Why does New Zealand have such poor outcomes from colorectal cancer?: the importance of the pre-diagnostic period
Melissa Firth 1 , Tania Blackmore 1 , Lynne Chepulis 1 , Rawiri Keenan 1 , Tim Stokes 2 , Mark Elwood 3 , David Weller 4 , Jon Emery 5 , Ross Lawrenson 1 61 Medical Research Centre, University of Waikato, Hamilton, New Zealand
2 Department of General Practice and Rural Health, University of Otago, Dunedin, New Zealand
3 School of Population Health, University of Auckland, Auckland, New Zealand
4 Centre for Population Health Sciences, The University of Edinburgh, Scotland, UK
5 Medicine, Dentistry and Health Sciences, The University of Melbourne, Victoria, Australia
6 Corresponding author. Email: ross.lawrenson@waikato.ac.nz
Journal of Primary Health Care 13(1) 15-26 https://doi.org/10.1071/HC20049
Published: 31 March 2021
Journal Compilation © Royal New Zealand College of General Practitioners 2021 This is an open access article licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License
Abstract
INTRODUCTION: Over 3000 cases of colorectal cancer (CRC) are diagnosed annually in New Zealand. The proportion of late stage diagnoses is higher than in similar countries, and highest in Māori and Pacific patients. Survival outcomes are poorer than for people in Australia and poor for Māori and Pacific peoples. A regional screening programme is not yet available to the entire target population (60–74 years).
AIM: This study reviews research investigating the pre-diagnostic pathway for CRC in New Zealand and how this may contribute to poorer outcomes.
METHODS: This was a scoping review of original articles examining the pre-diagnostic period for CRC published on the PubMed database between 2009 and 2019. Findings were interpreted within the Model of Pathways to Treatment framework and in context of international evidence.
RESULTS: In total, 83 publications were assessed; eight studies were included. Studies were mainly older than 5 years, qualitative, and focused on screening. Facilitatory factors for the appraisal and help-seeking intervals increased CRC public awareness and the critical role of general practitioners. No specific facilitatory or inhibitory factors were identified for the diagnostic interval, but two studies found that time frames did not meet national and international targets. One study discovered longer pre-diagnostic intervals were associated with younger age at diagnosis.
DISCUSSION: Limited recent research has investigated the CRC pre-diagnostic pathways in NZ. Identification of facilitatory and inhibitory factors and implementation of appropriate strategies to improve them alongside the wider uptake of the screening programme may improve stage at diagnosis and outcomes for New Zealand CRC patients.
KEYwords: Bowel cancer; equity; primary health care
| WHAT GAP THIS FILLS |
| What is already known: Survival from colorectal cancer (CRC) in NZ is lower than in Australia and varies by ethnicity and socioeconomic status. Survival is related to stage at diagnosis, and correcting for stage at diagnosis in ethnic subgroups accounts for most of the survival disparity. The distribution of stage at CRC diagnosis for patients in NZ is worse than that for other countries. Indicators of advanced stage at diagnosis or late diagnosis including presentation to the emergency department and emergency surgery are higher in NZ. National benchmarking identifies regional, ethnic and age-based variation in routes of diagnoses for CRC in NZ. CRC screening in NZ is only for people aged ≥60 years, and an increasing number of patients are diagnosed with CRC at a younger age. This also affects populations with younger age distributions at diagnosis (eg Māori and Pacific) disproportionally. We do not yet know how to improve stage at diagnosis for all patients, regardless of their eligibility for screening. |
| What this study adds: There are few published studies of the NZ population investigating factors affecting the pre-diagnostic period and late diagnosis in patients diagnosed with CRC. Most of these are qualitative, do not explore stage and were undertaken >5 years ago. The lack of information regarding Māori and Pacific populations is identified. Large-scale international collaborations examining the diagnostic pathway for CRC do not include NZ. Currently, research is addressing knowledge gaps about the pre-diagnostic period and its impact on late diagnosis in the NZ population. |
Introduction
Colorectal cancer (CRC; cancer of the colon, rectosigmoid and rectum, or bowel cancer) is common in New Zealand (NZ), with over 3000 new cases diagnosed annually.1 Survival post-diagnosis depends on the extent of disease (stage) at diagnosis, ranging from a 90% 5-year relative survival rate for early stage disease (localised disease, cancerous cells confined to the colon or rectum, American Joint Committee on Cancer (AJCC) stage I, IIA and IIB), to 14% for late or advanced-stage disease (distant disease, cancerous cells found in other organs or distant lymph nodes, AJCC stage IV).2 Diagnosis at an early stage and subsequent intervention are critical to ensuring positive outcomes for patients.
The distribution of stage at diagnosis for NZ patients diagnosed with CRC has been published by the PIPER (Presentations, Investigations, Pathways, Evaluation and Rx) project, the largest study of CRC in NZ to date.3 For colon cancer, the distribution of disease stage at diagnosis was 12% stage I, 27% stage II, 25% stage III and 24% stage IV. For rectal cancer (reported as non-metastatic vs. metastatic only), 19% had metastatic disease at diagnosis.4 These distributions are comparable to that of an unscreened United Kingdom population.5 Stage at diagnosis for NZ patients also varies by ethnicity, with Māori and Pacific patients having higher proportions of late-stage disease than non-Māori/non-Pacific (35%, 31% and 23% respectively).
Disease stage at diagnosis, survival outcomes and the poor distribution of stage at diagnosis, may explain why CRC survival in NZ is poor among international comparisons, particularly when compared to Australia.6–8 The CONCORD-3 study (an international comparison of 18 cancers across 71 countries) reports the 5-year net survival from colon and rectal cancer in 2010–14 as 64% and 66% respectively in NZ versus 71% for both in Australia.6 Similar 5-year net survival rates were also reported from the International Cancer Benchmarking Partnership SURVMARK-2 study, ranking NZ the third worst for survival from both colon and rectal cancer out of seven countries (Australia, Canada, Denmark, Ireland, NZ, Norway and the United Kingdom).7
These large-scale international studies support previous findings of NZ-based researchers who found that the 5-year relative survival for 2006–10 was 5% less than in Australia.8 Survival post-diagnosis also varies depending on patient ethnicity and socioeconomic status. The PIPER project identified significant survival disparities for Māori and Pacific patients, and for people living in areas of high deprivation.4 When investigating survival inequities between ethnic groups, controlling for disease stage significantly reduced the disparity for Māori patients, confirming the importance of early diagnosis in this population.4
Indicators of deficiencies in the pre-diagnostic pathway for CRC include diagnosis being made via emergency department (ED) and obstructive disease at initial diagnosis. In the PIPER study, 31% of patients were diagnosed following ED presentation, and 19% with obstruction.4 These indicators were worse for Māori patients living in areas with the greatest deprivation (socioeconomic status) and for rural patients.4 A NZ report on national performance indicators for bowel cancer between 2013 and 2016 found that 26% of patients were diagnosed following ED presentation and that this varied by District Health Board (DHB),9 and was higher for people aged <50 or >75 years, Māori, Pacific, and people living in areas of high social deprivation, confirming that inequalities in access to primary care and diagnostic services exist.
A staged roll out of a national bowel screening programme has been under way in NZ since July 2017, following a 6-year pilot in Waitemata DHB.10 At the time of writing, 10 of the 20 DHBs are participating in the programme (Hutt Valley, Wairarapa, Waitemata, Southern, Counties Manukau, Nelson Marlborough, Hawkes Bay, MidCentral, Whanganui and Lakes).10 However, even with a screening programme, most bowel cancers are still diagnosed symptomatically and limitations to access remain, including the age band covered by the programme (60–74 years) and disparities in participation.10 CRC occurs across all age groups, and there are patient groups who are more likely to be diagnosed at a younger age, particularly Māori and Pacific peoples.3 A position statement from Te Ohu Rata O Aotearoa, Māori Medical Practitioners Association, highlights that more than half of all cases of CRC occurring in Māori patients are diagnosed before age 60 years.11 As they emphasise, ignoring the different distributions in age at diagnosis between populations will result in increased inequities for Māori patients diagnosed with CRC.11 The incidence of CRC in patients aged <50 years is increasing in both the NZ population12 and internationally.2 Thus, the known limitations of screening coverage combined with increasing incidence in younger patients is reflected in our high rates of late stage at diagnosis and poor survival outcomes. This makes it important to deepen our understanding of the pathway to diagnosis for NZ patients diagnosed with CRC, and what we can do to intervene.
The aim of this paper is to identify and summarise research undertaken in NZ to investigate factors affecting the pre-diagnostic period for patients with CRC, which may contribute to late stage at diagnosis and poor survival.
Methods
This scoping review searched for published studies of factors contributing to late stage at diagnosis that included NZ patients’ data. Both qualitative and quantitative studies were included. Original research articles examining the pre-diagnostic period for patients diagnosed with CRC in NZ published between 2009 and 2019 were searched using the PubMed Central database. The pre-diagnostic period was defined as the time between the discovery of symptoms (or receipt of the invitation letter for bowel screening) and diagnosis. A Medical Subject Heading (MeSH) term search of the PubMed database for ‘colorectal neoplasms’ was combined with additional headings or subheadings including ‘diagnosis’ and ‘primary care’ and ‘New Zealand’ (see Appendix 1). Abstracts were reviewed for all articles, where available.
Editorials, letters to editors, and review articles were excluded. Full-text articles for all relevant studies were obtained, reviewed and data abstracted by one author (MJF). Reference lists of the full-text articles were also reviewed to identify any additional studies to be assessed for inclusion.
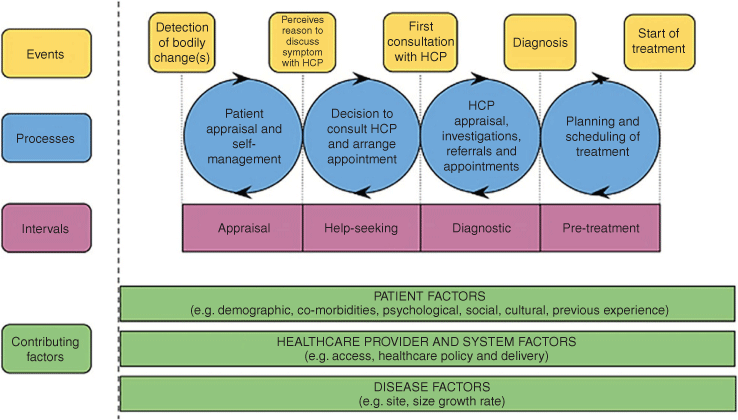
Data were abstracted into a pre-populated proforma for each study. Results were considered within the Model of Pathways to Treatment framework (Fig. 1),13 an internationally recognised theoretical framework for examining pathways to diagnosis. The framework considers four key intervals in the pre-treatment period: appraisal, help-seeking, diagnostic and pre-treatment. The framework allows for the consideration of contributing factors (patient, health-care provider and systems, and disease) and their impact on the intervals as facilitating or impeding progress through the pathway.13 For this study, only the first three intervals are relevant.
|
Results
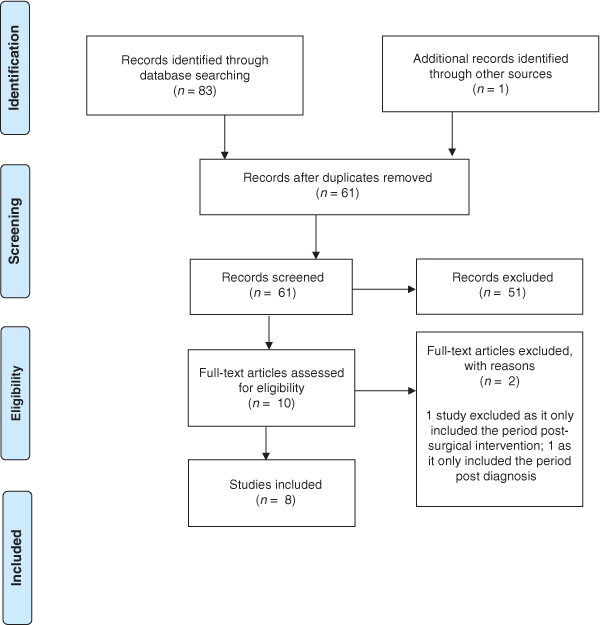
Database searching yielded 83 results. Following removal of duplicate records (n = 22) and 53 exclusions, eight relevant studies were reviewed (see Fig. 2). Reasons for exclusion were (in frequency order): study examining post-diagnostic pathway, diagnostic test parameters or secondary care; editorial; study examining a related diagnosis; letter to editor; review article; clinical guideline document; health economics study; pharmacy-based study; summary paper post conference. One search result could not be accessed for review and one article was identified through reference searching. Included studies were mainly qualitative (five of eight) and conducted >5 years ago (pre-implementation of the screening pilot). Five studies addressed research questions specific to CRC screening. However, the topics explored in these studies included factors that are relevant to the appraisal, help-seeking, and diagnostic intervals, hence their inclusion in this study. Four studies included Māori in their design (see Table 1). Collated findings are grouped into subheadings based on the intervals of the Models of Pathways to Treatment framework.
|

|
Appraisal interval
Studies examining perceptions to CRC screening identified the need to raise awareness of CRC in the public profile.14–17 They suggested that a multiple media campaign to raise awareness of CRC was necessary and could address many of the perceived inhibitory factors to screening, including patient factors surrounding reticence and concern regarding ability to collect faecal specimens, and health-system factors including perceived poor test reliability. Disease factors relating to lack of specific symptoms and perceived slow development of CRC were seen by patients as positive reasons to undergo screening.
In a qualitative study by Windner et al., 95% of participants reported being symptomatic, with 73% reporting more than one symptom. The most common ‘trigger’ symptom was rectal bleeding.18 In considering the pathways within this interval, this research found that most patients consulted a non-health-care professional before consulting a health-care professional, usually a general practitioner (GP).
The critical role of GPs in CRC diagnosis and screening was re-emphasised multiple times by earlier studies examining screening perceptions. No studies aimed to quantify the specific time frame of this interval, but one study examined time frames that included this interval.18 Windner et al. captured self-reported symptom-to-diagnosis interval for all symptomatic patients in their cohort. This time frame includes the appraisal period, the help-seeking interval; and the diagnostic interval: 25% reported <3 months, 44% <6 months and 71% <12 months. Patients aged <50 years were statistically significantly more likely to report a symptom-to-diagnosis interval of ≥6 months than patients in the screening programme range of ≥60 years.18 However, although this study is the most recent and one of the most in-depth, its design means it is probably not representative of the NZ population.
Help-seeking interval
Windner et al. asked direct questions about the help-seeking interval.18 Disease factors identified as facilitating help-seeking behaviour were non-specific symptom concern. Conversely, an acceptable alternative benign explanation for symptoms was the most commonly identified inhibitory factor. Raising public awareness of CRC in the appraisal interval would likely also have an impact on the help-seeking interval, as would the role and relationship with patients’ GPs. No studies attempted to quantify the time frame of this interval, while recognising the challenges of measuring this interval specifically.
Diagnostic interval
Windner et al. reported 54% of participants had zero to one and 6% had four or more visits with a health-care professional before diagnosis. The two quantitative studies largely focused on this interval. Tiong et al. compared their cohort to national and international targets for wait-times between referral to colonoscopy and referral to first treatment, and found that 44% and 21% met the 42 day and 62 day targets respectively. They also identified an increased pre-hospital delay (symptom onset to first specialist appointment) for patients with systemic symptoms and altered bowel habit.19 Murray et al. also report on the period between referral to first treatment, with 68% meeting the comparative UK target of 62 days (median length 35 days).20 There were no significant differences between 2001 and 2005 cohorts or by ethnicity. The greatest delays in this study were seen in the interval from initial referral to first specialist appointment.20
Discussion
This study found that little research has been undertaken about the pre-diagnostic period and its relationship to late diagnosis of CRC in NZ patients. Most studies we found are nearly 10 years old and repeatedly highlight the need for increased public awareness of CRC in NZ to assist self-appraisal, help-seeking and screening participation. They also emphasised the fundamental role GPs and primary health play in a CRC diagnosis and facilitating screening. Qualitative studies demonstrated a failure to meet national and international targets for timeliness, particularly when looking at the period from referral to first specialist appointment, diagnosis or treatment, although delays were not shown to be associated with late-stage diagnosis. A comprehensive mixed-methods approach, including analysis of time frames and qualitative assessment of factors influencing these time frames, has not been undertaken by any one study. Many gaps exist in our understanding of patient, health-care provider, system and disease factors that facilitate or inhibit the pathway to diagnosis for patients diagnosed with CRC in NZ. This study also highlights the lack of information on Māori and Pacific populations, who have poorer CRC outcomes.
Relevant research is being conducted internationally. The International Cancer Benchmarking Partnership is a collaboration to explore population- and health-care-related factors affecting cancer survival outcomes in Australia, Canada, Denmark, Norway, Sweden and the UK.22 Published work to date has studied primary care physician-reported access to investigations, timeliness of test results and wait times for secondary care specialist assessment, and the readiness of primary care physicians to investigate or refer to secondary care following symptoms indicative of cancer.23 They have also reported research on diagnostic routes and time intervals from first symptom to initiation of treatment.24 Both topics were assessed for differences between the six study countries and impact on reported survival figures. These large-scale studies identified a correlation between readiness to refer or investigate suspected cancer symptoms for CRC and survival,23 and showed that wide variations in time intervals exist between the countries, suggesting that improvements could be made in expediting diagnoses.24 They were unable to establish any correlation between greater time intervals and survival (countries with poorer survival did not consistently have longer time intervals).24 The authors of both studies acknowledged the need for more detailed examination and understanding of factors affecting readiness to refer (including changing access to investigations, quality and utility of clinical guidelines, and relationships between primary and secondary care)23 and length of time to diagnosis (noting that, in many cases, longer periods included more investigations).24
Many factors influencing the pre-diagnostic pathway are likely to be population- and health-system-specific. A 2014 NZ study used the International Cancer Benchmarking Partnership survey instrument to survey 192 GPs about a range of cancer types and found that NZ GPs have poor access to colonoscopy compared to other jurisdictions with similar primary care-led health services.25 This work also suggested that NZ GPs are less likely to refer patients at risk of CRC, although it could not address why this may be. Perhaps poorer access to colonoscopy means that GPs are more reluctant to refer and apply a higher threshold before referring for colonoscopy. The critical role of GPs and primary care was highlighted by several studies included in this review and is identified by the Ministry of Health as being ‘key’ in the success of the bowel screening programme.10 Accordingly, we urge support to facilitate GPs in the CRC pre-diagnostic pathway more effectively, through improving our knowledge and understanding of the current inhibitory factors and implementing evidence-based changes to mitigate these factors and improve timely diagnosis for patients.
Perceived delay in CRC diagnosis is important to NZ patients. The 2015 Health and Disability Commissioner report on delayed diagnosis of cancer in primary care between 2004 and 2013 indicated that delays in diagnosing CRC were a large source of complaint and were over-represented relative to its incidence in the population.26 Of 197 complaints, 54 (27%) pertained to a diagnosis of CRC.26 The report suggests that the most common issue for CRC complaints related to non-specific or atypical symptoms presentation.26 However, absence of appropriate examination where symptoms were present was significantly associated with delayed CRC diagnosis.26 For 72% of the CRC cases reviewed, the outcome was death or terminal illness, further emphasising impact of the late stage of diagnosis of CRC in NZ.26 The report found that the total number of cancer complaints made to the Health and Disability Commissioner over the 10-year period significantly increased from 2004 to 2013.26 Although this report is now 6 years old, it is likely that similar issues still exist, as evidenced by a 2019 article from the Associate Commissioner, Jane King, in NZ Doctor, describing a case seen four times over a 9-month period, initially for perianal itch and irritation, progressing to rectal bleeding and change in bowel habit.27 Failure to conduct a rectal examination and insufficient clinical records were found by the Health and Disability Commissioners’ clinical advisor to be a breach of the NZ code of Health and Disability Consumers’ Rights.27 Clear pathways and interventions based on knowledge of facilitatory and inhibitory factors to diagnosis, along with adequate support and prompt and appropriate follow-through from the secondary care sector, are needed to support the primary sector in this crucial role.
The authors are currently undertaking a project using both quantitative and qualitative research methods to examine the pre-diagnostic period for patients diagnosed with CRC in the Midland region of NZ. We hope that this research will identify where the greatest barriers are in the pre-diagnostic period, to drive targeted interventions to reduce late stage diagnosis of CRC. Given the critical role of GPs in the CRC pre-diagnostic pathway, this research, along with any subsequent potential interventions, will include input from colleagues working in primary care.
Conclusions
There is a paucity of recent research examining the pre-diagnostic period for patients in NZ diagnosed with CRC. Given the poor distribution in NZ of stage at diagnosis and survival outcomes known by international comparisons, inequities in outcomes by ethnicity, limitations of the current screening programme, differing age distributions for Māori and Pacific populations, and increasing rates of CRC diagnosis at younger ages, greater understanding is needed of how we can improve stage at diagnosis, via thorough examination of the pre-diagnostic pathway and implementation of facilitatory factors. Work to date highlights the critical role of GPs in this pathway, and the need for carefully designed and evaluated public awareness campaigns for CRC.
Competing interests
The authors declare no competing interests.
Funding
This study was supported by the Health Research Council of New Zealand (HRC grant number 17/147).
Acknowledgements
MJF wrote the main manuscript. All remaining authors edited and reviewed the manuscript.
References
[1] Ministry of Health. New cancer registrations 2016. Wellington: Ministry of Health; 2018 [cited 2018 December 12]. Available from: https://www.health.govt.nz/publication/new-cancer-registrations-2016.[2] Siegel RL, Miller KD, Fedewa SA, et al. Colorectal cancer statistics, 2017. CA Cancer J Clin. 2017; 67 177–93.
| Colorectal cancer statistics, 2017.Crossref | GoogleScholarGoogle Scholar | 28248415PubMed |
[3] Firth MJ, Sharples KJ, Hinder VA, et al. Methods of a national colorectal cancer cohort study: the PIPER Project. N Z Med J. 2016; 129 25–36.
| 27538037PubMed |
[4] Sharples KJ, Firth MJ, Hinder VA, et al. The New Zealand PIPER Project: colorectal cancer survival according to rurality, ethnicity and socioeconomic deprivation-results from a retrospective cohort study. N Z Med J. 2018; 131 24–39.
| 29879724PubMed |
[5] McClements PL, Madurasinghe V, Thomson CS, et al. Impact of the UK colorectal cancer screening pilot studies on incidence, stage distribution and mortality trends. Cancer Epidemiol. 2012; 36 e232–42.
| Impact of the UK colorectal cancer screening pilot studies on incidence, stage distribution and mortality trends.Crossref | GoogleScholarGoogle Scholar | 22425027PubMed |
[6] Allemani C, Matsuda T, Di Carlo V, et al. Global surveillance of trends in cancer survival 2000–14 (CONCORD-3): analysis of individual records for 37,513,025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet. 2018; 391 1023–75.
| Global surveillance of trends in cancer survival 2000–14 (CONCORD-3): analysis of individual records for 37,513,025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries.Crossref | GoogleScholarGoogle Scholar | 29395269PubMed |
[7] Arnold M, Rutherford MJ, Bardot A, et al. Progress in cancer survival, mortality, and incidence in seven high-income countries 1995–2014 (ICBP SURVMARK-2): a population-based study. Lancet Oncol. 2019; 20 1493–505.
| Progress in cancer survival, mortality, and incidence in seven high-income countries 1995–2014 (ICBP SURVMARK-2): a population-based study.Crossref | GoogleScholarGoogle Scholar | 31521509PubMed |
[8] Aye PS, Elwood JM, Stevanovic V. Comparison of cancer survival in New Zealand and Australia, 2006–2010. N Z Med J. 2014; 127 14–26.
| 25530328PubMed |
[9] Ministry of Health. Bowel Cancer Quality Improvement Report. Wellington, New Zealand: Ministry of Health; 2019.
[10] Ministry of Health NZG. About the National Bowel Screening Programme. Wellington: Ministry of Health; 2018. [cited 2018 December 12]. Available from: https://www.timetoscreen.nz/bowel-screening/about-the-national-bowel-screening-programme/.
[11] Hei Āhuru Mōwai, Māori Cancer Leadership Aotearoa. The National Bowel Screening Programme is exacerbating Maori health inequities [press release]. Te Ohu Rata O Aotearoa. Maori Medical Practitioners Association, 22 April 2019.
[12] Araghi M, Soerjomataram I, Bardot A, et al. Changes in colorectal cancer incidence in seven high-income countries: a population-based study. Lancet Gastroenterol Hepatol. 2019; 4 511–8.
| Changes in colorectal cancer incidence in seven high-income countries: a population-based study.Crossref | GoogleScholarGoogle Scholar | 31105047PubMed |
[13] Walter F, Webster A, Scott S, et al. The Andersen Model of Total Patient Delay: a systematic review of its application in cancer diagnosis. J Health Serv Res Policy. 2012; 17 110–8.
| The Andersen Model of Total Patient Delay: a systematic review of its application in cancer diagnosis.Crossref | GoogleScholarGoogle Scholar | 22008712PubMed |
[14] Thompson L, Reeder T, Abel G. I can’t get my husband to go and have a colonoscopy: gender and screening for colorectal cancer. Health. 2012; 16 235–49.
| I can’t get my husband to go and have a colonoscopy: gender and screening for colorectal cancer.Crossref | GoogleScholarGoogle Scholar | 21602246PubMed |
[15] Pitama S, Cave T, Huria T, et al. Exploring Maori health worker perspectives on colorectal cancer and screening. N Z Med J. 2012; 125 75–84.
| 22729062PubMed |
[16] Bong G, McCool J. Chinese peoples’ perceptions of colorectal cancer screening: a New Zealand perspective. N Z Med J. 2011; 124 29–38.
| 21725410PubMed |
[17] Reeder AI. “It’s a small price to pay for life”: faecal occult blood test (FOBT) screening for colorectal cancer, perceived barriers and facilitators. N Z Med J. 2011; 124 11–7.
| 21725408PubMed |
[18] Windner Z, Crengle S, de Graaf B, et al. New Zealanders’ experiences and pathways to a diagnosis of bowel cancer: a cross-sectional descriptive study of a younger cohort. N Z Med J. 2018; 131 30–9.
| 30286063PubMed |
[19] Tiong J, Gray A, Jackson C, et al. Audit of the association between length of time spent on diagnostic work-up and tumour stage in patients with symptomatic colon cancer. ANZ J Surg. 2017; 87 138–42.
| Audit of the association between length of time spent on diagnostic work-up and tumour stage in patients with symptomatic colon cancer.Crossref | GoogleScholarGoogle Scholar | 25091216PubMed |
[20] Murray M, Brown J, Hinder V, et al. The colorectal cancer patients’ journey: the Auckland region. N Z Med J. 2011; 124 18–28.
| 21725409PubMed |
[21] Abel GM, Thompson L. What do specialists and GPs think about the introduction of colorectal cancer screening? A qualitative study. N Z Med J. 2011; 124 89–95.
| 21946966PubMed |
[22] Weller D, Vedsted P, Anandan C, et al. An investigation of routes to cancer diagnosis in 10 international jurisdictions, as part of the International Cancer Benchmarking Partnership: survey development and implementation. BMJ Open. 2016; 6 e009641
| An investigation of routes to cancer diagnosis in 10 international jurisdictions, as part of the International Cancer Benchmarking Partnership: survey development and implementation.Crossref | GoogleScholarGoogle Scholar | 27836872PubMed |
[23] Rose PW, Rubin G, Perera-Salazar R, et al. Explaining variation in cancer survival between 11 jurisdictions in the International Cancer Benchmarking Partnership: a primary care vignette survey. BMJ Open. 2015; 5 e007212
| Explaining variation in cancer survival between 11 jurisdictions in the International Cancer Benchmarking Partnership: a primary care vignette survey.Crossref | GoogleScholarGoogle Scholar | 26017370PubMed |
[24] Weller D, Menon U, Zalounina Falborg A, et al. Diagnostic routes and time intervals for patients with colorectal cancer in 10 international jurisdictions; findings from a cross-sectional study from the International Cancer Benchmarking Partnership (ICBP). BMJ Open. 2018; 8 e023870
| Diagnostic routes and time intervals for patients with colorectal cancer in 10 international jurisdictions; findings from a cross-sectional study from the International Cancer Benchmarking Partnership (ICBP).Crossref | GoogleScholarGoogle Scholar | 30482749PubMed |
[25] Htun HW, Elwood JM, Ioannides SJ, et al. Investigations and referral for suspected cancer in primary care in New Zealand – a survey linked to the International Cancer Benchmarking Partnership. Eur J Cancer Care (Engl). 2017; 26 e12634
| Investigations and referral for suspected cancer in primary care in New Zealand – a survey linked to the International Cancer Benchmarking Partnership.Crossref | GoogleScholarGoogle Scholar |
[26] The Health and Disability Commissioner. Delayed Diagnosis of Cancer in Primary Care: Complaints to the Health and Disability Commissioner: 2004–2013. Auckland: Health and Disability Commissioner; 2015.
[27] King J. Clear patient protocols must be followed to avoid gaps in care. New Zealand Doctor; 2019.
[28] Walter F, Scott S, Webster A, Emery J. The Andersen Model of Total Patient Delay: a systematic review of its application in cancer diagnosis. J Health Services Research and Policy. 2012; 110–18.
Appendix 1. Database Search strategies

|


