Managing the misuse potential and risk of psychological harm from gabapentinoids in primary care in New Zealand
Shaun Aindow 1 , Rose Crossin 2 4 , Les Toop 3 , Ben Hudson 31 University of Otago Medical School, Christchurch, New Zealand.
2 Department of Population Health, University of Otago Medical School, 34 Gloucester St, Christchurch, New Zealand.
3 Department of General Practice, University of Otago Medical School, Christchurch, New Zealand.
4 Corresponding author. Email: rose.crossin@otago.ac.nz
Journal of Primary Health Care 13(4) 302-307 https://doi.org/10.1071/HC21011
Published: 23 December 2021
Journal Compilation © Royal New Zealand College of General Practitioners 2021 This is an open access article licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License
Abstract
Gabapentinoid prescribing is increasing in New Zealand. International evidence suggests that this prescribing trend is followed by increasing harms, including misuse, dependence, overdose, and psychological harms including suicidal thoughts or behaviours. However, there is limited guidance for prescribers on how to manage these potential harms. Here, we summarise the current international literature and identify three main risk factors that can be used for screening purposes when considering prescribing a gabapentinoid, to identify patients that may be at greater risk of harm. Based on current knowledge of harms, we provide guidance to prescribers on monitoring patients taking gabapentinoids. Finally, we summarise the evidence regarding tapering, and highlight key knowledge gaps including other interventions, referral, and data from primary care populations.
Keywords: Pregabalin; gabapentin; general practice; prescribing.
| WHAT GAP THIS FILLS |
| What is already known: Gabapentinoid prescribing is increasing in New Zealand. International evidence suggests that increased gabapentinoid prescribing leads to harms, including misuse, dependence, overdose and suicidal thoughts and behaviours. Despite this, guidance to prescribers on managing these potential harms in a primary care context is sparse. |
| What this study adds: We provide up-to-date evidence to guide prescribers on how to identify patients at high risk of harm from gabapentinoid use, and how to manage this risk in patients already taking gabapentinoids. |
Introduction
Gabapentinoids are a class of drugs including pregabalin and gabapentin, originally indicated for the treatment of epilepsy. Pregabalin became fully subsidised without restriction in New Zealand in 2018. Prior to this, gabapentin was the only subsidised gabapentinoid. Gabapentinoid dispensing grew steadily before pregabalin became subsidised;1 and has since accelerated (Figure 1).
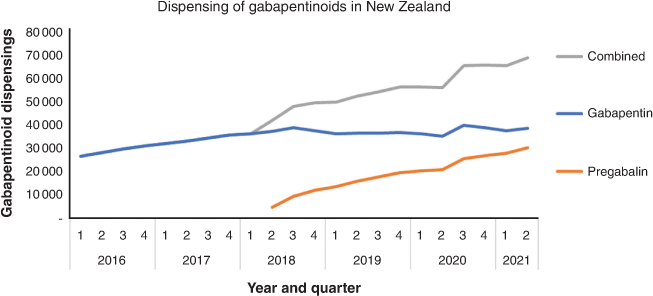
|
Approved indications for gabapentinoids in New Zealand are partial seizures and neuropathic pain,2,3 although findings of benefit for the latter are inconsistent and many patients will have no benefit and discontinue treatment.4 There are also several common ‘off label’ (unapproved) uses, including generalised anxiety disorder.5,6 Evidence of efficacy is limited for some of these unapproved uses.7 Despite the limited evidence of benefit for non-neuropathic pain, gabapentinoid prescribing for this unapproved indication has increased.8,9 The movement away from prescribing opioids in pain syndromes8 has left prescribers with a therapeutic gap, potentially contributing to this increase.
Internationally, in recent years, there have been significant increases in gabapentinoid prescribing, particularly of pregabalin, which has been associated with increasing harms, including misuse, dependence, overdose, and suicidal thoughts or behaviours.10–12 Extra-medical use (use without a prescription or not as directed by a prescriber) has been observed, particularly in high-risk and vulnerable populations.13,14 Dependence is a harm of concern in people using gabapentinoids medically and extra-medically, with evidence of euphoria during high-dose use and withdrawal symptoms.15,16 Pregabalin may be more addictive than gabapentin, but appears to be less addictive than opioids and benzodiazepines.15 Concern has grown internationally over the increase in drug-induced deaths (both intentional and unintentional) involving gabapentinoids.17,18 Although gabapentinoids alone are rarely detected post-mortem following overdose, concurrent use with other central nervous system depressants (particularly opioids, benzodiazepines, and alcohol) is frequently identified, and may additively increase overdose mortality risk.19,20 New onset or worsening suicidal thoughts or behaviours and depression have also emerged as possible harms associated with gabapentinoid use.21,22
With increasing awareness of the potential harms of gabapentinoids and limited effectiveness for some indications, prescribers need to be aware of how to identify patients who may be at greater risk of harm from gabapentinoids, how to monitor for increased harm, and when and how to discontinue these drugs. Discontinuing gabapentinoids may be associated with withdrawal symptoms,15,23 and as discontinuation rates are high,24,25 prescribers are likely to face this issue. A 2018 review outlined the misuse potential of pregabalin in New Zealand,26 but currently there is limited clinical guidance for prescribers on managing harms broader than misuse; a gap this paper aims to fill.
How can prescribers identify patients at risk of harm?
There is no specific screening process to identify gabapentinoid-related harms; however, a systematic review undertaken in 2017 identified young age, current or past opioid misuse, previous substance use disorder, psychiatric co-morbidity, and low income as risk factors for misuse.27 Since 2017, there has been a substantial increase in research examining gabapentinoid-related harms that are broader than misuse. International studies have been undertaken both at a population-level10,11,22,28–32 and in high-risk cohorts including people who use drugs,33,34 people with bipolar disorder,35 people with opioid use disorder,14,36,37 and in people who experience non-fatal17,38 and fatal18,39–41 overdoses; however, we could not identify any studies specifically conducted in a primary care context.
From these studies, we identified three factors that prescribers can use to screen for patients who may be at greater risk of harm from gabapentinoids:
-
Psychiatric history or co-morbidity (including suicidal thoughts or behaviour). Multiple studies have identified this risk factor10,11,14,22,28,29,35 and because of the potential for gabapentinoids to increase suicide risk,22,28 particular caution needs to be taken with patients who have a history of suicidal thoughts or behaviours.
-
Historical or concurrent substance use disorder. People with a historical or current substance use disorder (particularly opioid use disorder) are over-represented in gabapentinoid-related harms,10,22,29,30,32,33,36,37,41 and both gabapentin and pregabalin have been implicated in abuse-related adverse events.32
-
Concurrent use of other sedative medications, particularly opioids and benzodiazepines. Use of other sedative medications increases the risk of harm, particularly overdose mortality.10,11,18,30,31,33,34,37–41 This is of particular concern for patients who may misuse gabapentinoids to enhance the effects of opioids.33,34
Misuse of gabapentinoids appears to be more common in younger people,10,22,29 whereas people in middle-age are at greater risk of overdose-related harms.17,31 Sex does not appear to be a strong factor for predicting harm, with conflicting findings from different studies.17,22,23,40,42
Gabapentinoid misuse typically involves supra-therapeutic doses, so monitoring for dose escalation and development of tolerance are important to identify gabapentinoid use for reinforcing or pleasurable effects.27 Prescribers should also be alert to requests for other sedative medications. Gabapentinoid use is associated with suicide, although there is no current consensus on whether the link is causal.22,28,43,44 This association is stronger with pregabalin than gabapentin,22 which in some cases is not explained by suicidal behaviour or predisposition to psychiatric disorder before treatment initiation.28 Prescribers should therefore also monitor for mood changes or suicidal thoughts or behaviours emerging or worsening in patients using gabapentinoids, particularly pregabalin.
What should prescribers do if patients currently prescribed gabapentinoids are considered high-risk or experiencing harm?
Currently, tapering is the only intervention that can be identified from the literature for gabapentinoids. Three randomised controlled trials (RCTs) of pregabalin for generalised anxiety disorder describe a tapering protocol and the tolerability and effects of that protocol;45–47 however, these studies assessed tapering for people taking therapeutic doses of pregabalin (ranging from 150–600 mg/day) for relatively short time periods, and were not designed to investigate tapering for someone dependent on gabapentinoids or using supra-therapeutic doses. All three studies found that a week-long taper of pregabalin was well tolerated for doses up to 600 mg/day.45–47 Two of these studies reported withdrawal symptoms including anxiety or nervousness, headache, insomnia and irritability,45,46 which occurred in approximately one-third of patients following a 12-week treatment period.46 No RCTs of tapering versus abrupt cessation, or of different tapering protocols, were identified.
Although this evidence suggests that a 1-week taper is tolerated,45–47 this is based on a small number of studies, none of which were designed to assess an optimal approach to tapering. This timing has been reflected in clinical guidance,2,48 although other sources provide an alternate tapering protocol of a maximum reduction rate of 50–100 mg a week for pregabalin and 300 mg every 4 days for gabapentin.49,50 During discontinuation, patients should be monitored for withdrawal symptoms including anxiety, irritability, insomnia, depression and pain (including headache),45,46,51 which may develop even with tapering.45,46,52,53 A case of a patient with no seizure history who had a generalised seizure on gabapentinoid withdrawal has been reported,54 but this appears to be a rare outcome. There is no evidence to suggest that adjunctive treatment with benzodiazepines will attenuate gabapentinoid withdrawal,51 and concurrent use may increase risk of harm.10 There is currently no evidence for adjunctive treatment to support patients through gabapentinoid withdrawal.49
Health equity implications
Any changes to gabapentinoid prescribing practices must consider the health equity implications, particularly in respect to Te Tiriti o Waitangi. The prevalence of chronic pain is not evenly distributed across the New Zealand population, and is more common in people experiencing more socioeconomic deprivation.55 Additionally, the most commonly reported physical disorder in Māori is chronic pain, and among Māori with any 12-month mental health disorder, almost 50% report co-morbid chronic pain.56 A recent study of kaiāwhina (Māori community health workers) perspectives found that Māori have significant unmet need for pain management approaches that are culturally responsive and that treatment of chronic pain in primary care relies heavily on medication-based approaches.57 This is reflected in prescribing data, with older Māori more likely to be prescribed strong opioids than non-Māori of the same age.58 These findings present both a challenge and an opportunity. A challenge, in that if Māori are more likely to meet the screening factors discussed above, there may be a widening of the therapeutic gap in relation to treatment of chronic pain. However, there is an opportunity to develop and improve non-pharmaceutical pain management approaches by and for Māori that are culturally responsive.
What are the knowledge gaps?
To date, there has been no research into gabapentinoid prescribing and harms specifically in New Zealand. This gap could be addressed by studies at a population level, and in high-risk cohorts. Research should focus on primary care contexts, and could seek to validate the screening factors described, as well as improving understanding of prescribing practices and clinical decision-making in relation to gabapentinoid prescribing. Data linkage studies may be particularly useful to better understand gabapentinoid prescribing patterns in New Zealand.
More evidence is required to validate tapering protocols in different populations and with varied doses of gabapentinoids, including management of withdrawal symptoms. Beyond tapering, there is currently no evidence for interventions to address gabapentinoid-related harms, or on when referral for further treatment should occur. Research should focus on brief interventions utilised for other substance use disorders, particularly pharmaceutical opioids and benzodiazepines, for potential efficacy with gabapentinoids. These could include psychological interventions, motivational interviewing, or cognitive behavioural therapy,59 which could be employed alongside tapering. Filling these knowledge gaps may enable the development of a screening, brief intervention, referral to treatment (SBIRT)60,61 framework for gabapentinoids. We suggest there is sufficient evidence to populate the screening component of this model, but there is limited evidence for relevant brief interventions or referral to treatment.
Conclusions
In New Zealand, gabapentinoid dispensing continues to increase, primarily driven by pregabalin,62 and with it, the risk of harms including misuse, overdose, and suicidal thoughts or behaviours.10–12 Although gabapentinoids will be of benefit to some patients, others will experience harms or need to discontinue their use. Therefore, we have sought to provide prescribers with clear guidance on managing the risks associated with gabapentinoid prescribing. Through screening, potential harm from gabapentinoid prescribing can be reduced for high-risk patients in primary care, but further research is needed for effective interventions, improved guidance on tapering, and when referral for further treatment may be warranted.
Competing interests
The authors declare no competing interests for this study. Author RC has previously received funding as an untied educational grant from Seqirus for a project unrelated to this work.
Funding
This article did not receive any specific funding and was conducted as a Trainee Intern student project at the University of Otago.
Data availability
The primary data source for this paper was published academic literature. The data used to create Figure 1 are available from the National Pharmaceutical Data Warehouse (https://www.health.govt.nz/nz-health-statistics/national-collections-and-surveys/collections/pharmaceutical-collection).
Acknowledgements
The authors acknowledge Paul Bridgford of Pegasus Health for assistance with sourcing dispensing data, and Carol Davison of the University of Otago library for assistance with refining the literature search strategy.
References
[1] Pegasus. The Gabapentinoids Medicines Update Bulletin 2020. Christchurch: Pegasus Health; 2020.[2] Medsafe. Pregabalin Pfizer data sheet. Wellington: Medsafe; 2019. [cited 2020 December 21]. Available from: https://www.medsafe.govt.nz/profs/datasheet/l/Lyricacaps.pdf.
[3] Medsafe. Gabapentin data sheet. Wellington: Medsafe; 2019. [cited 2020 December 21]. Available from: https://www.medsafe.govt.nz/profs/Datasheet/a/arrowgabapentincap.pdf.
[4] Derry S, Bell RF, Straube S, et al. Pregabalin for neuropathic pain in adults. Cochrane Database Syst Rev. 2019; CD007076.
| 30673120PubMed |
[5] Generoso MB, Trevizol AP, Kasper S, et al. Pregabalin for generalized anxiety disorder: an updated systematic review and meta-analysis. Int Clin Psychopharmacol. 2017; 32 49–55.
| Pregabalin for generalized anxiety disorder: an updated systematic review and meta-analysis.Crossref | GoogleScholarGoogle Scholar | 27643884PubMed |
[6] Slee A, Nazareth I, Bondaronek P, et al. Pharmacological treatments for generalised anxiety disorder: a systematic review and network meta-analysis. Lancet. 2019; 393 768–77.
| Pharmacological treatments for generalised anxiety disorder: a systematic review and network meta-analysis.Crossref | GoogleScholarGoogle Scholar | 30712879PubMed |
[7] Federico CA, Wang T, Doussau A, et al. Assessment of pregabalin postapproval trials and the suggestion of efficacy for new indications: a systematic review. JAMA Intern Med. 2019; 179 90–7.
| Assessment of pregabalin postapproval trials and the suggestion of efficacy for new indications: a systematic review.Crossref | GoogleScholarGoogle Scholar | 30477010PubMed |
[8] Dyer O. Industry payments to doctors drive surge in gabapentinoid prescribing, study finds. BMJ. 2019; 366 l4672.
| Industry payments to doctors drive surge in gabapentinoid prescribing, study finds.Crossref | GoogleScholarGoogle Scholar | 31300395PubMed |
[9] Goodman CW, Brett AS. A clinical overview of off-label use of gabapentinoid drugs. JAMA Intern Med. 2019; 179 695–701.
| A clinical overview of off-label use of gabapentinoid drugs.Crossref | GoogleScholarGoogle Scholar | 30907944PubMed |
[10] Cairns R, Schaffer AL, Ryan N, et al. Rising pregabalin use and misuse in Australia: trends in utilization and intentional poisonings. Addiction. 2019; 114 1026–34.
| Rising pregabalin use and misuse in Australia: trends in utilization and intentional poisonings.Crossref | GoogleScholarGoogle Scholar | 30098227PubMed |
[11] Crossin R, Scott D, Arunogiri S, et al. Pregabalin misuse‐related ambulance attendances in Victoria, 2012–2017: characteristics of patients and attendances. Med J Aust. 2019; 210 75–9.
| Pregabalin misuse‐related ambulance attendances in Victoria, 2012–2017: characteristics of patients and attendances.Crossref | GoogleScholarGoogle Scholar | 30712302PubMed |
[12] Montastruc F, Loo SY, Renoux C. Trends in first gabapentin and pregabalin prescriptions in primary care in the United Kingdom, 1993–2017. JAMA. 2018; 320 2149–51.
| Trends in first gabapentin and pregabalin prescriptions in primary care in the United Kingdom, 1993–2017.Crossref | GoogleScholarGoogle Scholar | 30480717PubMed |
[13] Grosshans M, Lemenager T, Vollmert C, et al. Pregabalin abuse among opiate addicted patients. Eur J Clin Pharmacol. 2013; 69 2021–5.
| Pregabalin abuse among opiate addicted patients.Crossref | GoogleScholarGoogle Scholar | 23989299PubMed |
[14] Lancia M, Gambelunghe A, Gili A, et al. Pregabalin abuse in combination with other drugs: monitoring among methadone patients. Front Psychiatry. 2020; 10 1022.
| Pregabalin abuse in combination with other drugs: monitoring among methadone patients.Crossref | GoogleScholarGoogle Scholar | 32116826PubMed |
[15] Bonnet U, Scherbaum N. How addictive are gabapentin and pregabalin? A systematic review. Eur Neuropsychopharmacol. 2017; 27 1185–215.
| How addictive are gabapentin and pregabalin? A systematic review.Crossref | GoogleScholarGoogle Scholar | 28988943PubMed |
[16] Schifano F, Chiappini S. Pregabalin: a range of misuse‐related unanswered questions. CNS Neurosci Ther. 2019; 25 659.
| Pregabalin: a range of misuse‐related unanswered questions.Crossref | GoogleScholarGoogle Scholar | 30834646PubMed |
[17] Daly C, Griffin E, Ashcroft DM, et al. Intentional drug overdose involving pregabalin and gabapentin: findings from the National Self-Harm Registry Ireland, 2007–2015. Clin Drug Investig. 2018; 38 373–80.
| Intentional drug overdose involving pregabalin and gabapentin: findings from the National Self-Harm Registry Ireland, 2007–2015.Crossref | GoogleScholarGoogle Scholar | 29264838PubMed |
[18] Nahar LK, Murphy KG, Paterson S. Misuse and mortality related to gabapentin and pregabalin are being under-estimated: a two-year post-mortem population study. J Anal Toxicol. 2019; 43 564–70.
| Misuse and mortality related to gabapentin and pregabalin are being under-estimated: a two-year post-mortem population study.Crossref | GoogleScholarGoogle Scholar | 31062862PubMed |
[19] Elliott SP, Burke T, Smith C. Determining the toxicological significance of pregabalin in fatalities. J Forensic Sci. 2017; 62 169–73.
| Determining the toxicological significance of pregabalin in fatalities.Crossref | GoogleScholarGoogle Scholar | 27864947PubMed |
[20] Häkkinen M, Vuori E, Kalso E, et al. Profiles of pregabalin and gabapentin abuse by postmortem toxicology. Forensic Sci Int. 2014; 241 1–6.
| Profiles of pregabalin and gabapentin abuse by postmortem toxicology.Crossref | GoogleScholarGoogle Scholar | 24835028PubMed |
[21] Tracy DK. Gabapentinoids linked to new risks, including suicidal behaviour. BMJ. 2019; 365 l4021.
| Gabapentinoids linked to new risks, including suicidal behaviour.Crossref | GoogleScholarGoogle Scholar | 31189536PubMed |
[22] Molero Y, Larsson H, D’Onofrio BM, et al. Associations between gabapentinoids and suicidal behaviour, unintentional overdoses, injuries, road traffic incidents, and violent crime: population based cohort study in Sweden. BMJ. 2019; 365 l2147.
| Associations between gabapentinoids and suicidal behaviour, unintentional overdoses, injuries, road traffic incidents, and violent crime: population based cohort study in Sweden.Crossref | GoogleScholarGoogle Scholar | 31189556PubMed |
[23] Gahr M, Freudenmann RW, Hiemke C, et al. Pregabalin abuse and dependence in Germany: results from a database query. Eur J Clin Pharmacol. 2013; 69 1335–42.
| Pregabalin abuse and dependence in Germany: results from a database query.Crossref | GoogleScholarGoogle Scholar | 23292158PubMed |
[24] Chiu T, Brett J, Pearson SA, Schaffer AL. Patterns of pregabalin initiation and discontinuation after its subsidy in Australia. Br J Clin Pharmacol. 2020; 86 1882–7.
| Patterns of pregabalin initiation and discontinuation after its subsidy in Australia.Crossref | GoogleScholarGoogle Scholar | 32153053PubMed |
[25] Viniol A, Ploner T, Hickstein L, et al. Prescribing practice of pregabalin/gabapentin in pain therapy: an evaluation of German claim data. BMJ Open. 2019; 9 e021535.
| Prescribing practice of pregabalin/gabapentin in pain therapy: an evaluation of German claim data.Crossref | GoogleScholarGoogle Scholar | 30928920PubMed |
[26] Ponton R. Pregabalin misuse: preventing potential problems in New Zealand. N Z Med J. 2018; 131 50–4.
| 30001306PubMed |
[27] Evoy KE, Morrison MD, Saklad SR. Abuse and misuse of pregabalin and gabapentin. Drugs. 2017; 77 403–26.
| Abuse and misuse of pregabalin and gabapentin.Crossref | GoogleScholarGoogle Scholar | 28144823PubMed |
[28] Dreier JW, Pedersen CB, Gasse C, Christensen J. Antiepileptic drugs and suicide: role of prior suicidal behavior and parental psychiatric disorder. Ann Neurol. 2019; 86 951–61.
| Antiepileptic drugs and suicide: role of prior suicidal behavior and parental psychiatric disorder.Crossref | GoogleScholarGoogle Scholar | 31621936PubMed |
[29] Driot D, Jouanjus E, Oustric S, et al. Patterns of gabapentin and pregabalin use and misuse: Results of a population-based cohort study in France. Br J Clin Pharmacol. 2019; 85 1260–9.
| Patterns of gabapentin and pregabalin use and misuse: Results of a population-based cohort study in France.Crossref | GoogleScholarGoogle Scholar | 30737829PubMed |
[30] Peckham AM, Evoy KE, Covvey JR, et al. Predictors of gabapentin overuse with or without concomitant opioids in a commercially insured US population. Pharmacotherapy. 2018; 38 436–43.
| Predictors of gabapentin overuse with or without concomitant opioids in a commercially insured US population.Crossref | GoogleScholarGoogle Scholar | 29484686PubMed |
[31] Reynolds K, Kaufman R, Korenoski A, et al. Trends in gabapentin and baclofen exposures reported to US poison centers. Clin Toxicol (Phila). 2020; 58 763–72.
| Trends in gabapentin and baclofen exposures reported to US poison centers.Crossref | GoogleScholarGoogle Scholar | 31786961PubMed |
[32] Vickers-Smith R, Sun J, Charnigo RJ, et al. Gabapentin drug misuse signals: A pharmacovigilance assessment using the FDA adverse event reporting system. Drug Alcohol Depend. 2020; 206 107709
| Gabapentin drug misuse signals: A pharmacovigilance assessment using the FDA adverse event reporting system.Crossref | GoogleScholarGoogle Scholar | 31732295PubMed |
[33] Vickers Smith R, Boland EM, Young AM, et al. A qualitative analysis of gabapentin misuse and diversion among people who use drugs in Appalachian Kentucky. Psychol Addict Behav. 2018; 32 115.
| A qualitative analysis of gabapentin misuse and diversion among people who use drugs in Appalachian Kentucky.Crossref | GoogleScholarGoogle Scholar | 29239621PubMed |
[34] Applewhite D, Regan S, Koenigs K, et al. Use of promethazine, gabapentin and clonidine in combination with opioids or opioid agonist therapies among individuals attending a syringe service program. Int J Drug Policy. 2020; 79 102752.
| Use of promethazine, gabapentin and clonidine in combination with opioids or opioid agonist therapies among individuals attending a syringe service program.Crossref | GoogleScholarGoogle Scholar | 32330837PubMed |
[35] Leith WM, Lambert WE, Boehnlein JK, Freeman MD. The association between gabapentin and suicidality in bipolar patients. Int Clin Psychopharmacol. 2019; 34 27–32.
| The association between gabapentin and suicidality in bipolar patients.Crossref | GoogleScholarGoogle Scholar | 30383553PubMed |
[36] Stein MD, Kenney SR, Anderson BJ, et al. Prescribed and non-prescribed gabapentin use among persons seeking inpatient opioid detoxification. J Subst Abuse Treat. 2020; 110 37–41.
| Prescribed and non-prescribed gabapentin use among persons seeking inpatient opioid detoxification.Crossref | GoogleScholarGoogle Scholar | 31952626PubMed |
[37] Sason A, Adelson M, Schreiber S, Peles E. Pregabalin misuse in methadone maintenance treatment patients in Israel: prevalence and risk factors. Drug Alcohol Depend. 2018; 189 8–11.
| Pregabalin misuse in methadone maintenance treatment patients in Israel: prevalence and risk factors.Crossref | GoogleScholarGoogle Scholar | 29857329PubMed |
[38] Isoardi KZ, Polkinghorne G, Harris K, Isbister GK. Pregabalin poisoning and rising recreational use: a retrospective observational series. Br J Clin Pharmacol. 2020; 86 2435–40.
| Pregabalin poisoning and rising recreational use: a retrospective observational series.Crossref | GoogleScholarGoogle Scholar | 32374500PubMed |
[39] Gomes T, Greaves S, van den Brink W, et al. Pregabalin and the risk for opioid-related death: a nested case–control study. Ann Intern Med. 2018; 169 732–4.
| Pregabalin and the risk for opioid-related death: a nested case–control study.Crossref | GoogleScholarGoogle Scholar | 30140853PubMed |
[40] Slavova S, Miller A, Bunn TL, et al. Prevalence of gabapentin in drug overdose postmortem toxicology testing results. Drug Alcohol Depend. 2018; 186 80–5.
| Prevalence of gabapentin in drug overdose postmortem toxicology testing results.Crossref | GoogleScholarGoogle Scholar | 29554591PubMed |
[41] Thompson A, Morey S, Griffiths A. Pregabalin and its involvement in coronial cases. J Anal Toxicol. 2020; 44 29–35.
| 31095711PubMed |
[42] Bodén R, Wettermark B, Brandt L, Kieler H. Factors associated with pregabalin dispensing at higher than the approved maximum dose. Eur J Clin Pharmacol 2014; 70 197–204.
| Factors associated with pregabalin dispensing at higher than the approved maximum dose.Crossref | GoogleScholarGoogle Scholar | 24141597PubMed |
[43] Patorno E, Bohn RL, Wahl PM, et al. Anticonvulsant medications and the risk of suicide, attempted suicide, or violent death. JAMA. 2010; 303 1401–9.
| Anticonvulsant medications and the risk of suicide, attempted suicide, or violent death.Crossref | GoogleScholarGoogle Scholar | 20388896PubMed |
[44] Grimaldi‐Bensouda L, Nordon C, Rossignol M, et al. Antiepileptic drugs and risk of suicide attempts: a case–control study exploring the impact of underlying medical conditions. Pharmacoepidemiol Drug Saf. 2017; 26 239–47.
| Antiepileptic drugs and risk of suicide attempts: a case–control study exploring the impact of underlying medical conditions.Crossref | GoogleScholarGoogle Scholar | 28052554PubMed |
[45] Feltner DE, Crockatt JG, Dubovsky SJ, et al. A randomized, double-blind, placebo-controlled, fixed-dose, multicenter study of pregabalin in patients with generalized anxiety disorder. J Clin Psychopharmacol. 2003; 23 240–9.
| A randomized, double-blind, placebo-controlled, fixed-dose, multicenter study of pregabalin in patients with generalized anxiety disorder.Crossref | GoogleScholarGoogle Scholar | 12826986PubMed |
[46] Kasper S, Iglesias-García C, Schweizer E, et al. Pregabalin long-term treatment and assessment of discontinuation in patients with generalized anxiety disorder. Int J Neuropsychopharmacol. 2014; 17 685–95.
| Pregabalin long-term treatment and assessment of discontinuation in patients with generalized anxiety disorder.Crossref | GoogleScholarGoogle Scholar | 24351233PubMed |
[47] Pande AC, Crockatt JG, Feltner DE, et al. Pregabalin in generalized anxiety disorder: a placebo-controlled trial. Am J Psychiatry. 2003; 160 533–40.
| Pregabalin in generalized anxiety disorder: a placebo-controlled trial.Crossref | GoogleScholarGoogle Scholar | 12611835PubMed |
[48] Sussex Partnership. Protocol for the management of Pregabalin and Gabapentin use in HMP Lewes United Kingdom: NHS Foundation Trust. Available from: https://www.sps.nhs.uk/wp-content/uploads/2016/08/M-Protocol_for_Mgmt_of_pregab-gaba_Sussex.pdf.
[49] Parsons G. Guide to the management of gabapentinoid misuse. Prescriber. 2018; 29 25–30.
| Guide to the management of gabapentinoid misuse.Crossref | GoogleScholarGoogle Scholar |
[50] Public Health England. Advice for prescribers on the risk of the misuse of pregabalin and gabapentin. United Kingdom: NHS England; 2014.
[51] Hellwig TR, Hammerquist R, Termaat J. Withdrawal symptoms after gabapentin discontinuation. Am J Health Syst Pharm. 2010; 67 910–2.
| Withdrawal symptoms after gabapentin discontinuation.Crossref | GoogleScholarGoogle Scholar | 20484214PubMed |
[52] Gahr M, Franke B, Freudenmann RW, et al. Concerns about pregabalin: further experience with its potential of causing addictive behaviors. J Addict Med. 2013; 7 147–9.
| Concerns about pregabalin: further experience with its potential of causing addictive behaviors.Crossref | GoogleScholarGoogle Scholar | 23519046PubMed |
[53] Tran KT, Hranicky D, Lark T, Jacob N. Gabapentin withdrawal syndrome in the presence of a taper. Bipolar Disord. 2005; 7 302–4.
| Gabapentin withdrawal syndrome in the presence of a taper.Crossref | GoogleScholarGoogle Scholar | 15898970PubMed |
[54] Barrueto F, Green J, Howland MA, et al. Gabapentin withdrawal presenting as status epilepticus. J Toxicol Clin Toxicol. 2002; 40 925–8.
| Gabapentin withdrawal presenting as status epilepticus.Crossref | GoogleScholarGoogle Scholar | 12507063PubMed |
[55] Dominick C, Blyth F, Nicholas M. Patterns of chronic pain in the New Zealand population. N Z Med J. 2011; 124 63–76.
| 21946879PubMed |
[56] Baxter J, Kingi TK, Tapsell R, Durie M. Māori. In: Te Rau Hinengaro: The New Zealand Mental Health Survey. Oakley Browne MA, Wells JE, Scott KM. editors. Wellington: Ministry of Health; 2006.
[57] Devan H, Jones B, Davies C, et al. Are we just dishing out pills constantly to mask their pain? Kaiāwhina Māori health workers’ perspectives on pain management for Māori. N Z Med J. 2021; 134 19–29.
| 34695073PubMed |
[58] Health Quality and Safety Commission New Zealand. Atlas of Healthcare Variation (Opioids). Wellington: Health Quality and Safety Commission; 2021. [cited 2021 November 17]. Available from: https://www.hqsc.govt.nz/our-programmes/health-quality-evaluation/projects/atlas-of-healthcare-variation/opioids/
[59] Parr JM, Kavanagh DJ, Cahill L, et al. Effectiveness of current treatment approaches for benzodiazepine discontinuation: a meta‐analysis. Addiction. 2009; 104 13–24.
| Effectiveness of current treatment approaches for benzodiazepine discontinuation: a meta‐analysis.Crossref | GoogleScholarGoogle Scholar | 18983627PubMed |
[60] Agerwala SM, McCance-Katz EF. Integrating screening, brief intervention, and referral to treatment (SBIRT) into clinical practice settings: a brief review. J Psychoactive Drugs. 2012; 44 307–17.
| Integrating screening, brief intervention, and referral to treatment (SBIRT) into clinical practice settings: a brief review.Crossref | GoogleScholarGoogle Scholar | 23210379PubMed |
[61] Babor TF, McRee BG, Kassebaum PA, et al. Screening, Brief Intervention, and Referral to Treatment (SBIRT) toward a public health approach to the management of substance abuse. Subst Abus. 2007; 28 7–30.
| Screening, Brief Intervention, and Referral to Treatment (SBIRT) toward a public health approach to the management of substance abuse.Crossref | GoogleScholarGoogle Scholar | 18077300PubMed |
[62] Ministry of Health. Pharmaceutical Collection 2021. Wellington: Ministry of Health; 2021. [cited 2021 November 17]. Available from: https://www.health.govt.nz/nz-health-statistics/national-collections-and-surveys/collections/pharmaceutical-collection


