High prevalence of malnutrition and frailty among older adults at admission to residential aged care
Idah Chatindiara 1 3 , Jacqueline Allen 2 , Dushanka Hettige 1 , Stacey Senior 1 , Marilize Richter 1 , Marlena Kruger 1 , Carol Wham 11 College of Health, Massey University, Turitea Placem Albany, Auckland, New Zealand
2 Department of Surgery, University of Auckland, Auckland, New Zealand
3 Corresponding author. Email: ichatindi@gmail.com
Journal of Primary Health Care 12(4) 305-317 https://doi.org/10.1071/HC20042
Published: 22 December 2020
Journal Compilation © Royal New Zealand College of General Practitioners 2020 This is an open access article licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License
Abstract
INTRODUCTION: Malnutrition is an under-recognised and under-treated problem often affecting older adults.
AIM: The aim of this study was to evaluate the prevalence of and factors associated with malnutrition and frailty among older adults at early admission to residential aged care.
METHODS: A cross-sectional study was undertaken among eligible older adults within the first week of admission to residential aged care. Participants were assessed for malnutrition risk using the Mini Nutritional Assessment Short Form, frailty using the Fried phenotype criterion, muscle strength using a grip strength dynamometer and gait speed using a 2.4-m walk test. A Cox regression analysis was conducted to identify factors associated with malnutrition risk and frailty status.
RESULTS: Of 174 participants (mean age 85.5 years, 61% women), two-thirds (66%) were admitted to residential aged care from the community. Most (93%) were either malnourished (48%) or at risk of malnutrition (45%). A total of 76% of participants were frail and 24% were pre-frail. Forty-three percent were both malnourished and frail. Low risk of malnutrition was associated with increases in muscle strength [0.96 (0.93–0.99)], gait speed [0.27 (0.10–0.73)] and pre-frailty status [0.32 (0.12–0.83)].
DISCUSSION: This study provides preliminary evidence for high prevalence of malnutrition and frailty at admission to residential aged care. Almost all participants were malnourished or at nutrition risk. Findings highlight the need for strategies to prevent, detect and treat malnutrition in community health care and support nutrition screening at admission to residential aged care.
Keywords: Malnutrition; frailty; residential aged care
| WHAT GAP THIS FILLS |
| What is already known: Poor nutrition prevents healthy ageing and approximately one-in-three older adults living in the community are at risk of malnutrition. |
| What this study adds: This study reports the prevalence of both malnutrition and frailty at admission to residential aged care facilities in the Waitemata District Health Board area. The findings help inform primary health-care clinicians about the utility of nutrition screening and early intervention. |
Introduction
Malnutrition is a common geriatric syndrome, which is associated with loss of independence, poor quality of life and high mortality risk.1–8 Loss of independence is a key factor that necessitates placement to residential aged care.9,10 Residential aged care placement is less preferable than living in the community for many older adults,9–11 as well as an economic burden to the health system. Currently, older adults use ~42% of district health board expenses, of which over half (60%) is used for support services in residential aged care.12 In New Zealand, approximately half of older adults enter residential aged care before they die13 and almost 13% die (all cause mortality) within the first 6 months of placement.14
Nutrition screening is recommended across all health-care settings by international and national nutrition organisations to allow early identification of malnutrition risk.15,16 A plethora of nutrition screening tools have been developed to identify people at risk of malnutrition who might benefit from full nutrition assessment and intervention. Based on its validity, reliability, sensitivity and specificity testing, the Mini Nutritional Assessment Short Form (MNA®-SF) is the most frequently used tool for nutrition risk screening in older adults.17 In 2009, the MNA®-SF was revised to include use of calf circumference as an alternative to body mass index (BMI),18 improving its helpfulness in residential aged care where measurement of height and weight may be difficult. Among older adults who live in residential care, a high prevalence (30–50%) of malnutrition (inadequate nutrient and energy intake) has been reported.19–21
Malnourished older adults tend to have more co-morbidities than their peers who are well nourished, and the prevalence of malnutrition is likely to increase with increasing frailty.22 Age-related loss of muscle mass and strength (sarcopenia) is considered to be both a component23 and an additional cause of physical frailty with advancing age.4 By definition, frailty is a framework for identifying increased vulnerability resulting from failure of multiple physiologic systems.24 The Fried phenotype criterion24 is a robust and frequently used frailty assessment instrument.25,26 Older adults in residential aged care are frequently considered frail, but few studies have objectively reported the prevalence of frailty in this setting. In a systematic review and meta-analysis, only nine studies assessing frailty among residential aged care residents were identified, reporting a wide range of frailty prevalence (19–76%).27
Both malnutrition and frailty are linked to a general decline in health, increased medication use and comorbidities, poor dentition, swallowing difficulties, low cognition,22,28,29 loss of independence and poor quality of life.6,7 Similarities in symptoms and factors associated with malnutrition and frailty explain why sometimes similar intervention strategies are implemented for people who appear malnourished or frail.2 However, the differences in aetiology of these conditions5 means a minimum set of indicators that capture both conditions is required to develop complementary interventions that address mechanisms for both frailty and malnutrition.2 The importance of considering the overlap and distinctiveness of these conditions in research and clinical practice is recognised,1,2 but such studies in residential aged care are limited.2 The aim of this study was to evaluate the prevalence and factors associated with malnutrition and frailty at early admission to residential aged care.
Methods
Study design
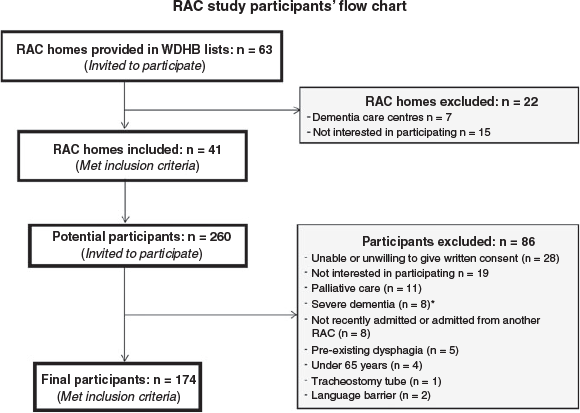
A cross-sectional study was conducted among 174 adults aged ≥65 years (≥55 years for Māori and Pacific participants as they have a lower life expectancy than people of other ethnicities30), within the first week of admission to residential aged care. There are four main types of residential aged care depending on the level of care required; rest home, long-stay hospital, dementia and psycho-geriatric units.31 The study included residents admitted for rest home or hospital level of care, at facilities of the Waitemata District Health Board (DHB) region of Auckland, New Zealand. Participants were excluded if they were in palliative care or previously diagnosed with dementia, swallow disorders, malabsorptive disorders, cancer of the larynx or psychiatric eating disorders. An opportunistic sample of 174 older adults was included as no prior sample size calculation was conducted.
The Health and Disability Ethics Committee: Northern A (Application 14/NTA/70) approved the study.
Recruitment and data collection
Forty-one of the 63 residential aged care facilities registered with the Waitemata DHB agreed to participate. Weekly calls to facility managers were made to check for new admissions. Investigators visited potential participants to provide study details and seek written consent. Family members were proxies for participants who were unable to provide written consent. Demographic and health data were recorded from the participants and their medical files. The health data included co-morbidities, medications and nutritional supplements taken. Participants were asked if they were able to perform activities of daily living (ADL) such as shopping, cleaning and cooking, before residential care admission. Self-reported dental status was recorded as either dentate (able to chew food without appliances) or non-dentate (missing teeth contributing to chewing problems or usage of dental appliance). All data were collected during a single visit between April and October 2017 and all assessments were conducted by three nutrition and dietetics researchers (IC, DH, SS).
Malnutrition status
Using the Mini Nutritional Assessment Short Form (MNA®SF) cut-off points,18 nutrition status was defined as ‘well nourished’ (MNA®SF score ≥12), ‘at risk of malnutrition’ (MNA®SF 8–11) or ‘malnourished’ (MNA®SF score 0–7). Subsequently, malnutrition status was categorised as ‘malnourished’ (MNA®SF score 0–7) and ‘non-malnourished’ (MNA®SF score 8–14). All assessments were performed as per the MNA®SF user guide.32
Dysphagia risk
The Eating Assessment Tool-10 (EAT-10) is a 10 item self-reported validated questionnaire that assesses perception of swallowing difficulty (dysphagia). An increased EAT-10 score indicates increasing dysphagia risk or swallowing difficulties and an EAT-10 score ≥3 is suggestive of swallow impairment.33
Cognitive status
Cognitive status was determined using the Montreal Cognitive Assessment (MoCA) tool.34 A standardised protocol using the MoCA screened for mild cognitive impairment.35 The total possible score is 30 points, and a score ≤26 indicates some level of cognitive impairment.34
Muscle strength
Muscle strength was assessed using hand grip strength36 measured by the Jamar Hydraulic Dynamometer (model #5030J1; Sammons Preston, USA). The measurement procedure followed the standard approved by the American Society of Hand Therapists.37 We recorded the mean of three measurements from the dominant hand. A cut-off point of <20 kg for women and <30 kg for men indicates low muscle strength and risk for sarcopenia,36 so it was used to indicate a positive score for the frailty low muscle strength criterion.
Gait speed
Mobility was assessed by a 2.4-m (8-foot) walk test. Two cones were placed 0.6 m apart at one end of an unobstructed area of floor. A third cone was placed 2.4 m from the second cone, and a fourth cone was placed 0.6 m from the third. A stopwatch (Accusplit, Survivor, Pleasanton, CA, USA) measured the time taken to complete the 2.4-m walk between the second and third cone.38 The 2.4-m walk was performed three times and the mean and fastest time taken to complete the walk were recorded in seconds. The fastest 2.4-m walk (s) was converted to a 2.4-m gait speed (m/s) and the 2.4-m gait speed was converted to a 4-m gait speed using the following equations:39
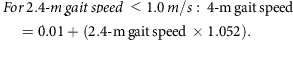
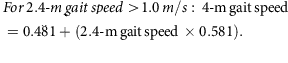
From the 4-m gait speed, a cut-off point of ≤0.8 m/s indicates low gait speed and sarcopenia risk,36 so it was used to indicate a positive score for the frailty low gait speed criterion. Participants who were chair bound or whose clinical notes indicated a recent fall or ‘risk of fall’ were not asked to perform the walk test and were also recorded as having a low gait speed.
Frailty status
Frailty assessment was based on the Fried phenotype criterion, which describes frailty as having three or more positive frailty scores of the following: low gait speed, low muscle strength, low physical activity, extreme exhaustion and unintentional weight loss.24 Having one or two of the conditions was considered ‘pre-frail’.24 If it was not feasible to record a score for one or two components, the data were recorded as ‘not scored’ and the total frailty score was taken from the remaining criterion.40
Positive frailty scores for low gait speed and low muscle strength were recorded from the assessments above. A positive score for the frailty ‘low physical activity’ criterion was recorded for participants responding: ‘one to three times a month’ or ‘hardly ever or never’ to the question: ‘How often do you engage in activities that require a low or moderate level of energy such as gardening, cleaning the car, or going for a walk?’41 (response options were: 1 = ‘more than once a week’; 2 = ‘once a week’; 3 = ‘one to three times a month’ and 4 = ‘hardly ever or never’). As recommended by Fried et al.,24 exhaustion was assessed using the two statements from the Centre for Epidemiologic Studies Depression (CES-D) Scale.42 A positive score for the frailty exhaustion criterion was recorded if participants answered ‘moderate or most of the time’ to either of the two questions: ‘how often in the last week did you feel that everything you did was an effort’ and ‘how often in the last week did you feel that you could not get going’ (responses: 1 = rarely or none of the time [<1 day]; 2 = some or little of the time [1 – 2 days]; moderate amount of the time [3 – 4 days]; 4 = most of the time [5 – 7 days]). Finally, unintentional weight loss was self-reported or if previous weight was available in the medical record, it was calculated through subtracting the ‘current bodyweight’ on the data collection day from records made in the previous 3 months. A positive score for the frailty unintentional weight loss criterion was met for participants who had lost >3 kg within the preceding 3 months.
Statistical analysis
All statistical analyses were completed using SPSS version 24 (SPSS Inc., Chicago, IL, USA). Results were considered significant at P < 0.05. Analyses compared participant characteristics by malnutrition status (non-malnourished vs. malnourished) and by frailty status (non-frail vs. frail). Continuous data were checked for normality. Independent t-tests were performed to compare the differences between normally distributed data (mean ± s.d.) and non-parametric data (median and interquartile range [IQR]) were analysed using the Mann–Whitney U-test. Categorical data are presented as frequencies and percentages, and chi-squared tests of independence or the Fisher’s exact test were used to compare the differences between categories. To assess for factors associated with malnutrition and frailty, we performed Cox regression analyses, adjusting for non-modifiable human factors (age, gender and ethnicity), number of medications and comorbidities, as these factors are known to influence malnutrition and institutionalisation. To assess whether an independent association existed between malnutrition and frailty, an additional Cox regression analysis adjusting for all variables assessed was conducted. Prevalence ratios (PRs) and 95% confidence intervals (CIs) were reported from the regression analyses.
Due to the high prevalence of malnutrition and frailty, a Cox regression with equal follow-up time was conducted, as there was a high chance for logistic regression to overestimate PRs and inadequately control for confounding factors.43 Findings from the logistic regression analyses confirmed the overestimation of the PR, and when adjusting for similar confounding factors used in the Cox regression model, more factors were identified as statistically associated with malnutrition and frailty.
Results
Participant characteristics
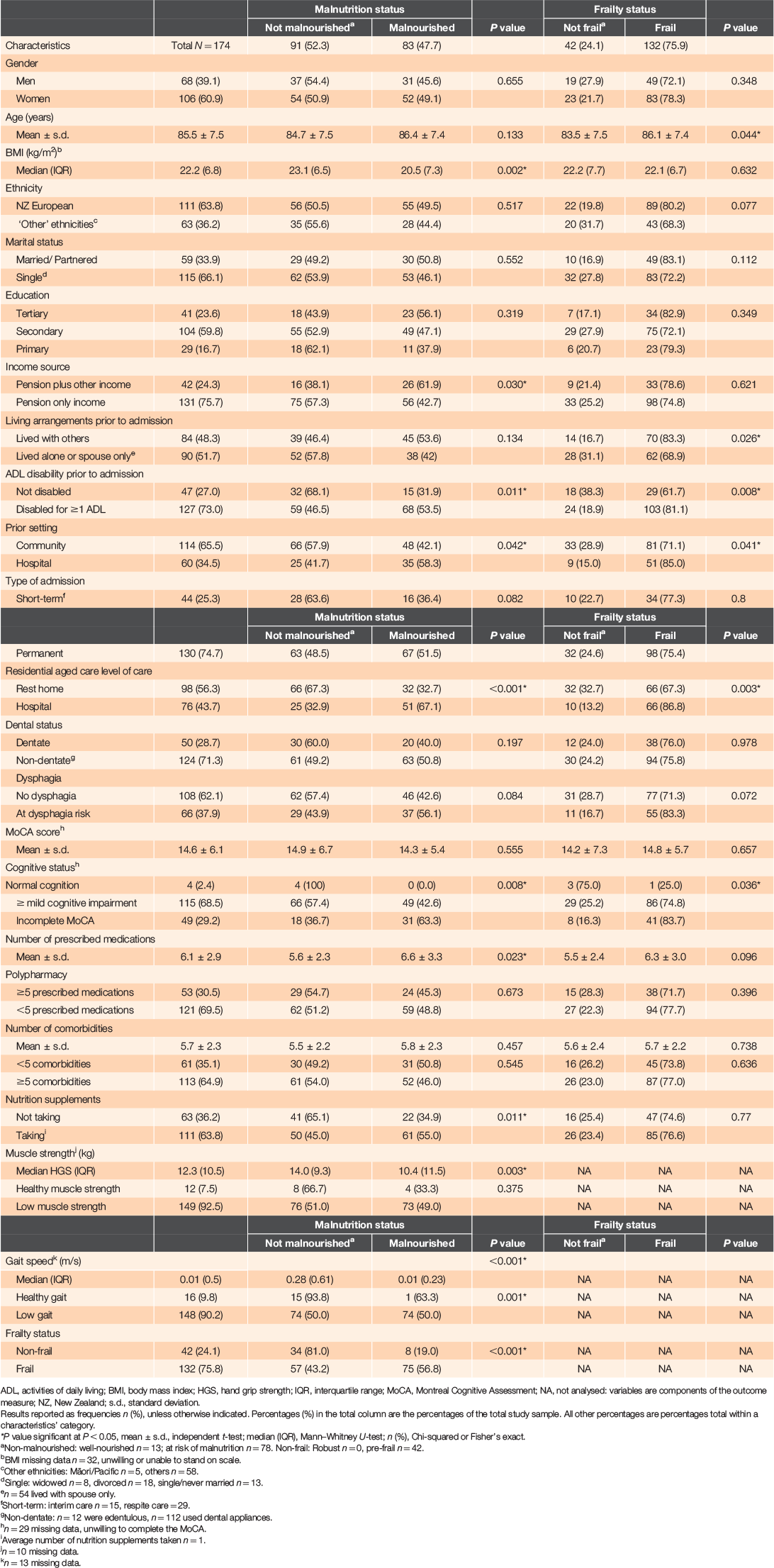
Figure 1 shows the participant recruitment process. A total of 174 older adults were recruited, of whom 75% were entering residential aged care as permanent residents and 25% were short-term (interim or respite) residents. Their mean age was 85.5 years (± 7.5 years) and median BMI was 22.1 (6.8) kg/m2. Approximately 61% were women, 66% were single, widowed or divorced and 64% were of New Zealand European ethnicity. Most participants had a low gait speed (90%), low muscle strength (93%), mild cognitive impairment (69%), were non-dentate (71%), with polypharmacy treatments (70%) and ≥5 comorbidities (65%). Over half (64%) of the participants took nutrition supplements – on average, one supplement, mostly vitamin D, an oral nutrition supplement or multivitamins. Table 1 shows the participant characteristics by malnutrition and frailty status.
|
|
Malnutrition and frailty
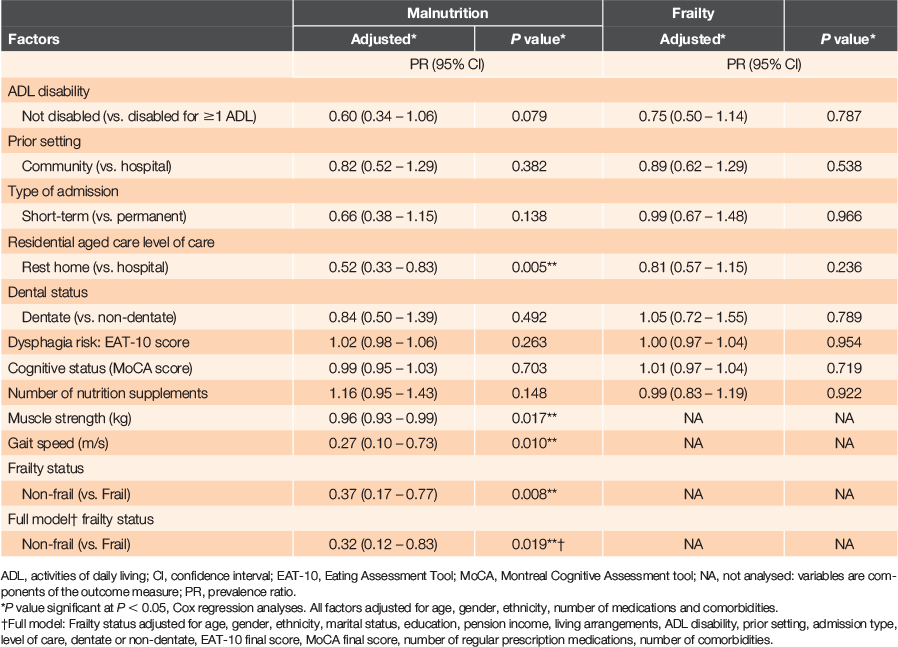
Most participants (92.5%) were either malnourished (83; 47.7%) or at risk of malnutrition (78; 44.8%) and 13 (7.5%) were well nourished. Three-quarters were frail, 42 (24.1%) were pre-frail and none were robust. Table 2 shows the results from a Cox regression analysis of factors associated with malnutrition and frailty. After adjusting for age, gender, ethnicity, number of medications and comorbidities, lower risk for malnutrition was associated with level of residential care: rest home versus hospital, PR = 0.52 (CI: 0.33–0.83); unit increases in participants’ muscle strength (kg), PR = 0.96 (CI: 0.93–0.99) and gait speed (m/s), PR = 0.27 (CI: 0.10–0.73); and frailty status: non-frail versus frail, PR = 0.37 (CI: 0.17–0.77). An independent association between malnutrition and frailty was found after adjusting for several additional factors (full Cox regression model, Table 2), whereby non-frail older adults had ~68% lower risk for malnutrition (PR = 0.32 [CI: 0.12–0.83]), when compared to frail participants.
|
Overlapping prevalence of malnutrition and frailty
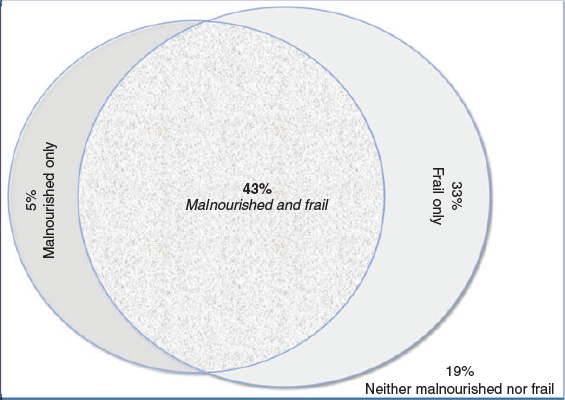
Almost half (75; 43.1%) of the participants had coexisting malnutrition and frailty, 65 (37.4%) were either frail or malnourished and the remaining 34 (19.5%) were neither malnourished nor frail. Figure 2 illustrates the coexistence of malnutrition and frailty. Ninety percent of the malnourished participants were identified as frail and 55% of frail participants were malnourished.
|
Discussion
This study reports the prevalence of both malnutrition and frailty at admission to residential aged care in the Waitemata DHB. The prevalence of malnutrition (48%) and frailty (76%) in the current study is similar to other observations among residential aged care residents, where up to 50% of older adults have been reported to be malnourished19–21 and up to 76% as frail.27 We also found nearly half (45%) of the participants were at risk of malnutrition and approximately one-quarter (24%) were at risk of frailty (pre-frail). This indicates the substantial proportion of older adults where risk for malnutrition or frailty could be lowered with early screening and effective nutrition or frailty interventions.
Our study shows that 4 in 10 (43%) participants were both malnourished and frail at admission to residential aged care. This overlap is similar to the 47% prevalence reported among nursing home residents in Japan, where the coexistence of malnutrition and frailty predicted mortality.8 The current study found no significant difference in the prevalence of malnutrition or frailty between participants requiring short-term versus permanent admission. Although adults entering short-term residential care are usually considered more independent than those entering residential aged care permanently, our finding suggests that both groups had a significant decline in physical function and nutritional status. During the 7-month data collection period, we identified several participants initially admitted for a short-term placement, who returned for permanent placement. Screening for malnutrition and frailty at admission to residential aged care, regardless of term of placement, will identify these conditions and enable support to be given to people returning to the community. Early identification and intervention may reduce the health burden and risk of complications for both community-dwelling older adults and aged care residents. As most participants (92.5%) were either malnourished (47.7%) or at risk (44.8%), the current study provides evidence for the importance of malnutrition screening at both admission to residential aged care and in all settings, as recommended by the Australian and New Zealand Society for Geriatric Medicine.15
Timely screening for malnutrition has some potential workforce and fiscal constraints. The opportunity to screen vulnerable older adults in primary care arises at routine health checks or influenza vaccination and may mitigate decline in nutrition status, especially if it leads to a care plan. Furthermore, in New Zealand, the interRAI (international Resident Assessment Instrument) has been adopted and mandated as an assessment tool by the Ministry of Health; risk of malnutrition is being assessed as part of the interRAI suite.44 The adoption of the interRAI occurred after the current study designing, so interRAI data were not collected. However, the interRAI nutrition indicators have a low sensitivity compared to the validated malnutrition screening tools (MNA®SF and SCREENII),45 so we recommend that a short malnutrition screening tool such as the MNA®SF be administered alongside the interRAI, or at least to improve the sensitivity of the interRAI by changing the BMI cut-off point for detecting malnutrition risk to <23 kg/m2 versus the current <20 kg/m2. A BMI >23 kg/m2 has been found to have a protective effect in older adults so that cut-off is used in nutrition tools such as the MNA®SF.18
The current study demonstrates that after adjusting for several factors, non-frail older adults had ~68% lower risk for malnutrition than frail participants. An independent association between malnutrition and frailty has previously been reported among older adults (mean age 6746 and 76 years47) residing in the community. In our study, malnutrition was found to be associated with low gait speed and low muscle strength (hand grip strength), which are both components of the Fried phenotype.24 Although the Fried phenotype is the most widely used frailty assessment tool, consensus has not been reached on the gold standard for frailty assessment in older adults.23 Some studies suggest use of one or two physical performance measures such as hand grip strength,48 timed up and go and gait speed49 as adequate markers for frailty. A bi-directional hazardous relationship exists between malnutrition and frailty. Although malnutrition is a major cause of muscle loss and frailty50,51 there are several mechanisms by which frailty can also lead to inadequate nutrient and energy intake. Frail older adults may have inadequate muscle strength or mobility24 for meal preparation and are likely to have low appetite52 and swallowing difficulties,53 reducing food intake. Associations between malnutrition and some individual components of physical frailty, including lower physical health related quality of life,54 low muscle strength55 and low gait speed,56 have been reported among older New Zealanders. The current study’s findings provide support for co-existence of malnutrition and frailty among older New Zealanders across residential settings.
When adjusting for covariates, no statistically significant associations were found between malnutrition or frailty and potential risk factors including dental status, dysphagia risk, taking nutrition supplements and cognitive status. This is inconsistent with previous observations, where these factors have been associated with malnutrition22,28 and frailty.29,57 As our study design included an in-person clinic visit at residential aged care centres, with assessments over ~1 h, older adults with dementia, terminal illnesses or under palliative care were excluded, and we acknowledge prevalence ratios would have been higher with the inclusion of those conditions. Initial exclusion criteria reduced the number of participants who would have shown pre-existing dysphagia complaints or cognitive impairments. In future, we would like to examine associations of malnutrition and frailty with these high-risk factors.
The following study limitations are noted. Although a snapshot of prevalence and coexistence of malnutrition and frailty at admission to residential aged care is provided, the sample size may not be statistically powered for external generalisability or preventing type two errors (failure to reject a false null hypothesis that there is no association between the risk factors and malnutrition or frailty). This may explain some of the non-statistically significant associations reported. Two-thirds of the participants showed some level of cognitive impairment; the possibility of recall bias cannot be completely ruled out for the assessments that relied on participants’ memory. To increase reliability and accuracy of the subjective data collected from participants with some level of cognitive impairment, the data were cross-checked with clinical notes, a family member or a registered nurse at the residential care facility. Although the study design included body composition assessment using bio-impedance analysis scales, only 42 participants completed this assessment, as most participants had metal implants or were unable to stand still on the scale. In future studies, the use of body composition assessment equipment where older adults lie prone may assist with measurement.
Conclusion and implications
This study found that at admission to residential aged care, most (93%) older adults were either malnourished (48%) or at risk of malnutrition (45%) and 76% were frail. Four in 10 older adults had both malnutrition and frailty, indicating a wide overlap of the conditions. However, because distinct prevalence ratios were observed (48% for malnutrition and 76% for frailty), this study supports emerging research highlighting the importance of understanding the overlap and distinctiveness of both conditions, to design the most appropriate complementary interventions. Our findings support screening for early identification and intervention at admission to residential care. As two-thirds of the participants were admitted from the community, they may have benefited from screening in primary care to identify and treat malnutrition. Considering the co-occurrence of frailty and malnutrition, appropriate complementary interventions are needed.
Competing interests
All authors declare no potential conflicts of interest with respect to the research, authorship or publication of this article.
Funding
This research did not receive any funding from agencies in the public, commercial or not-for-profit sectors.
Acknowledgements
Data collection from only newly admitted residents required a lot of coordination among several people from different organisations. The authors greatly appreciate the Waitemata DHB representatives, Ms Teresa Stanbrook and Ms Karla Powell, and several residential aged care staff members who all participated in data collection. Further appreciations go to all the participants and Waitemata DHB residential aged care facilities that agreed to participate.
References
[1] Verlaan S, Ligthart-Melis GC, Wijers SL, et al. High prevalence of physical frailty among community-dwelling malnourished older adults–A systematic review and meta-analysis. J Am Med Dir Assoc. 2017; 18 374–82.| High prevalence of physical frailty among community-dwelling malnourished older adults–A systematic review and meta-analysis.Crossref | GoogleScholarGoogle Scholar | 28238676PubMed |
[2] Laur CV, McNicholl T, Valaitis R, Keller HH. Malnutrition or frailty? Overlap and evidence gaps in the diagnosis and treatment of frailty and malnutrition. Appl Physiol Nutr Metab. 2017; 42 449–58.
| Malnutrition or frailty? Overlap and evidence gaps in the diagnosis and treatment of frailty and malnutrition.Crossref | GoogleScholarGoogle Scholar | 28322060PubMed |
[3] Hu X, Zhang L, Wang H, et al. Malnutrition-sarcopenia syndrome predicts mortality in hospitalized older patients. Sci Rep. 2017; 7 3171
| Malnutrition-sarcopenia syndrome predicts mortality in hospitalized older patients.Crossref | GoogleScholarGoogle Scholar | 28600505PubMed |
[4] Cruz-Jentoft AJ, Kiesswetter E, Drey M, Sieber CC. Nutrition, frailty, and sarcopenia. Aging Clin Exp Res. 2017; 29 43–8.
| Nutrition, frailty, and sarcopenia.Crossref | GoogleScholarGoogle Scholar | 28155181PubMed |
[5] Jeejeebhoy KN. Malnutrition, fatigue, frailty, vulnerability, sarcopenia and cachexia: overlap of clinical features. Curr Opin Clin Nutr Metab Care. 2012; 15 213–9.
| Malnutrition, fatigue, frailty, vulnerability, sarcopenia and cachexia: overlap of clinical features.Crossref | GoogleScholarGoogle Scholar | 22450775PubMed |
[6] Rasheed S, Woods RT. Malnutrition and quality of life in older people: a systematic review and meta-analysis. Ageing Res Rev. 2013; 12 561–6.
| Malnutrition and quality of life in older people: a systematic review and meta-analysis.Crossref | GoogleScholarGoogle Scholar | 23228882PubMed |
[7] Kojima G, Iliffe S, Jivraj S, Walters K. Association between frailty and quality of life among community-dwelling older people: a systematic review and meta-analysis. J Epidemiol Community Health. 2016; 70 716–21.
| Association between frailty and quality of life among community-dwelling older people: a systematic review and meta-analysis.Crossref | GoogleScholarGoogle Scholar | 26783304PubMed |
[8] Kamo T, Takayama K, Ishii H, et al. Coexisting severe frailty and malnutrition predict mortality among the oldest old in nursing homes: a 1-year prospective study. Arch Gerontol Geriatr. 2017; 70 99–104.
| Coexisting severe frailty and malnutrition predict mortality among the oldest old in nursing homes: a 1-year prospective study.Crossref | GoogleScholarGoogle Scholar | 28126636PubMed |
[9] Wiles JL, Leibing A, Guberman N, et al. The meaning of “ageing in place” to older people. Gerontologist. 2011; 52 gnr098
| The meaning of “ageing in place” to older people.Crossref | GoogleScholarGoogle Scholar | 21983126PubMed |
[10] Jorgensen D, Arksey H, Parsons M, et al. Why do older people in New Zealand enter residential care rather than choosing to remain at home, and who makes that decision? Ageing Int. 2009; 34 15–32.
| Why do older people in New Zealand enter residential care rather than choosing to remain at home, and who makes that decision?Crossref | GoogleScholarGoogle Scholar |
[11] Chatindiara I, Sheridan N, Kruger M, Wham C. Eating less the logical thing to do? Vulnerability to malnutrition with advancing age: a qualitative study. Appetite. 2019; 146 104502
| Eating less the logical thing to do? Vulnerability to malnutrition with advancing age: a qualitative study.Crossref | GoogleScholarGoogle Scholar | 31678148PubMed |
[12] Ministry of Health. DHB spending on services for older people. Wellington, New Zealand: Ministry of Health; 2016. [cited 2018 January 31]. Available from: http://www.health.govt.nz/nz-health-statistics/health-statistics-and-data-sets/older-peoples-health-data-and-stats/dhb-spending-services-older-people.
[13] Broad JB, Ashton T, Gott M, et al. Likelihood of residential aged care use in later life: a simple approach to estimation with international comparison. Aust N Z J Public Health. 2015; 39 374–9.
| Likelihood of residential aged care use in later life: a simple approach to estimation with international comparison.Crossref | GoogleScholarGoogle Scholar | 26095070PubMed |
[14] Connolly MJ, Broad JB, Boyd M, et al. Residential aged care: the de facto hospice for New Zealand’s older people. Australas J Ageing. 2014; 33 114–20.
| Residential aged care: the de facto hospice for New Zealand’s older people.Crossref | GoogleScholarGoogle Scholar | 24521449PubMed |
[15] Australian and New Zealand Society for Geriatric Medicine Position Statement: undernutrition and the older person. Australas J Ageing. 2017; 36 75
| Position Statement: undernutrition and the older person.Crossref | GoogleScholarGoogle Scholar | 27357752PubMed |
[16] Thiyagarajan JA, Cesari M, Kumar S, et al. Diagnostic accuracy of screening tool for non-specialist health care settings: a summary of findings from ICOPE rapid reviews. Geneva, Switzerland: World Health Organization; 2017.
[17] Kaiser MJ, Bauer JM, Rämsch C, et al. Frequency of malnutrition in older adults: a multinational perspective using the mini nutritional assessment. J Am Geriatr Soc. 2010; 58 1734–8.
| Frequency of malnutrition in older adults: a multinational perspective using the mini nutritional assessment.Crossref | GoogleScholarGoogle Scholar | 20863332PubMed |
[18] Kaiser MJ, Bauer J, Ramsch C, et al. Validation of the Mini Nutritional Assessment Short-Form (MNA®-SF): a practical tool for identification of nutritional status. J Nutr Health Aging. 2009; 13 782–8.
| Validation of the Mini Nutritional Assessment Short-Form (MNA®-SF): a practical tool for identification of nutritional status.Crossref | GoogleScholarGoogle Scholar | 19812868PubMed |
[19] Gaskill D, Black LJ, Isenring EA, et al. Malnutrition prevalence and nutrition issues in residential aged care facilities. Australas J Ageing. 2008; 27 189–94.
| Malnutrition prevalence and nutrition issues in residential aged care facilities.Crossref | GoogleScholarGoogle Scholar | 19032620PubMed |
[20] Keller H, Vucea V, Slaughter SE, et al. Prevalence of malnutrition or risk in residents in long term care: comparison of four tools. J Nutr Gerontol Geriatr. 2019; 38 329–44.
| Prevalence of malnutrition or risk in residents in long term care: comparison of four tools.Crossref | GoogleScholarGoogle Scholar | 31335280PubMed |
[21] Suominen M, Muurinen S, Routasalo P, et al. Malnutrition and associated factors among aged residents in all nursing homes in Helsinki. Eur J Clin Nutr. 2005; 59 578–83.
| Malnutrition and associated factors among aged residents in all nursing homes in Helsinki.Crossref | GoogleScholarGoogle Scholar | 15744328PubMed |
[22] Fávaro-Moreira NC, Krausch-Hofmann S, Matthys C, et al. Risk factors for malnutrition in older adults: a systematic review of the literature based on longitudinal data. Adv Nutr. 2016; 7 507–22.
| Risk factors for malnutrition in older adults: a systematic review of the literature based on longitudinal data.Crossref | GoogleScholarGoogle Scholar | 27184278PubMed |
[23] Morley JE, Vellas B, Van Kan GA, et al. Frailty consensus: a call to action. J Am Med Dir Assoc. 2013; 14 392–7.
| Frailty consensus: a call to action.Crossref | GoogleScholarGoogle Scholar | 23764209PubMed |
[24] Fried LP, Tangen CM, Walston J, et al. Frailty in older adults evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001; 56 M146–57.
| Frailty in older adults evidence for a phenotype.Crossref | GoogleScholarGoogle Scholar | 11253156PubMed |
[25] Buta BJ, Walston JD, Godino JG, et al. Frailty assessment instruments: systematic characterization of the uses and contexts of highly-cited instruments. Ageing Res Rev. 2016; 26 53–61.
| Frailty assessment instruments: systematic characterization of the uses and contexts of highly-cited instruments.Crossref | GoogleScholarGoogle Scholar | 26674984PubMed |
[26] Dent E, Kowal P, Hoogendijk EO. Frailty measurement in research and clinical presidential aged caretice: a review. Eur J Intern Med. 2016; 31 3–10.
| 27039014PubMed |
[27] Kojima G. Prevalence of frailty in nursing homes: a systematic review and meta-analysis. J Am Med Dir Assoc. 2015; 16 940–5.
| 26255709PubMed |
[28] Agarwal E, Miller M, Yaxley A, Isenring E. Malnutrition in the elderly: a narrative review. Maturitas. 2013; 76 296–302.
| 23958435PubMed |
[29] Pegorari MS, Tavares DMdS. Factors associated with the frailty syndrome in elderly individuals living in the urban area. Rev Lat Am Enfermagem. 2014; 22 874–82.
| Factors associated with the frailty syndrome in elderly individuals living in the urban area.Crossref | GoogleScholarGoogle Scholar | 25493685PubMed |
[30] Statistics New Zealand. Population. Wellington, New Zealand: Stats NZ; 2018. [cited 2017 November 22]. Available from: https://www.stats.govt.nz/topics/population.
[31] Ministry of Health. Long-term residential care for older people: what you need to know. Wellington: Ministry of Health; 2012.
[32] Rubenstein LZ, Harker JO, Salvà A, et al. Screening for undernutrition in geriatric practice developing the short-form mini-nutritional assessment (MNA-SF). J Gerontol A Biol Sci Med Sci. 2001; 56 M366–72.
| Screening for undernutrition in geriatric practice developing the short-form mini-nutritional assessment (MNA-SF).Crossref | GoogleScholarGoogle Scholar | 11382797PubMed |
[33] Belafsky PC, Mouadeb DA, Rees CJ, et al. Validity and reliability of the Eating Assessment Tool (EAT-10). Ann Otol Rhinol Laryngol 2008; 117 919–24.
| Validity and reliability of the Eating Assessment Tool (EAT-10).Crossref | GoogleScholarGoogle Scholar | 19140539PubMed |
[34] Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc 2005; 53 695–9.
| The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment.Crossref | GoogleScholarGoogle Scholar | 15817019PubMed |
[35] Nasreddine Z. Montreal Cognitive Assessment (MoCA): administration and scoring instructions. Canada; 2004. Available from: http://www.mocatest.org/.
[36] Cruz-Jentoft AJ, Baeyens JP, Bauer JM, et al. Sarcopenia: European consensus on definition and diagnosis Report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010; 39 412–23.
| Sarcopenia: European consensus on definition and diagnosis Report of the European Working Group on Sarcopenia in Older People.Crossref | GoogleScholarGoogle Scholar | 20392703PubMed |
[37] Roberts HC, Denison HJ, Martin HJ, et al. A review of the measurement of grip strength in clinical and epidemiological studies: towards a standardised approach. Age Ageing. 2011; 40 afr051
| A review of the measurement of grip strength in clinical and epidemiological studies: towards a standardised approach.Crossref | GoogleScholarGoogle Scholar | 21624928PubMed |
[38] Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994; 49 M85–94.
| A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission.Crossref | GoogleScholarGoogle Scholar | 8126356PubMed |
[39] Guralnik JM, Ferrucci L, Pieper CF, et al. Lower extremity function and subsequent disability: consistency across studies, predictive models, and value of gait speed alone compared with the Short Physical Performance Battery. J Gerontol A Biol Sci Med Sci. 2000; 55 M221–31.
| Lower extremity function and subsequent disability: consistency across studies, predictive models, and value of gait speed alone compared with the Short Physical Performance Battery.Crossref | GoogleScholarGoogle Scholar | 10811152PubMed |
[40] Bieniek J, Wilczyński K, Szewieczek J. Fried frailty phenotype assessment components as applied to geriatric inpatients. Clin Interv Aging. 2016; 11 453
| 27217729PubMed |
[41] Santos-Eggimann B, Cuénoud P, Spagnoli J, Junod J. Prevalence of frailty in middle-aged and older community-dwelling Europeans living in 10 countries. J Gerontol A Biol Sci Med Sci. 2009; 64A 675–81.
| Prevalence of frailty in middle-aged and older community-dwelling Europeans living in 10 countries.Crossref | GoogleScholarGoogle Scholar |
[42] Orme JG, Reis J, Herz EJ. Factorial and discriminant validity of the Center for Epidemiological Studies Depression (CES-D) scale. J Clin Psychol. 1986; 42 28–33.
| Factorial and discriminant validity of the Center for Epidemiological Studies Depression (CES-D) scale.Crossref | GoogleScholarGoogle Scholar | 3950011PubMed |
[43] Barros AJ, Hirakata VN. Alternatives for logistic regression in cross-sectional studies: an empirical comparison of models that directly estimate the prevalence ratio. BMC Med Res Methodol. 2003; 3 21
| Alternatives for logistic regression in cross-sectional studies: an empirical comparison of models that directly estimate the prevalence ratio.Crossref | GoogleScholarGoogle Scholar | 14567763PubMed |
[44] interRAI New Zealand. [cited 2019 September 18]. Available from: https://www.interrai.co.nz/about/
[45] Radich MJ. Comparison of nutritional components of InterRAI-HC to validated nutrition screening tools (Thesis, Master of Dietetics). Dunedin: University of Otago; 2014.
[46] Wei K, Nyunt MSZ, Gao Q, et al. Frailty and malnutrition: related and distinct syndrome prevalence and association among community-dwelling older adults: Singapore Longitudinal Ageing Studies. J Am Med Dir Assoc. 2017; 18 1019–28.
| Frailty and malnutrition: related and distinct syndrome prevalence and association among community-dwelling older adults: Singapore Longitudinal Ageing Studies.Crossref | GoogleScholarGoogle Scholar | 28804010PubMed |
[47] Boulos C, Salameh P, Barberger-Gateau P. Malnutrition and frailty in community dwelling older adults living in a rural setting. Clin Nutr. 2016; 35 138–43.
| Malnutrition and frailty in community dwelling older adults living in a rural setting.Crossref | GoogleScholarGoogle Scholar | 25649256PubMed |
[48] Syddall H, Cooper C, Martin F, et al. Is grip strength a useful single marker of frailty? Age Ageing. 2003; 32 650–6.
| Is grip strength a useful single marker of frailty?Crossref | GoogleScholarGoogle Scholar | 14600007PubMed |
[49] Clegg A, Rogers L, Young J. Diagnostic test accuracy of simple instruments for identifying frailty in community-dwelling older people: a systematic review. Age Ageing. 2014; 44 148–52.
| Diagnostic test accuracy of simple instruments for identifying frailty in community-dwelling older people: a systematic review.Crossref | GoogleScholarGoogle Scholar | 25355618PubMed |
[50] Kaiser M, Bandinelli S, Lunenfeld B. Frailty and the role of nutrition in older people. A review of the current literature. Acta Biomed. 2010; 81 37–45.
| 20518190PubMed |
[51] Goisser S, Guyonnet S, Volkert D. The role of nutrition in frailty: an overview. J Frailty Aging. 2016; 5 74–7.
| 27224496PubMed |
[52] Martone AM, Onder G, Vetrano D, et al. Anorexia of aging: a modifiable risk factor for frailty. Nutrients. 2013; 5 4126–33.
| Anorexia of aging: a modifiable risk factor for frailty.Crossref | GoogleScholarGoogle Scholar | 24128975PubMed |
[53] Payne M, Morley JE. Dysphagia, dementia and frailty. J Nutr Health Aging 2018; 22 562–565.
| Dysphagia, dementia and frailty.Crossref | GoogleScholarGoogle Scholar | 29717753PubMed |
[54] Wham CA, Teh R, Moyes S, et al. Health and social factors associated with nutrition risk: results from life and living in advanced age: a cohort study in New Zealand (LILACS NZ). J Nutr Health Aging. 2015; 19 637–45.
| Health and social factors associated with nutrition risk: results from life and living in advanced age: a cohort study in New Zealand (LILACS NZ).Crossref | GoogleScholarGoogle Scholar | 26054500PubMed |
[55] Chatindiara I, Allen J, Popman A, et al. Dysphagia risk, low muscle strength and poor cognition predict malnutrition risk in older adults at hospital admission. BMC Geriatr. 2018; 18 78
| Dysphagia risk, low muscle strength and poor cognition predict malnutrition risk in older adults at hospital admission.Crossref | GoogleScholarGoogle Scholar | 29562879PubMed |
[56] Chatindiara I, Williams V, Sycamore E, et al. Associations between nutrition risk status, body composition and physical performance among community-dwelling older adults. Aust N Z J Public Health. 2018; 43 56–62.
| Associations between nutrition risk status, body composition and physical performance among community-dwelling older adults.Crossref | GoogleScholarGoogle Scholar | 30457191PubMed |
[57] Bahat G, Yilmaz O, Kilic C, et al. Association between dysphagia and frailty. Clin Nutr. 2018; 37 S181


