 54–69.
[27] Sheridan J, Butler R. Prescription drug misuse in New Zealand: challenges for primary health care professionals. Res Social Adm Pharm 2011; 7 281–93.
| Prescription drug misuse in New Zealand: challenges for primary health care professionals.Crossref | GoogleScholarGoogle Scholar |
[28] Withanarachchie V, Rychert M, Wilkins C. The role of cannabis clinics in the health system: a qualitative study of physicians’ views in New Zealand. BMC Health Serv Res 2023; 23 10
| The role of cannabis clinics in the health system: a qualitative study of physicians’ views in New Zealand.Crossref | GoogleScholarGoogle Scholar |
[29] Keen S, Lomeli-Rodriguez M, Joffe H. From challenge to opportunity: virtual qualitative research during COVID-19 and beyond. Int J Qual Methods 2022; 21 16094069221105075
| From challenge to opportunity: virtual qualitative research during COVID-19 and beyond.Crossref | GoogleScholarGoogle Scholar |
[30] Rychert M, Wilkins C, Parker K, et al. Predictors of medicinal cannabis users’ willingness to utilise a new prescription medicinal cannabis scheme in New Zealand. N Z Med J 2021; 134 66–75.
[31] Braun V, Clarke V, Terry G, et al. Thematic analysis. In: Liamputtong P, editor. Handbook of research methods in health and social sciences. Singapore: Springer; 2018. pp. 843–60.
[32] Sandelowski M. Whatever happened to qualitative description? Res Nurs Health 2000; 23 334–40.
| Whatever happened to qualitative description?Crossref | GoogleScholarGoogle Scholar |
[33] Felnhofer A, Kothgassner OD, Stoll A, et al. Knowledge about and attitudes towards medical cannabis among Austrian university students. Complement Ther Med 2021; 58 102700
| Knowledge about and attitudes towards medical cannabis among Austrian university students.Crossref | GoogleScholarGoogle Scholar |
[34] Benavides A, Gregorio N, Gupta P, et al. Medical students are unprepared to counsel patients about medical cannabis and want to learn more. Complement Ther Med 2020; 48 102237
| Medical students are unprepared to counsel patients about medical cannabis and want to learn more.Crossref | GoogleScholarGoogle Scholar |
[35] Jain R, Chang CC, Koto MA, et al. Cannabis use and knowledge among medical students at the University of the Free State, Bloemfontein, South Africa. J Child Adolesc Ment Health 2018; 30 19–26.
| Cannabis use and knowledge among medical students at the University of the Free State, Bloemfontein, South Africa.Crossref | GoogleScholarGoogle Scholar |
[36] Chung AK-K, Tse C-Y, Law JK-C. Attitudes and beliefs of medical students on cannabis in Hong Kong. Complement Ther Med 2022; 70 102870
| Attitudes and beliefs of medical students on cannabis in Hong Kong.Crossref | GoogleScholarGoogle Scholar |
[37] Weisman JM, Rodríguez M. A systematic review of medical students’ and professionals’ attitudes and knowledge regarding medical cannabis. J Cannabis Res 2021; 3 47
| A systematic review of medical students’ and professionals’ attitudes and knowledge regarding medical cannabis.Crossref | GoogleScholarGoogle Scholar |
[38] Bawa Z, McCartney D, Manocha R, et al. Knowledge, experiences, and attitudes of Australian General Practitioners towards medicinal cannabis: a 2021–2022 survey. BMC Prim Care 2022; 23 330
| Knowledge, experiences, and attitudes of Australian General Practitioners towards medicinal cannabis: a 2021–2022 survey.Crossref | GoogleScholarGoogle Scholar |
[39] The Best Practice Advocacy Centre New Zealand. An overview of medicinal cannabis for health practitioners. Best Practice Advocacy Centre New Zealand; 2022. Available at https://bpac.org.nz/2022/medicinal-cannabis.aspx [Accessed 9 November 2022].
[40] Fuertes JN, Toporovsky A, Reyes M, et al. The physician-patient working alliance: theory, research, and future possibilities. Patient Educ Couns 2017; 100 610–5.
| The physician-patient working alliance: theory, research, and future possibilities.Crossref | GoogleScholarGoogle Scholar |
[41] Birkhäuer J, Gaab J, Kossowsky J, et al. Trust in the health care professional and health outcome: a meta-analysis. PLoS One 2017; 12 e0170988
| Trust in the health care professional and health outcome: a meta-analysis.Crossref | GoogleScholarGoogle Scholar |
[42] Olaisen RH, Schluchter MD, Flocke SA, et al. Assessing the longitudinal impact of physician-patient relationship on functional health. Ann Fam Med 2020; 18 422–9.
| Assessing the longitudinal impact of physician-patient relationship on functional health.Crossref | GoogleScholarGoogle Scholar |
[43] Chandra S, Mohammadnezhad M, Ward P. Trust and communication in a doctor-patient relationship: a literature review. J Healthc Commun 2018; 3 36
[44] Prosk E, Arboleda MF, Rapin L, et al. The model of care at a leading medical cannabis clinic in Canada. Complement Ther Med 2021; 60 102740
| The model of care at a leading medical cannabis clinic in Canada.Crossref | GoogleScholarGoogle Scholar |
[45] Rapin L, Gamaoun R, El Hage C, et al. Cannabidiol use and effectiveness: real-world evidence from a Canadian medical cannabis clinic. J Cannabis Res 2021; 3 19
| Cannabidiol use and effectiveness: real-world evidence from a Canadian medical cannabis clinic.Crossref | GoogleScholarGoogle Scholar |
[46] Ng JY, Gilotra K, Usman S, et al. Attitudes toward medical cannabis among family physicians practising in Ontario, Canada: a qualitative research study. CMAJ Open 2021; 9 E342–8.
| Attitudes toward medical cannabis among family physicians practising in Ontario, Canada: a qualitative research study.Crossref | GoogleScholarGoogle Scholar |
[47] Donohue JM, Guclu H, Gellad WF, et al. Influence of peer networks on physician adoption of new drugs. PLoS One 2018; 13 e0204826
| Influence of peer networks on physician adoption of new drugs.Crossref | GoogleScholarGoogle Scholar |
[48] Zolotov Y, Vulfsons S, Sznitman S. Predicting physicians’ intentions to recommend medical cannabis. J Pain Symptom Manage 2019; 58 400–7.
| Predicting physicians’ intentions to recommend medical cannabis.Crossref | GoogleScholarGoogle Scholar |
[49] McGettigan P, Golden J, Fryer J, et al. Prescribers prefer people: the sources of information used by doctors for prescribing suggest that the medium is more important than the message. Br J Clin Pharmacol 2001; 51 184–9.
| Prescribers prefer people: the sources of information used by doctors for prescribing suggest that the medium is more important than the message.Crossref | GoogleScholarGoogle Scholar |
[50] Jacoby A, Smith M, Eccles M. A qualitative study to explore influences on general practitioners’ decisions to prescribe new drugs. Br J Gen Pract 2003; 53 120–5.
[51] Jones MI, Greenfield SM, Bradley CP. Prescribing new drugs: qualitative study of influences on consultants and general practitioners. BMJ 2001; 323 378
| Prescribing new drugs: qualitative study of influences on consultants and general practitioners.Crossref | GoogleScholarGoogle Scholar |
[52] Manchanda P, Xie Y, Youn N. The role of targeted communication and contagion in product adoption. Market Sci 2008; 27 961–76.
| The role of targeted communication and contagion in product adoption.Crossref | GoogleScholarGoogle Scholar |
[53] Lin SJ, Jan KA, Kao JT. Colleague interactions and new drug prescribing behaviour: the case of the initial prescription of antidepressants in Taiwanese medical centres. Soc Sci Med 2011; 73 1208–13.
| Colleague interactions and new drug prescribing behaviour: the case of the initial prescription of antidepressants in Taiwanese medical centres.Crossref | GoogleScholarGoogle Scholar |
[54] Tamblyn R, McLeod P, Hanley JA, et al. Physician and practice characteristics associated with the early utilization of new prescription drugs. Med Care 2003; 41 895–908.
| Physician and practice characteristics associated with the early utilization of new prescription drugs.Crossref | GoogleScholarGoogle Scholar |
[55] Bourke J, Roper S. In with the new: the determinants of prescribing innovation by general practitioners in Ireland. Eur J Health Econ 2012; 13 393–407.
| In with the new: the determinants of prescribing innovation by general practitioners in Ireland.Crossref | GoogleScholarGoogle Scholar |
[56] Lublóy Á. Factors affecting the uptake of new medicines: a systematic literature review. BMC Health Serv Res 2014; 14 469
| Factors affecting the uptake of new medicines: a systematic literature review.Crossref | GoogleScholarGoogle Scholar |
[57] Harrison M, Marra CA, Bansback N. Preferences for ‘new’ treatments diminish in the face of ambiguity. Health Econ 2017; 26 743–52.
| Preferences for ‘new’ treatments diminish in the face of ambiguity.Crossref | GoogleScholarGoogle Scholar |
[58] de Almedia Neto AC, Tobin L, Wutzke S, et al. Influences on the prescribing of new drugs. Aust Fam Physician 2008; 37 78–81.
| Influences on the prescribing of new drugs.Crossref | GoogleScholarGoogle Scholar |
[59] Liu Q, Gupta S. A micro-level diffusion model for new drug adoption. J Prod Innov Manage 2012; 29 372–84.
| A micro-level diffusion model for new drug adoption.Crossref | GoogleScholarGoogle Scholar |
[60] Prosser H, Almond S, Walley T. Influences on GPs’ decision to prescribe new drugs—the importance of who says what. Fam Pract 2003; 20 61–8.
| Influences on GPs’ decision to prescribe new drugs—the importance of who says what.Crossref | GoogleScholarGoogle Scholar |
[61] Russo EB. Current therapeutic cannabis controversies and clinical trial design issues. Front Pharmacol 2016; 7 309
| Current therapeutic cannabis controversies and clinical trial design issues.Crossref | GoogleScholarGoogle Scholar |
[62] Braithwaite I, Bhagavan C, Doppen M, et al. Medicinal applications of cannabis/cannabinoids. Curr Opin Psychol 2021; 38 1–10.
| Medicinal applications of cannabis/cannabinoids.Crossref | GoogleScholarGoogle Scholar |
[63] Graham M, Lucas CJ, Schneider J, et al. Translational hurdles with cannabis medicines. Pharmacoepidemiol Drug Saf 2020; 29 1325–30.
| Translational hurdles with cannabis medicines.Crossref | GoogleScholarGoogle Scholar |
[64] Banerjee R, Erridge S, Salazar O, et al. Real world evidence in medical cannabis research. Ther Innov Regul Sci 2022; 56 8–14.
| Real world evidence in medical cannabis research.Crossref | GoogleScholarGoogle Scholar |
[65] Sakal C, Lynskey M, Schlag AK, et al. Developing a real-world evidence base for prescribed cannabis in the United Kingdom: preliminary findings from Project Twenty21. Psychopharmacology 2022; 239 1147–55.
| Developing a real-world evidence base for prescribed cannabis in the United Kingdom: preliminary findings from Project Twenty21.Crossref | GoogleScholarGoogle Scholar |
[66] Rychert M, Wilkins C, Withanarachchie V. Why it’s time to treat medicinal cannabis as an alternative therapy, not a pharmaceutical. The Conversation, October 2021. Available at https://theconversation.com/why-its-time-to-treat-medicinal-cannabis-as-an-alternative-therapy-not-a-pharmaceutical-169458 [Accessed 17 August 2022].


